Abstract
Helicobacter pylori is a spiral, gram-negative bacterium that specifically and persistently infects the human stomach. In some individuals, H. pylori-induced chronic gastritis may progress to gastroduodenal ulcers and gastric cancer. Currently, the host-microbe interactions that determine the clinical outcome of infection are not well defined. H. pylori strains capable of disrupting the gastric epithelial barrier may increase the likelihood of developing serious disease. In this study, H. pylori strain SS1 increased gastric, but not small intestinal, permeability in C57BL/6 mice. H. pylori strain SS1 was able to directly increase paracellular permeability, in the absence of host inflammatory cells, by disrupting the tight-junctional proteins occludin, claudin-4, and claudin-5 in confluent nontransformed epithelial cells. H. pylori SS1 also reduced claudin-4 protein levels in human gastric AGS cells. The ability of H. pylori SS1 to increase permeability appeared to be independent of the well-characterized virulence factors vacuolating cytotoxin and CagA protein. H. pylori activated myosin light-chain kinase in epithelial cells to phosphorylate myosin light chain and increase permeability by disrupting claudin-4 and claudin-5. The bacterial factor responsible for increasing epithelial permeability was heat sensitive, membrane bound, and required apical contact with monolayers. In conclusion, disruptions of the tight junctions observed in this study implicate host cell signaling pathways, including the phosphorylation of myosin light chain and the regulation of tight-junctional proteins claudin-4 and claudin-5, in the pathogenesis of H. pylori infection.
Helicobacter pylori colonizes the stomachs of over half of the human population. While in most cases, chronic infection with H. pylori causes only gastritis, its interactions with the host may lead to gastroduodenal ulcers, gastric adenocarcinoma, and mucosal lymphomas (9, 56). Despite intensive study, the host-microbe interactions that determine the clinical outcome of infection remain unknown. Consistent with recent observations, we hypothesized that H. pylori strains possess virulence factors capable of disrupting epithelial barrier function, a process which may increase the likelihood of developing serious disease.
The gastrointestinal epithelium, among its other functions, acts as a selective barrier and prevents potentially harmful luminal agents such as microbial products, food antigens, or toxins from penetrating underlying tissues, while allowing for exchanges of ions and small molecules. Tight junctions between epithelial cells play a key role in this barrier function and consist of a complex interaction between several protein families. Transcellular tight-junctional proteins, including occludin, junctional adhesion molecule (JAM), and claudins, are anchored to actin filaments and myosin light chain (MLC) of the perijunctional actinomyosin ring by the linker protein zonula occludens 1 (ZO-1) (17, 18, 29). Phosphorylation of MLC by myosin light-chain kinase (MLCK) physiologically regulates paracellular permeability by placing tension on the tight-junctional complexes (55). It has been well established that the paracellular permeability offered by tight junctions can be altered in response to physiological and pathological stimuli (7, 30), and a variety of enteric microbes have been found to directly alter tight-junction permeability, a change suggested to contribute to disease symptoms (11, 16, 46, 48, 51, 57). H. pylori strains producing the vacuolating cytotoxin (VacA) have the ability to modulate the integrity of the epithelium by increasing tight-junction permeability to small ions and molecules (41, 42). H. pylori preferentially adheres near the tight junctions of gastric mucous cells (13, 21). Strains possessing the Cag pathogenicity island translocate the CagA protein into host cells via a type IV secretory system (5, 39). Findings from recent studies using Madin-Darby canine kidney cells or gastric adenocarcinoma cells indicate that translocated CagA increases paracellular permeability by recruiting ZO-1 and JAM to sites of bacterial attachment (1). Yet, while the host inflammatory response may contribute to an increase in permeability in human subjects (19, 38), the ability of the bacterium to directly disrupt tight junctions of the human gastric epithelium remains controversial (2, 44). Recent findings showed that H. pylori has the ability to increase the passage of food antigens across human gastric biopsies (33), as well as in animal model systems (34). These observations are consistent with observations that the infection may be associated with the development of food allergy (8, 15). However, the epithelial mechanisms leading to H. pylori-induced permeability defects remain obscure, and the clinical significance of these abnormalities warrants further investigation.
