Abstract
Human infection with Leishmania braziliensis can lead to cutaneous leishmaniasis (CL) or mucosal leishmaniasis (ML). We hypothesize that the intense tissue destruction observed in ML is a consequence of an uncontrolled exacerbated inflammatory immune response, with cytotoxic activity. For the first time, this work identifies the cellular sources of inflammatory and antiinflammatory cytokines, the expression of effector molecules, and the expression of interleukin-10 (IL-10) receptor in ML and CL lesions by using confocal microscopy. ML lesions displayed a higher number of gamma interferon (IFN-γ)-producing cells than did CL lesions. In both ML and CL, CD4+ cells represented the majority of IFN-γ-producing cells, followed by CD8+ cells and CD4− CD8− cells. The numbers of tumor necrosis factor alpha-positive cells, as well as those of IL-10-producing cells, were similar in ML and CL lesions. The effector molecule granzyme A showed greater expression in ML than in CL lesions, while inducible nitric oxide synthase did not. Finally, the expression of IL-10 receptor was lower in ML than in CL lesions. Thus, our data identified distinct cytokine and cell population profiles for CL versus ML patients and provide a possible mechanism for the development of ML disease through the demonstration that low expression of IL-10 receptor is present in conjunction with a cytotoxic and inflammatory profile in ML.
The control of a given infection relies on the ability of our organism to mount an efficient immune response that leads to the control of the infectious agent. However, in addition to the need for an early response that will trigger killing mechanisms, further control of the response is critical for the establishment of pathology or cure. This coordinated action of the immune system involves mobilization of inflammatory mechanisms andantiinflammatory, modulatory responses. Thus, a failure to control an exacerbated inflammatory response can be a major cause of pathology and morbidity. Determination of the mechanisms involved with the development of pathology in human disease will open new possibilities of immunological intervention for prevention of pathology.
A great deal of information concerning the dynamics of immune responses to infectious agents, particularly the role of T helper 1 (Th1) and Th2 populations, have come from studies of cellular reactivity to Leishmania parasites (33). The insights obtained from these experimental studies provided critical knowledge toward the understanding of several other diseases. Thus, study of the immune response in human leishmaniasis, while clarifying the mechanisms involved in the establishment of protective and pathogenic responses in this important disease, will also aid in the comprehension of other diseases where the control of inflammatory responses is crucial.
While it is generally accepted that infection with different species of Leishmania leads to the establishment of different clinical forms, the same species of this parasite may also lead to different diseases, demonstrating that the host's immune response is essential for disease pathogenesis. In humans, infection with Leishmania braziliensis causes different forms of American cutaneous leishmaniasis, such as the localized and disseminated forms, as well as mucosal disease. Localized cutaneous leishmaniasis (CL) is characterized by the appearance of a single or a few ulcerated skin lesions and a relatively effective response to conventional antimonial treatment (19). Approximately 3% of patients previously affected by CL may develop the mucosal disease (29). Mucosal leishmaniasis (ML) is marked by the disfiguring nature of the associated lesions, usually involving nasal or oropharyngeal mucosal areas. Treatment of ML often requires more than one course of conventional antimonial therapy or even the use of more-toxic drugs such as amphotericin B or immunomodulatory approaches (20).
Previous studies have demonstrated that CL is associated with high in vitro proliferative responses to parasite-derived antigens (12, 13, 14). Moreover, increased production of gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) has been observed in both in vitro responses and in situ analysis of CL lesions (5, 31). Interestingly, interleukin-10 (IL-10) production was also detected in patients with CL by several different methodological approaches (4, 9). An increase in the frequency of IL-10- as well as IFN-γ-producing cells was recently seen (3). These findings suggest that the mild nature of CL may be related to the early establishment of efficient parasite-killing mechanisms, associated with the control of exacerbated inflammatory responses. Comparative analysis of the immune responses of CL and ML patients has shown that peripheral blood mononuclear cells (PBMC) from individuals that develop ML display a higher proliferative response to parasite antigens and higher levels of IFN-γ and TNF-α production, associated with lower levels of IL-10, than those from CL patients (5). Furthermore, addition of IL-10 to in vitro cultures led to modulation of antigen-induced IFN-γ production by PBMC from CL but not ML patients, suggesting that an uncontrolled inflammatory response is related to the severe tissue destruction observed in ML (5). However, the mechanisms responsible for this uncontrolled response have not yet been determined.
In this work, we investigated the question of whether the destructive ML lesions are associated with a high inflammatory response induced by the local production of inflammatory cytokines, cytotoxic molecules, and decreased modulatory responses. Thus, we evaluated the expression and cellular sources of the inflammatory cytokines IFN-γ and TNF-α, and of the antiinflammatory cytokine IL-10, in lesions from patients with CL and ML by using triple-staining confocal microscopy analysis. We also evaluated the expression of the effector molecules granzyme A and inducible nitric oxide synthase (iNOS) by cells from inflammatory lesions of CL and ML patients, correlating their expression with cytotoxic mechanisms involved in tissue damage in both clinical forms. Finally, we analyzed the expression of the IL-10 receptor as a possible mechanism for the exacerbated in situ response in ML. Our results clearly characterize ML as an example of how an uncontrolled response can lead to pathology, and they should be taken into consideration when vaccines and/or therapies based on the induction of inflammatory responses are proposed.