Factors unique to the bacterial strain and the genetic susceptibility of the host may explain the spectrum of gastric disease seen during H. pylori infection. The present study found that the SS1 strain of H. pylori increased gastric permeability in a mouse model of infection and disrupted specific tight-junctional proteins of nontransformed confluent intestinal epithelial monolayers. The disruption was caused independently of previously reported bacterial virulence factors, was strain specific, and functioned by signaling through host cell pathways involving the phosphorylation of MLC and the regulation of tight-junctional proteins claudin-4, claudin-5, and occludin.
MATERIALS AND METHODS
Bacterial culture.
H. pylori strains LC11 (VacA+ CagA+) and LC20 (VacA− CagA−) were originally isolated from children with gastritis (37). Strain SS1 (VacA+ CagA+) was initially obtained from a human gastric biopsy and subsequently passaged through mice (27). For use in all experiments, bacteria were grown from frozen stock on Columbia blood agar (Becton Dickinson Co., Sparks, MD) supplemented with 7% heat-inactivated horse serum (Sigma Chemical Co., St. Louis, MO.) for 3 to 5 days and single colonies were transferred to brucella broth (Becton Dickinson) supplemented with 10% heat-inactivated fetal bovine serum (Sigma) and grown on a shaker (150 rpm) for 48 h in a microaerophilic atmosphere (10% CO2, 4% O2, balance N2) at 37°C. Bacteria cultured in broth for 48 h were used as inocula in all in vivo and in vitro experiments. For in vitro experiments, a multiplicity of infection of 100:1 was used. Heat-killed bacteria were prepared by boiling a broth culture for 2 min. Conditioned, sterile bacterial broth was prepared by passing broth cultures through a 0.2-μm-pore filter (Acrodisc; Pall Corp., Ann Arbor, MI). For other experiments, H. pylori cells were killed by 10 min of formalin fixation, exposure to antibiotics (100 μg/ml streptomycin, 100 U/ml penicillin, 0.8 mg/ml tylosin; Sigma) or exposure to room air for 24 h. All inocula were tested for live bacterial numbers by serial dilution on Columbia blood agar plates and cultured as described above.
Animals.
Female C57BL/6 mice (4 weeks old; Charles River Laboratories, Montreal, Canada) were housed in microisolator cages (22°C, 40% humidity, 12-h photoperiods) with free access to sterile food and water. Animals were infected with H. pylori strain SS1 as described previously (27). Briefly, mice were orogastrically gavaged with a 200-μl inoculum of bacterial broth containing 1 × 108 live bacteria on days 0, 2, and 4 using a blunt feeding needle. Animals were cared for and experimental practices were conducted according to the standards of the Canadian Council on Animal Care and approved by the Life and Environmental Sciences Animal Care Committee of the University of Calgary.
In vivo studies.
Animals were sacrificed by cervical dislocation 14, 70, and 100 days after infection. Stomachs were removed and rinsed with sterile phosphate-buffered saline (PBS). Bacterial colonization was measured by serially diluting homogenized gastric tissue on agar plates. Gastric tissue was fixed in 2% paraformaldehyde, embedded in paraffin, and cut into 5-μm sections. Sections stained with hematoxylin and eosin were used to assess inflammatory cell infiltration. Gastritis was scored using the modified Sydney system (degree of leukocyte infiltration scored as follows: 0 = normal, 1 = mild, 2 = moderate, and 3 = marked) (12). On days 0, 7, 15, 24, 31, 48, 60, 73, 90, 94, and 98, mice were fasted for 4 h and then orogastrically gavaged with 200 μl of a solution containing 500 mg/ml sucrose, 60 mg/ml lactulose, and 20 mg/ml mannitol (all from BDH, Inc., Toronto, Canada) suspended in sterile distilled water. Animals were then individually housed in metabolic cages with access to water only. Urine was collected for 22 h and analyzed for the presence of excreted sugars by high-performance liquid chromatography (Dionex; Mississauga, Ontario, Canada) as described previously (52). Sucrose was used as a measure of gastric permeability, and lactulose/mannitol ratios were used to determine small-intestinal permeability (48, 52).