MATERIALS AND METHODS
Patients.
The patients analyzed in this study were from Corte de Pedra, an area of endemicity for Leishmania braziliensis infection, located 280 km southwest of Salvador, the capital of Bahia state, Brazil. All patients were volunteers, and informed consent was obtained from all individuals prior to collection of lesion material. Medical care and patient evaluation and characterization were under the responsibility of Edgar Carvalho, with participation of a dermatologist and ear-nose-throat specialists to identify typical leishmaniasis skin or mucosal lesions, respectively. Diagnosis of leishmaniasis was performed based on clinical and laboratory criteria. Detection of suggestive skin or mucosal lesions was associated with a positive skin Montenegro test, parasite isolation, and/or histopathological analysis to confirm a diagnosis of CL or ML. For all CL and ML cases, parasite species were typed to confirm that the disease was due to L.braziliensis infection. CL patients enrolled in this study (n = 14) presented with a single ulcerated lesion and had not been previously diagnosed with or treated for leishmaniasis. ML patients (n = 7) presented with nasal lesions and, at the time of biopsy collection, did not display concomitant cutaneous disease. At the time of sample collection, the ages of the active lesions were estimated at 30 to 45 days for both CL and ML lesions. While the estimated times of CL and ML lesion development were comparable, ML patients had been exposed to leishmaniasis previously, since they had previously had cutaneous disease, which was healed at the time when mucosal disease was diagnosed. The interval between the cure of CL and the diagnosis of ML was variable. The estimated time of lesion development was based on questioning of the patients, together with the intervals of patient examinations in the area of endemicity. Treatment was offered to all patients as needed despite their enrollment in this project. However, CL and ML patients were not under treatment when samples were collected. Lesions were collected either at the Corte de Pedra health care facility or at the Serviço de Imunologia, Hospital Universitário Edgar Santos, UFBA, in Salvador. Skin biopsy specimens were taken from the borders of active lesions, using a 4-mm-diameter punch, after the application of a local anesthetic. Mucosal lesions were obtained by excision of a small fragment (approximately 3 mm, on average) using a scalpel, after local anesthetic application. Lesions were maintained in a 30% sucrose solution for approximately 30 min at 4°C; then they were transferred to OCT Tissue Tek freezing medium and immediately placed in dry ice. The material was stored at −70°C until analysis. The Ethical Committees of the Bahia and Minas Gerais Federal Universities approved all procedures involved in this study.
Histological and immunofluorescence staining.
Individual 4- to 5-μm cryosections were placed in saline-precoated slides and fixed for 10 min with acetone. Slides were incubated with phosphate-buffered saline for 15 min and subjected either to hematoxylin-eosin staining or to immunofluorescence using specific monoclonal antibodies. Standard hematoxylin-eosin staining was performed to ensure tissue integrity as well as for evaluation of the intensity and location of the inflammatory infiltrate. Immunofluorescence reactions involved incubation with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-labeled monoclonal antibodies directed to surface receptors (CD4 clone S3.5, CD8 clone 3B5, CD68 clone Ki-M7, or IL-10 receptor clone B7-H1) or intracellular molecules (granzyme A clone CLB-GA28, iNOS or IFN-γ clone B27, IL-10 clone 9D7, or TNF-α clone 20A4), respectively. Sections were incubated with antibody mixtures overnight at 4°C. After staining, preparations were extensively washed with phosphate-buffered saline, counterstained with 4′,6′-diamidino-2-phenylindole (DAPI), and mounted using antifade mounting medium (Molecular Probes). Slides were kept at 4°C, protected from light, until acquisition in a laser scanning confocal microscope (Zeiss). Isotype controls were analyzed separately to confirm the lack of nonspecific staining. Monoclonal antibodies were purchased from Caltag (Burlingame, CA), except for the anti-IL-10 receptor monoclonal antibody, which was purchased from Becton Dickinson (San Jose, CA).
Confocal analysis.
Imaging was performed with a Bio-Rad MRC 1024 laser scanning confocal system running LaserSharp 3.0 software coupled to a Zeiss microscope (Axiovert 100) with a water immersion objective (40×, 1.2 numerical aperture). A water-cooled argon UV laser (488 nm) or a krypton/argon laser was used to excite the preparation (through its 363-nm, 488-nm, or 568-nm line), and light emitted was selected with band-pass filters (522/35 for FITC and DAPI, 598/40 for PE). For each section, the inflammatory infiltrate present in the connective tissue adjacent to the epithelia was located and an area presenting with a uniform infiltrate was selected for analysis. Within this inflammatory area, a minimum of three images (fields) were collected. Image analysis and processing were performed with LaserSharp (Bio-Rad), Confocal Assistant, Adobe Photoshop, and Image Tool software. Analyses were performed by counting the total number of cells in the three fields acquired and calculating the average number of cells per section for each patient. This calculation was performed for each parameter analyzed, allowing for determination of the total number of inflammatory cells (total number of DAPI+ cells within the inflammatory infiltrate), the number of FITC or PE single-positive cells, and the number of double-positive cells. The counts were performed blindly, the results were expressed as the average number of cells per field for each parameter for each patient, and then the values were averaged for each group. The results are representative of two experiments per patient. Intensities of IFN-γ and IL-10 receptor expression were determined using Pseudocolor software. This analysis allows for the conversion of pixels into numbers, providing a numerical analysis of intensity in relation to the number of pixels in the area analyzed. This number was then corrected for the number of cells present in the area analyzed.