In vitro cell culture.
In vitro studies were performed using a nontumorigenic intestinal epithelial cell line with a canine genotype (SCBN) and, for some of the experiments, a human gastric adenocarcinoma cell line, AGS (ATCC, Manassas, VA). SCBN grows into polarized confluent monolayers; possesses cytokeratins, mucin, and sucrase-isomaltase antigens; has mRNA for epidermal growth factor, interleukin-6, vascular cell adhesion molecule 1, and cytoskeletal proteins sensitive to microbes; shows calcium-dependent chloride secretion; and expresses protease-activated receptor 1 (4, 6, 40, 48). SCBN cells were used between passages 21 and 25 and grown in Dulbecco's modified Eagle's medium (Sigma), and AGS cells were used between passages 43 and 47 and raised in F-12K medium (Sigma). Cell culture medium was supplemented with fetal bovine serum (5% for SCBN and 10% for AGS), 100 μg/ml streptomycin, 100 U/ml penicillin, 0.8 mg/ml tylosin, and 200 mM l-glutamine (all from Sigma) and maintained at 37°C in 5% CO2 with 96% humidity. Culture medium was replenished every 2 to 3 days, and cells were passaged using 5× trypsin-EDTA (Sigma). Trypsinized cells were added to Lab-Tek chamber slides (400 μl; 2.0 × 104 cells/ml) (Nalge Nunc International, Naperville, IL), sterile tissue culture dishes (4.0 ml; 2.0 × 104 cells/ml) (60 mm by 15 mm; Becton Dickinson), or Transwell filter units (500 μl; 2.0 × 105 cells/ml) (Costar, Corning Inc., Corning, NY) that contained a 1.13-cm2 filter membrane with 0.4-μm pores.
Immunocytochemistry.
Confluent SCBN monolayers grown on chamber slides (Nalge Nunc) were used to assess cytoskeletal protein expression in response to H. pylori exposure. After the indicated time of bacterial exposure, SCBN cells were washed once in sterile PBS and fixed with −20°C methanol for 20 min. Cells were permeabilized with 1% Triton X-100 (Sigma), and nonspecific binding was blocked using fetal bovine serum (Sigma) for 15 min at 20°C. Monolayers were incubated with the primary antibodies polyclonal rabbit anti-ZO-1 (1/100), polyclonal rabbit anti-JAM-A (1/100), polyclonal mouse anti-occludin, monoclonal mouse anti-claudin-4 (1/200), and mouse monoclonal anti-claudin-5 (1/200) (all from Zymed Laboratories, San Francisco, CA). Cells were then incubated with the appropriate anti-rabbit immunoglobulin G (IgG) or anti-mouse IgG conjugated to Cy3 (1/100; Sigma). Nuclei were counterstained using 1 μM Hoechst fluorescence staining 33258 (Molecular Probes, Eugene, OR). All labeling incubations were performed for 1 h at 37°C.
Quantification of tight-junctional proteins.
Confluent SCBN monolayers or PGS cells grown on sterile culture dishes and challenged with H. pylori for 12 h were used for Western blotting. Cells were removed using 300 μl of lysis buffer (PBS with 1% Igepal CA-630, 0.1% sodium dodecyl sulfate, 0.5% sodium deoxycholate; all from Sigma) containing a protease inhibitor tablet (Complete, Mini; Roche Diagnostics, Mannheim, Germany) and centrifuged at 10,000 × g for 10 min. Cytoplasmic and membrane-associated protein fractions were separated by differential detergent extraction as previously described (48). Protein levels were determined (Bradford Bio-Rad protein assay; Bio-Rad, Hercules, CA), and 25 μg of protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Bio-Rad). Membranes were incubated with primary antibodies to ZO-1 (1/500), JAM-A (1/125), occludin (1/500), claudin-4 (1/250), claudin-5 (1/250) (all from Zymed), myosin light chain (1/500; clone MY-21; Sigma) and phospho-myosin light chain 2 (1/1,000; Cell Signaling Technology, Beverly, MA). Cells were then incubated with the appropriate anti-rabbit IgG or anti-mouse IgG conjugated to horseradish peroxidase (1/10,000; Santa Cruz Biotechnologies, Santa Cruz, CA). Blots were visualized using a chemiluminescence detection system (ECL; Amersham, Buckinghamshire, England). Band intensity was determined using densitometry software and a GS-710 scanner (both from Bio-Rad). Western blotting and densitometric analysis were also performed with human gastric AGS cells, treated as described above.