Statistical analysis.
Statistical analysis of the data was performed using JMP statistical software from SAS. The comparisons of means for a given parameter were performed using a nonparametric (one tailed, considering unequal variance of groups) t test. Results were considered statistically different when the analysis returned a P value of <0.05.
RESULTS
ML lesions display a more intense inflammatory infiltrate due to recruitment of CD4+ and CD8+ cells than CL lesions.
The intensity of the inflammatory infiltrate in lesions from CL and ML patients was determined using conventional histological analysis, as well as by counting the number of DAPI-positive cells per field using confocal microscopy, as described in Materials and Methods. The average number of cells was significantly higher in ML lesions than in CL lesions, demonstrating the occurrence of a more intense inflammation in ML (Table 1). The inflammatory infiltrates were predominantly composed of mononuclear cells in both CL and ML lesions. Moreover, while the numbers of CD68+ cells in CL and ML lesions were not statistically different, the numbers of CD4+ and CD8+ cells were higher in ML than in CL lesions (Table 1). Although increases in CD4+ and CD8+ cells were observed in ML lesions, the CD4/CD8 ratio was similar between groups. Lastly, the relative percentages of CD4+, CD8+, and CD68+ cells did not differ between groups (data not shown).
TABLE 1.
Total numbers of inflammatory cells and numbers of CD4+, CD8+, and CD68+ cells in CL and ML lesions
| Clinical form | Total no. of cellsa
|
|||
|---|---|---|---|---|
| DAPI+ | CD4+ | CD8+ | CD68+ | |
| CL (n = 14) | 4,580 ± 1,784 | 1,059 ± 757 | 1,069 ± 542 | 1,313 ± 580 |
| ML (n = 7) | 6,664 ± 1,821 | 1,671 ± 480 | 1,846 ± 801** | 1,822 ± 908 |
Counted based on expression of specific fluorescent markers as described in Materials and Methods. Double asterisks indicate that the difference between ML and CL lesions is statistically significant at a P value of 0.05.
Cellular sources of cytokines in ML and CL lesions.
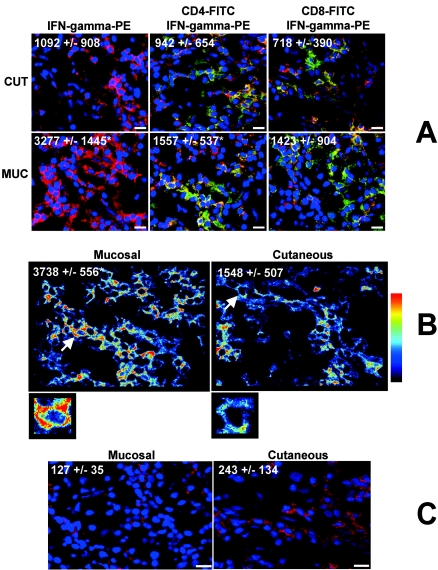
The expression of the inflammatory cytokines TNF-α and IFN-γ was evaluated in lesions from CL and ML patients. The analysis showed that the total numbers of cells expressing TNF-α were not statistically different between groups (Table 2). Evaluation of the cellular sources of TNF-α demonstrated that CD68+ cells are the main cell population expressing this cytokine in both CL and ML lesions (Table 2) and that approximately 50% of the CD68+ cells are committed to expression of TNF-α in CL and ML lesions (Table 2). While the total expression of TNF-α was not statistically different when ML and CL lesions were compared (Table 2), the total number of IFN-γ-expressing cells was higher in lesions from ML than from CL patients (Fig. 1A). Not only the number of IFN-γ+ cells, but also the intensity of expression of this cytokine, was higher in lesions from patients with ML than in those from patients with CL (Fig. 1B). We also observed that ML patients displayed a statistically higher frequency of CD4+ IFN-γ+ cells than CL patients (Fig. 1A), and although the average numbers of CD8+ IFN-γ+ cells were higher in ML than in CL lesions, statistical significance was not achieved (P < 0.06) (Fig. 1A). Our analysis of the contributions of CD4+, CD8+, and CD4− CD8− cells toward the production of IFN-γ showed that CD4+ cells are the main source of IFN-γ in ML and CL lesions (43%± 21% and 51% ± 11%, respectively), followed by CD8+ (38% ± 10% and 40% ± 15% for CL and ML, respectively) and CD4− CD8− (21% ± 15% and 10% ± 4% for CL and ML, respectively) cells.
TABLE 2.
Numbers of TNF-α+ and IL-10+ cells and relative contributions of the CD68+ population to the production of these cytokines in lesions from patients with CL and ML
| Clinical form | TNF-α production
|
IL-10 production
|
||||||
|---|---|---|---|---|---|---|---|---|
| No. of TNF-α+ cellsa
|
% Contribution of CD68+ cellsb | % Commitment of CD68+ cellsc | No. of IL-10+ cellsa
|
% Contribution of CD68+ cellsb | % Commitment of CD68+ cellsc | |||
| Total | CD68+ | Total | CD68+ | |||||
| CL (n = 14) | 1,144 ± 691 | 909 ± 547 | 78 ± 12 | 65 ± 21 | 2,025 ± 785 | 1,267 ± 598 | 62 ± 13 | 79 ± 16 |
| ML (n = 7) | 1,354 ± 993 | 1,148 ± 937 | 80 ± 12 | 56 ± 23 | 2,468 ± 1,597 | 1,148 ± 1,158 | 53 ± 17 | 91 ± 4d |
Counted based on expression of specific fluorescent markers for TNF-α or IL-10 either alone or together with CD68, as described in Materials and Methods.
Calculated as the percentage of CD68+ cells expressing the cytokine in relation to the total expression of the cytokine.
Calculated as the percentage of CD68+ cells expressing the cytokine in relation to total CD68+ cells.
Statistically significantly different from the value for CL (P < 0.05).
FIG. 1.
Differential expression of IFN-γ and IL-10 receptor in CL and ML lesions. (A) Representative images from confocal microscopy analyses for determination of the numbers of total IFN-γ+ cells, CD4+ IFN-γ+ cells, and CD8+ IFN-γ+ cells in CL (CUT) and ML (MUC) lesions. Tissue sections were stained with FITC-labeled anti-CD4 or anti-CD8 monoclonal antibodies and with PE-labeled anti-IFN-γ and were counterstained with DAPI as described in Materials and Methods. The three optical sections for each patient were obtained simultaneously with lines 363, 488, and 568 of the argon/krypton laser and the proper set of filters. Overlays for CD4 or CD8 (green), IFN-γ (red), and DAPI (blue) in CL and ML lesions are shown. Cells that are double positive for CD4 or CD8 and IFN-γ appear in yellow. These images are representative of each group. Values are the average ± standard deviation for each group following numerical determination of the number of positive cells for the indicated molecule(s). Asterisks indicate statistically significant differences (ML > CL) at a P value of <0.05. CL and ML lesions from 14 and 7 patients, respectively, were analyzed. Bar, 10 μm. (B) Representative analysis of the intensity of expression of IFN-γ in CL and ML lesions by using Pseudocolor software as described in Materials and Methods. The predominance of red indicates a higher intensity of expression than the predominance of blue (see the color scale on the right). White arrows indicate the cells enlarged below. The asterisk indicates a statistically significant difference (ML > CL) at a P value of <0.05. Fourteen CL and seven ML lesions were analyzed. (C) Representative images from the confocal microscopy analysis for determination of the numbers of total IL-10 receptor-positive cells in CL and ML lesions. Tissue sections were stained with a PE-labeled monoclonal antibody against the IL-10 receptor as described in Materials and Methods. A total of nine CL and seven ML lesions were analyzed. Bars, 10 μm. The images are representative of one of two independent experiments for each individual lesion. Values are the average ± standard deviation for each group from the numerical analysis of the number of cells expressing the indicated molecule(s).
Analysis of the antiinflammatory cytokine IL-10 was also performed. We observed that the total number of IL-10-expressing cells and the number of CD68+ IL-10+ cells did not differ between groups (Table 2). CD68+ cells account for approximately 62 and 53% of the total expression of IL-10 in CL and ML lesions, respectively (Table 2), suggesting that 40 to 50% of the IL-10 present in the lesion sites comes from other cellular sources. These percentages were statistically equivalent when the two groups were compared. Interestingly, while approximately 80% of the CD68+ cells expressed IL-10 in CL lesions, more than 90% of the CD68+ cells from ML lesions expressed IL-10, showing a higher commitment of the monocytic population to the production of IL-10 in ML than in CL lesions (Table 2).
Cells from ML lesions display a lower intensity of expression of the IL-10 receptor than cells from CL lesions.
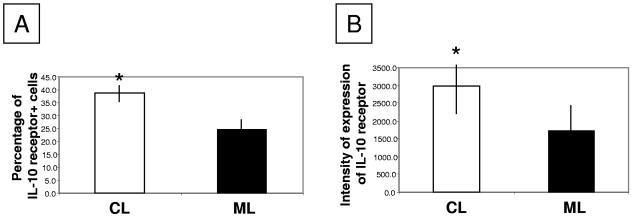
Previous studies performed by our group demonstrated that addition of IL-10 to in vitro cultures led to modulation of antigen-induced IFN-γ production by PBMC from CL but not ML patients, suggesting a deficient response to IL-10 in ML. This finding, together with the fact that we observed an intense inflammatory response in ML lesions, led to the hypothesis that IL-10 unresponsiveness was due to low IL-10 receptor expression, which would lead to an exacerbated inflammatory response. Thus, we evaluated the expression of the IL-10 receptor in ML and CL lesions, determining the numbers and percentages of cells expressing this molecule, as well as its intensity of expression per cell. We observed that while the numbers of cells expressing the IL-10 receptor were similar in ML and CL lesions (Fig. 1C), the percentage of cells expressing this molecule (Fig. 2A) and the intensity of expression of the IL-10 receptor on a cell-by-cell basis (Fig. 2B) were lower in ML than in CL lesions.
FIG. 2.