In vitro permeability.
SCBN cells reached confluence 7 to 9 days after passage into Transwells (Costar) and had an electrical resistance of >1,000 Ω/cm2 (Electrovoltohmeter; World Precision Instruments, Sarasota, FL). Effects of apical H. pylori exposure on epithelial permeability were assessed in the presence or absence of the caspase 3 inhibitor Z-DEVD-FMK (100 μM; Calbiochem, La Jolla, CA) or the specific myosin light-chain kinase inhibitor ML-9 (40 μM; Sigma) as previously described (7, 48). The effects of heat-inactivated (100°C, 2 min), filtered (0.2-μm pore size), killed (formalin, antibiotics, aeration), or basolaterally administered bacterial cultures on permeability were also assessed. Cells were pretreated for 30 min with inhibitors or the dimethyl sulfoxide vehicle before bacterial challenge. After coincubation, Transwell compartments were washed two times with sterile Ringer's solution (115 mM NaCl, 50 mM NaHCO3, 2.8 mM KH2PO4, 2.8 mM K2HPO4, 1.2 mM CaCl2, 1.2 mM MgCl2, 10 mM glucose, pH 7.4) and a nonabsorbable fluorescein isothiocyanate (FITC)-conjugated 3,000-molecular-weight (MW) dextran probe (500 μl; 100 μM in Ringer's solution; Molecular Probes) was added to the apical compartment, while 1,000 μl of Ringer's was added to the basolateral compartment. After a 3-h incubation (37°C, 5% CO2, 96% humidity), relative fluorescent units of basolateral samples were calculated with a microplate fluorometer (Spectra Max Gemini; Molecular Devices) (7). Values were expressed as % apical FITC-dextran × 10−3 per cm2 per h that crossed the Transwell membrane.
Statistical analysis.
Gastritis scores were expressed as median ± upper and lower quartiles. All other results were expressed as means ± standard error and tested for normality. Comparisons were made using a one-way analysis of variance followed by Tukey's post hoc test for parametric data and Dunn's test for nonparametric data. Gastritis scores were compared using a Mann-Whitney rank sum test. Densitometry data were compared using Student's t test. Statistical significance was established at P < 0.05.
RESULTS
Infection with H. pylori SS1 causes inflammation and increases gastric permeability in mice.