Analysis of the expression of the IL-10 receptor in CL and ML lesions. (A) Percentage of cells expressing the IL-10 receptor in CL and ML lesions; (B) intensity of expression of the IL-10 receptor per cell. Tissue sections were stained with PE-labeled monoclonal antibodies against the IL-10 receptor and counterstained with DAPI, and the number and percentage of cells expressing the IL-10 receptor, as well as the intensity of its expression, were calculated as described in Materials and Methods. Asterisks indicate statistically significant differences between groups at a P value of <0.05. Nine CL and seven ML lesions were analyzed.
Expression of granzyme A and iNOS in ML and CL lesions.
We determined the expression of two effector molecules, granzyme A and iNOS, in lesions from CL and ML patients. Although the total number of cells expressing iNOS was apparently higher in CL lesions, our analyses did not show statistical significance (P = 0.2) (Table 3). However, the percentage of expression of iNOS+ cells was higher in CL than in ML (Table 3).The total number of cells expressing granzyme A and the number of granzyme A+ CD8+ T cells were determined using double-staining confocal analysis. These studies demonstrated that ML lesions display a significantly higher number of granzyme A+ cells than CL lesions (Table 3). Interestingly, the numbers of CD8+ granzyme A+ cells were similar in the two groups (Table 3). However, the commitment of the CD8+ cell population to cytotoxic activity was higher in ML lesions, as shown by the higher percentage of CD8+ cells expressing granzyme A (Table 3). Moreover, a statistically significant positive correlation was seen between total IFN-γ+ and total granzyme A+ cells in lesions from ML but not CL patients (data not shown). Further analysis also showed that while in CL lesions CD8+ cells are responsible for 70% of the total granzyme A expression, in ML lesions this population contributes to approximately 50% of the total granzyme A expression. This shows that in CL the majority of the cells producing granzyme A are CD8+, while in ML other cell populations are responsible for 50% of the total production.
TABLE 3.
Expression of iNOS and granzyme A in lesions from patients with CL and ML
| Clinical form | iNOS expression
|
Granzyme A expression
|
||||
|---|---|---|---|---|---|---|
| No. of iNOS+ cellsa | % iNOS+ cells | No. of granzyme A+ cellsa
|
% Contribution of CD8+ cellsb | % Commitment of CD8+ cellsc | ||
| Total | CD8+ | |||||
| CL (n = 9) | 1,274 ± 230 | 87 ± 6 | 924 ± 466 | 687 ± 391 | 70 ± 21 | 58 ± 13 |
| ML (n = 7) | 1,159 ± 231 | 64 ± 19d | 1,562 ± 610e | 950 ± 587 | 56 ± 14d | 74 ± 17e |
Counted based on expression of specific fluorescence for either granzyme A or iNOS alone or granzyme A and CD8, as described in Materials and Methods.
Calculated as the percentage of CD8+ cells expressing granzyme A in relation to total granzyme A expression.
Calculated as the percentage of CD8+ cells expressing granzyme A in relation to total CD8+ cells.
Significantly lower than value for CL (P < 0.05).
Significantly higher than value for CL (P < 0.05).
DISCUSSION
Analyses of the immunological profile of PBMC from ML patients have suggested that the tissue pathology associated with this severe form of leishmaniasis is related to the establishment of an exacerbated inflammatory response, representing a polar hypersensitivity reaction to Leishmania infection (5, 12, 32). In this study, in which an extensive multiparameter confocal microscopy analysis of the inflammatory infiltrate in ML and CL lesions was performed, several points of evidence in favor of this hypothesis were revealed: (i) the inflammatory infiltrate in ML lesions is more intense than that observed in CL lesions; (ii) higher expression of the inflammatory cytokine IFN-γ and of the cytotoxic molecule granzyme A was observed in ML than in CL lesions; and (iii) the intensity of expression of the IL-10 receptor was lower in ML than in CL lesions. Thus, our work demonstrates the occurrence of in situ hyperactivation in ML lesions, likely due to down-regulation of the IL-10 receptor, and provides new insights toward the understanding of the complex mechanisms of immunopathology related to ML and CL.
Previous studies have demonstrated that the inflammatory infiltrate of CL and ML lesions is primarily composed of T cells, followed by macrophages and very few or no B cells or NK cells (9, 15, 32). These studies strengthen the argument that T cells play a critical role in leishmaniasis. A mixed cytokine profile, including expression of TNF-α, IFN-γ, IL-10, and IL-4, was detected in CL and ML lesions by using PCR and immunohistochemistry techniques (1, 11, 24, 25, 30, 31). The use of PCR, while allowing for a sensitive analysis, does not provide information on the intensity, composition, and architecture of the inflammatory infiltrate or on the cellular sources of the cytokines analyzed. By use of multiparameter confocal microscopy, we determined that CD4+ T cells were the primary source of IFN-γ in both CL and ML, followed by CD8+ T cells and CD4− CD8− cells. These results are similar to those we found previously when determining the sources of IFN-γ in PBMC from ML patients (5). However, CD4− CD8− cells appeared as the second major source of IFN-γ in PBMC from CL patients (9). This difference may reflect distinctive recruitment of T-cell subpopulations to lesion sites. We also determined that monocytes are the main source of TNF-α in CL and ML lesions, a finding that differs from those of our previous studies in that CD4+ T cells were the main source of TNF-α among the PBMC from CL and ML (5, 9). It is possible that the local cytokine environment and the presence of parasites activate more TNF-α production by macrophages in the tissue. Our analysis showed that CD68+ and CD68− cells contribute equally to the expression of IL-10 in CL and ML lesions, suggesting that T cells are also important in IL-10 production in the lesions.