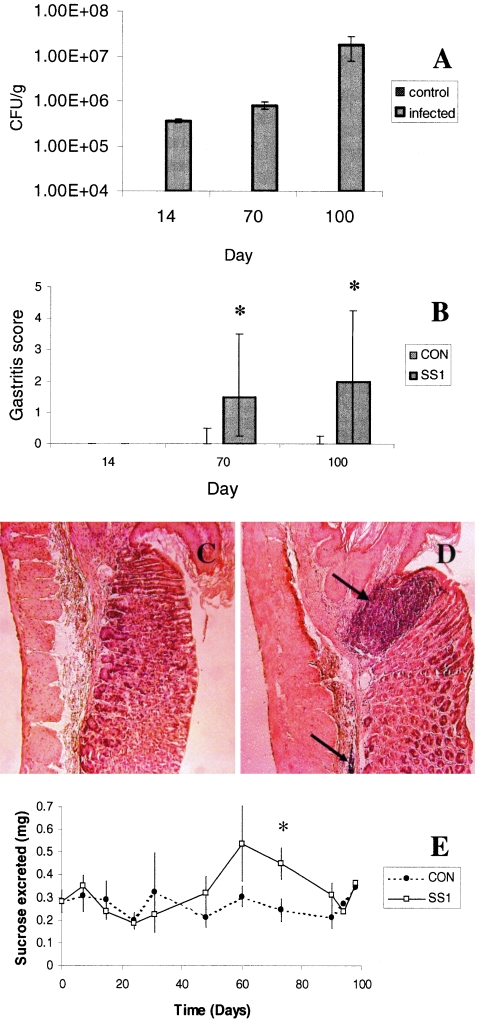
Inflammation and gastric and duodenal permeability were assessed in a previously validated mouse model (27). Gastric tissues from infected mice were colonized with increasing numbers of H. pylori 14, 70, and 100 days postinoculation (Fig. 1A). H. pylori was not recovered from duodenal tissues or from gastric tissues of noninfected control mice (data not shown). Inflammatory cells were seen infiltrating the gastric glands and submucosa after 70 and 100 days of infection (Fig. 1B). Immune cell infiltration was particularly evident at the transition zone between the nonglandular and glandular mucosa (Fig. 1C and D). After 73 days of infection, mice exhibited a transient increase in gastric (Fig. 1E) but not duodenal permeability (data not shown).
FIG. 1.
H. pylori SS1 causes inflammation and increases gastric permeability in mice. Increasing numbers of H. pylori organisms were recovered from the stomachs of mice 14, 70, and 100 days after infection (A). At days 70 and 100, infected animals had gastritis as measured by light microscopy (B). Uninfected animals showed no histologic signs of inflammation, (C) whereas infected animals had dense inflammatory cell infiltrates at the transition zone (large arrow) between nonglandular and glandular mucosa and in submucosal regions (small arrow) of the stomach (D). Gastric permeability as measured by the amount of sucrose excreted in the urine was increased in infected animals after 73 days of infection (E). Gastritis score (B) values are expressed as the median ± upper and lower quartiles. Bacterial count (A) and gastric permeability (E) values are means ± standard error from replicates run concurrently in one study: n = 5 for controls (CON) and n = 8 for infected animals in panels A, B, C, and D; and n = 5 for controls and n = 16 for infected animals in panel E. *, P < 0.05 compared with control. Photomicrographs were taken at an original magnification of ×200.
H. pylori SS1 increases epithelial permeability in vitro.
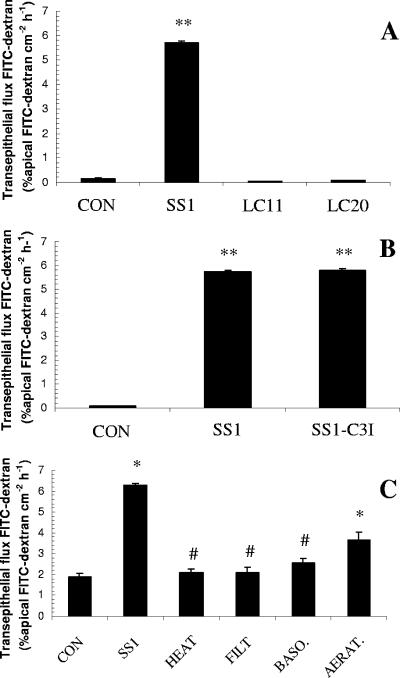
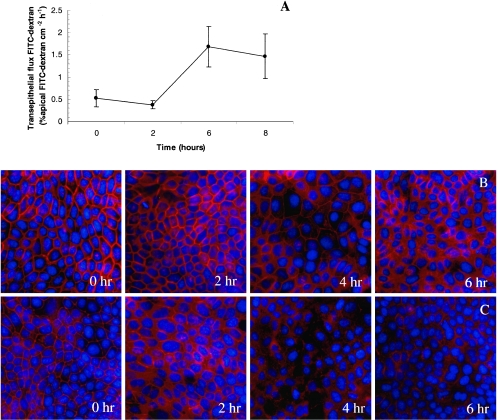
SCBN epithelial monolayers exposed to apical H. pylori SS1 (CagA+ VacA+) had a significantly increased permeability to 3,000-MW dextran (Fig. 2A). The increase in permeability was bacterial strain specific and apparently independent of the well-characterized virulence factors CagA and VacA as H. pylori strains LC11 (CagA+ VacA+) and strain LC20 (CagA− VacA−) did not disrupt the epithelial barrier (Fig. 2A). H. pylori is able to induce apoptosis in epithelial cells, and recent findings indicate that loss of epithelial barrier function could be caspase-3 dependent (6, 7, 25). The disruption of epithelial barrier function was not prevented by caspase 3 inhibition (Fig. 2B). Cells exposed to H. pylori for 12 h remained confluent by light microscopy (data not shown). Increased epithelial permeability was not observed when monolayers were exposed to heat-inactivated bacterial inocula or to cell-free bacterial culture filtrates (Fig. 2C). The H. pylori-induced barrier defect required apical cellular contact, as basolateral challenge had no effect on epithelial permeability (Fig. 2C). Bacteria killed by aeration (Fig. 2C), formalin fixation (data not shown), and antibiotics (data not shown) were still able to increase permeability.
FIG. 2.
H. pylori strain SS1 increases the permeability of SCBN cell monolayers. Apical exposure (12 h) to strain SS1 (CagA+ VacA+) but not strains LC11 (CagA+ VacA+) and LC20 (CagA− VacA−) increases epithelial permeability to 3,000-MW dextran (A). The disruption of epithelial barrier function is not prevented by caspase 3 inhibition (B). Heat killing and filtration (<0.2 μm) of SS1 broth cultures remove the factor responsible for increasing epithelial permeability (C). Basolateral exposure of epithelial cells to strain SS1 also has no effect on barrier function (C). Bacteria killed by aeration do not lose their ability to increase epithelial permeability (C). Values are means ± standard error; n = 6 per group. * and **, P < 0.05 and P < 0.001, respectively, compared with control (CON); #, P < 0.05 compared with SS1.