IFN-γ, an inflammatory cytokine produced predominantly by T cells, is critical in eliciting cellular responses and is a potent mediator of Leishmania killing in synergy with other cytokines and effector mediators, such as nitric oxide (NO) (21, 22). On the other hand, it has been shown that NO can cause tissue damage (17, 28). Since similar frequencies of iNOS+ cells were found in CL and ML lesions, it is possible that NO is not the only molecule involved in parasite killing or tissue damage in human leishmaniasis. In addition to NO induction, another important biological function of IFN-γ is the induction of cytotoxic activity, which is directly correlated with granzyme A and TIA-1 expression (2, 25, 36). We have previously shown that CL lesions display a high number of TIA-1+ cells (23) and that cytotoxic activity is exacerbated in PBMC from ML patients (6). In this work, we observed that the number of granzyme A+ cells was significantly higher in ML than in CL lesions. These data are consistent with the extensive tissue destruction observed in areas of mucosal commitment. Interestingly, while the main cells expressing granzyme A in CL lesions are CD8+ T cells, a significant number of CD8− cells express granzyme A in ML lesions, pointing to the participation of other cell types in cytotoxic functions in ML. It is likely that CD4+ T cells would account for the expression of granzyme A in ML lesions, since NK cells seem to be scarce in the ML inflammatory infiltrate (8). Moreover, it has been shown that the presence of inflammatory cytokines from Th1 cells can favor the development of CD4+ cells that display cytotoxic functions (34). Also, granzyme A activity was previously assigned to Leishmania-reactive CD4+ T cells in experimental models (16). These data suggest that cytotoxic CD4+ T cells could play an important role in the pathogenesis of ML. Further studies need to be done to clarify this point.
While IFN-γ expression was increased in ML lesions over that in CL lesions, the expression of TNF-α, another important inflammatory cytokine, was not. Since the main sources of IFN-γ were CD4+ and CD8+ T cells, while the main sources of TNF-α were CD68+ macrophages, it is possible that different mechanisms could be involved in the production of the two inflammatory cytokines. Recent studies have demonstrated that TNF-α is indeed present in ML lesions prior to and after specific treatment (1). The involvement of TNF-α in ML pathology was suggested previously, when pentoxifylline, a TNF-α inhibitor, was shown to be an efficient adjuvant treatment for ML patients refractory to conventional therapy (20). Previous studies have shown that inflammatory cytokines such as IFN-γ are able to induce expression of the TNF-α receptor (35). Since ML lesions display higher IFN-γ expression, it is possible that this cytokine is acting to increase the expression of the TNF-α receptor, which will be investigated in the future.
A potent antagonist of IFN-γ activities is the cytokine IL-10 (27). Our analysis of in situ IL-10 expression showed that CL and ML lesions have similar numbers of IL-10-expressing cells. Since the inflammatory infiltrate of ML lesions is significantly more intense than that of CL lesions, one would expect that the percentage of IL-10-expressing cells would be lower in ML than in CL lesions. Although statistical analysis of these data did not show significance, the IFN-γ/IL-10 ratio is higher in ML patients (0.4 and 0.7 for CL and ML, respectively), showing that the proportion of IFN-γ+ cells is indeed higher in ML patients. Moreover, the finding that ML patients display low levels of IL-10 receptor adds supportive evidence to the hypothesis that ML patients have a poorly controlled inflammatory environment. Previous studies have shown that proinflammatory cytokines induce down-regulation of the IL-10 receptor (26), and we have shown that in vitro blocking of IL-10 is able to restore the production of IFN-γ in cultures of cells from CL but not ML patients stimulated with soluble leishmania antigen (5). Other antiinflammatory cytokines, such as IL-13 (10), IL-4 (31), and transforming growth factor β (18), may also be involved in the control of ML. Recent studies with the experimental model of Leishmania major infection have shown that CD4+ CD25+ regulatory T cells are accumulated in lesions and exert IL-10-dependent as well as IL-10-independent suppressive functions (7). The involvement of CD4+ CD25+ regulatory cells in human leishmaniasis has not been clarified to date and could present an important regulatory mechanism.
We show evidence that high expression of IFN-γ, increased cytotoxic activity, and low expression of the IL-10 receptor may be responsible for the lack of control of the inflammatory response in ML. By adding to our knowledge of immunoregulatory and pathogenic cellular mechanisms in American cutaneous leishmaniasis, these findings will be critical when interventions involving modulation of specific cell populations are considered.
Acknowledgments
We are thankful to Roque Almeida for critical review of the manuscript.
This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Disease (TDR), TMRC/NIH, the Howard Hughes Medical Institute, and PADCT/CNPq. W.O.D., E.M.C., M.A.R.-S., K.J.G., and A.R.D.J. are CNPq fellows, and D.R.F. is a CAPES fellow.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Amato, V. S., H. F. Andrade, V. Amato Neto, and M. L. Duarte. 2003. Persistence of tumor necrosis factor-alpha in situ after lesion healing in mucosal leishmaniasis. Am. J. Trop. Med. Hyg. 68:527-528. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, P., C. Nagler-Anderson, C. O'Brien, H. Levine, S. Watkins, H. S. Slayter, M. L. Blue, and S. F. Schlossman. 1990. A monoclonal antibody reactive with a 15-kDa cytoplasmic granule-associated protein defines a subpopulation of CD8+ T lymphocytes. J. Immunol. 144:574-582. [PubMed] [Google Scholar]
- 3.Antonelli, L. R., W. O. Dutra, R. P. Almeida, O. Bacellar, and K. J. Gollob. 2004. Antigen specific correlations of cellular immune responses in human leishmaniasis suggests mechanisms for immunoregulation. Clin. Exp. Immunol. 136:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azulay, R. D., and D. R. Azulay, Jr. 1995. Immune-clinical-pathologic spectrum of leishmaniasis. Int. J. Dermatol. 34:303-307. [DOI] [PubMed] [Google Scholar]
- 5.Bacellar, O., H. Lessa, A. Schriefer, P. Machado, A. R. Jesus, W. O. Dutra, K. J. Gollob, and E. M. Carvalho. 2002. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect. Immun. 70:6734-6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barral-Netto, M., A. Barral, C. Brodskyn, E. M. Carvalho, and S. G. Reed. 1995. Cytotoxicity in human mucosal and cutaneous leishmaniasis. Parasite Immunol. 17:21-28. [DOI] [PubMed] [Google Scholar]
- 7.Belkaid, Y., C. A. Piccirillo, S. Mendez, E. M. Shevach, and D. L. Sacks. 2002. CD4+ CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420:502-507. [DOI] [PubMed] [Google Scholar]
- 8.Bittencourt, A. L., and A. Barral. 1991. Evaluation of the histopathological classifications of American cutaneous and mucocutaneous leishmaniasis. Mem. Inst. Oswaldo Cruz 86:51-56. [DOI] [PubMed] [Google Scholar]
- 9.Bottrel, R. L., W. O. Dutra, F. A. Martins, B. Gontijo, E. M. Carvalho, M. Barral-Netto, A. Barral, R. P. Almeida, W. Mayrink, R. Locksley, and K. J. Gollob. 2001. Flow cytometric determination of cellular sources and frequencies of key cytokine-producing lymphocytes directed against recombinant LACK and soluble Leishmania antigen in human cutaneous leishmaniasis. Infect. Immun. 69:3232-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourreau, E., G. Prevot, R. Pradinaud, and P. Launois. 2001. Interleukin (IL)-13 is the predominant Th2 cytokine in localized cutaneous leishmaniasis lesions and renders specific CD4+ T cells unresponsive to IL-12. J. Infect. Dis. 183:953-959. [DOI] [PubMed] [Google Scholar]
- 11.Caceres-Dittmar, G., F. J. Tapia, M. A. Sanchez, M. Yamamura, K. Uyemura, R. L. Modlin, B. R. Bloom, and J. Convit. 1993. Determination of the cytokine profile in American cutaneous leishmaniasis using the polymerase chain reaction. Clin. Exp. Immunol. 91:500-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carvalho, E. M., W. D. Johnson, E. Barreto, P. D. Marsden, J. L. Costa, S. Reed, and H. Rocha. 1985. Cell mediated immunity in American cutaneous and mucosal leishmaniasis. J. Immunol. 135:4144-4148. [PubMed] [Google Scholar]
- 13.Castes, M., A. Agnelli, and A. J. Rondon. 1984. Mechanisms associated with immunoregulation in human American cutaneous leishmaniasis. Clin. Exp. Immunol. 57:279-286. [PMC free article] [PubMed] [Google Scholar]
- 14.Castes, M., A. Agnelli, O. Verde, and A. J. Rondon. 1983. Characterization of the cellular immune response in American cutaneous leishmaniasis. Clin. Immunol. Immunopathol. 27:176-186. [DOI] [PubMed] [Google Scholar]
- 15.Esterre, P., S. Guerret, P. Ravisse, L. Dimier-David, J. P. Dedet, and J. A. Grimaud. 1994. Immunohistochemical analysis of the mucosal lesion in mucocutaneous leishmaniasis. Parasite 1:305-309. [DOI] [PubMed] [Google Scholar]
- 16.Frischholz, S., M. Rollinghoff, and H. Moll. 1994. Cutaneous leishmaniasis: co-ordinate expression of granzyme A and lymphokines by CD4+ T cells from susceptible mice. Immunology 82:255-260. [PMC free article] [PubMed] [Google Scholar]
- 17.Grisham, M. B., K. P. Pavlick, F. S. Laroux, J. Hoffman, S. Bharwani, and R. E. Wolf. 2002. Nitric oxide and chronic gut inflammation: controversies in inflammatory bowel disease. J. Investig. Med. 50:272-283. [DOI] [PubMed] [Google Scholar]
- 18.Joyce, D. A., J. H. Steer, and A. Kloda. 1996. Dexamethasone antagonizes IL-4 and IL-10-induced release of IL-1RA by monocytes but augments IL-4-, IL-10-, and TGF-β-induced suppression of TNF-α release. J. Interferon Cytokine Res. 16:511-517. [DOI] [PubMed] [Google Scholar]
- 19.Lee, S. A., and R. Hasburn. 2003. Therapy of cutaneous leishmaniasis. Int. J. Infect. Dis. 7:86-93. [DOI] [PubMed] [Google Scholar]
- 20.Lessa, H. A., P. Machado, F. Lima, A. A. Cruz, O. Bacellar, J. Guerreiro, and E. M. Carvalho. 2001. Successful treatment of refractory mucosal leishmaniasis with pentoxifylline plus antimony. Am. J. Trop. Med. Hyg. 65:87-89. [DOI] [PubMed] [Google Scholar]
- 21.Liew, F. Y. 1995. Regulation of lymphocyte functions by nitric oxide. Curr. Opin. Immunol. 7:396-399. [DOI] [PubMed] [Google Scholar]
- 22.Liew, F. Y., Y. Li, and S. Millott. 1990. Tumor necrosis factor-alpha synergizes with IFN-γ in mediating killing of Leishmania major through the induction of nitric oxide. J. Immunol. 145:4306-4310. [PubMed] [Google Scholar]
- 23.Machado, P., J. Kanitakis, R. Almeida, A. Chalon, C. Araújo, and E. M. Carvalho. 2002. Evidence of in situ cytotoxicity in American cutaneous leishmaniasis. Eur. J. Dermatol. 12:449-451. [PubMed] [Google Scholar]
- 24.Melby, P. C., B. J. Darnell, and V. V. Tryon. 1993. Quantitative measurement of human cytokine gene expression by polymerase chain reaction. J. Immunol. Methods 159:235-244. [DOI] [PubMed] [Google Scholar]
- 25.Melby, P. C., F. J. Andrade-Narvaez, B. J. Darnell, G. Valencia-Pacheco, V. V. Tryon, and A. Palomo-Cetina. 1994. Increased expression of proinflammatory cytokines in chronic lesions of human cutaneous leishmaniasis. Infect. Immun. 62:837-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michel, G., A. Mirmohammadsadegh, E. Olasz, B. Jarzebska-Deussen, A. Muschen, L. Kemeny, H. F. Abts, and T. Ruzicka. 1997. Demonstration and functional analysis of IL-10 receptors in human epidermal cells: decreased expression in psoriatic skin, down-modulation by IL-8, and up-regulation by an antipsoriatic glucocorticosteroid in normal cultured keratinocytes. J. Immunol. 159:6291-6297. [PubMed] [Google Scholar]
- 27.Mosmann, T. R., and K. W. Moore. 1991. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol. Today 12:49-53. [DOI] [PubMed] [Google Scholar]
- 28.Napoli, C. 2002. Nitric oxide and atherosclerotic lesion progression: an overview. J. Card. Surg. 17:355-362. [DOI] [PubMed] [Google Scholar]
- 29.Netto, E. M., P. D. Marsden, E. A. Llanos-Cuentas, J. M. Costa, C. C. Cuba, A. C. Barreto, R. Badaro, W. D. Johnson, and T. C. Jones. 1990. Long-term follow-up of patients with Leishmania (Viannia) braziliensis infection and treated with Glucantime. Trans. R. Soc. Trop. Med. Hyg. 84:367-370. [DOI] [PubMed] [Google Scholar]
- 30.Pirmez, C., C. Cooper, M. Paes-Oliveira, A. Schubach, V. K. Torigian, and R. L. Modlin. 1990. Immunologic responsiveness in American cutaneous leishmaniasis lesions. J. Immunol. 145:3100-3104. [PubMed] [Google Scholar]
- 31.Pirmez, C., M. Yamamura, K. Uyemura, M. Paes-Oliveira, F. Conceição-Silva, and R. L. Modlin. 1993. Cytokine patterns in the pathogenesis of human leishmaniasis. J. Clin. Investig. 91:1390-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ribeiro-de-Jesus, A., R. P. Almeida, H. Lessa, O. Bacellar, and E. M. Carvalho. 1998. Cytokine profile and pathology in human leishmaniasis. Braz. J. Med. Biol. Res. 31:143-148. [DOI] [PubMed] [Google Scholar]
- 33.Sacks, D., and N. Noben-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2:845-858. [DOI] [PubMed] [Google Scholar]
- 34.Sasiain, M. C., S. de la Barrera, S. Fink, M. Finiasz, M. Aleman, M. H. Farina, G. Pizzariello, and R. Valdez. 1998. Interferon-gamma (IFN-γ) and tumour necrosis factor-alpha (TNF-α) are necessary in the early stages of induction of CD4 and CD8 cytotoxic T cells by Mycobacterium leprae heat shock protein (hsp) 65 kD. Clin. Exp. Immunol. 114:196-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tannenbaum, C. S., J. A. Major, and T. A. Hamilton. 1993. IFN-γ and lipopolysaccharide differentially modulate expression of tumor necrosis factor receptor mRNA in murine peritoneal macrophages. J. Immunol. 152:6833-6839. [PubMed] [Google Scholar]
- 36.Trapani, J. A., and M. J. Smyth. 2002. Functional significance of the perforin/granzyme cell death pathway. Nat. Rev. Immunol. 2:735-747. [DOI] [PubMed] [Google Scholar]