H. pylori SS1 disrupts tight-junctional proteins.
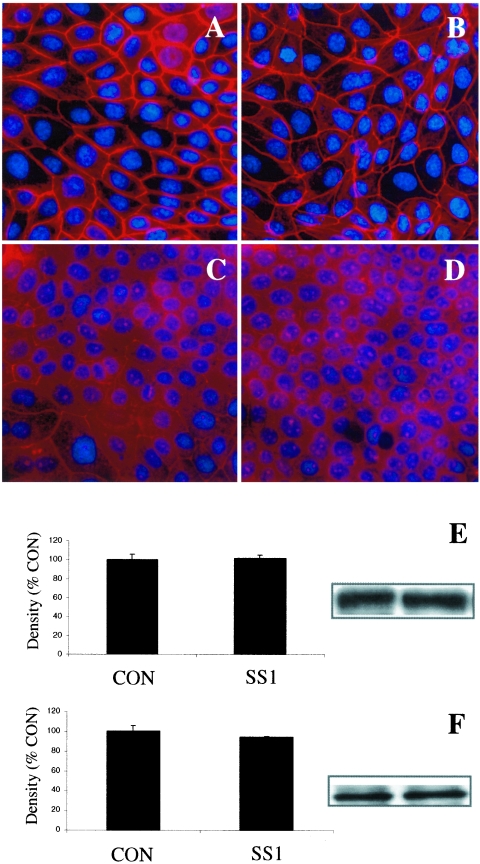
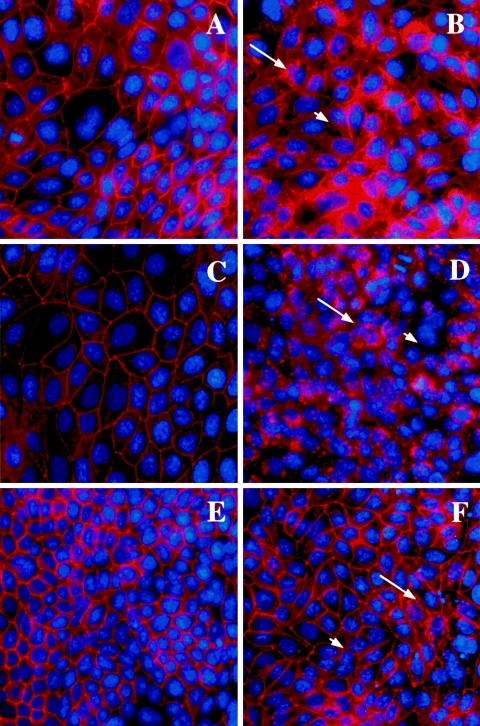
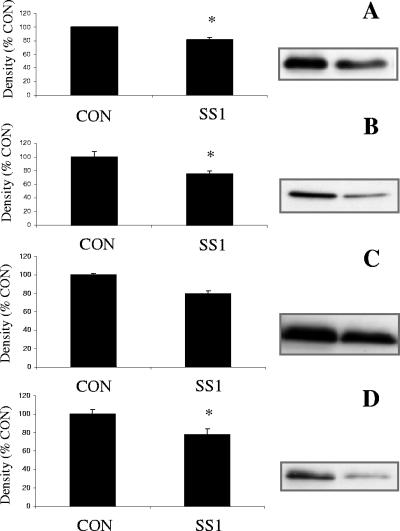
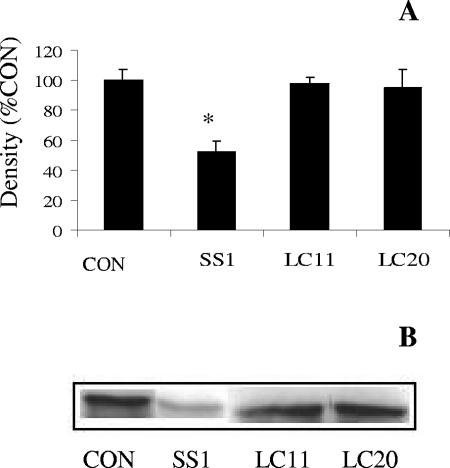
Apical exposure of epithelial monolayers to H. pylori SS1 had no effect on the staining pattern of ZO-1 or JAM-A (Fig. 3B and D) compared with controls (Fig. 3A and C). Western blotting revealed no difference in total cellular protein levels of ZO-1 or JAM-A in SCBN cells exposed to H. pylori SS1 compared with controls (Fig. 3E and F). In contrast, epithelial monolayers exposed to H. pylori SS1 exhibited dramatic rearrangements of claudin-4 (Fig. 4B), claudin-5 (Fig. 4D), and occludin (Fig. 4F). Immunocytochemistry revealed clear disruptions of claudin-4, claudin-5, and occludin staining at the level of the tight junctions (arrows and arrowheads, Fig. 4B, D, and F). To confirm the immunocytochemistry findings, effects of H. pylori SS1 on the cellular tight-junctional proteins were assessed using Western blotting. Claudin-4 (Fig. 5A) and claudin-5 (Fig. 5B) protein levels were significantly reduced following exposure of epithelial cells to H. pylori SS1. Total occludin levels did not change in response to H. pylori exposure (data not shown). However, there was a decrease in the membrane-associated occludin fraction (Fig. 5D), while the cytosolic occludin fraction remained unchanged (Fig. 5C). Time course experiments correlated the disruption of claudin-4 and claudin-5 with the increase in epithelial permeability to 3,000-MW dextran (Fig. 6A, B, and C). In an attempt to determine whether tight-junctional changes induced by H. pylori SS1 could also be observed in human gastric cells, the AGS cell line was used for Western blotting analysis of claudin-4. As AGS do not form confluent monolayers with organized tight junctions, the experiments assessed total claudin-4 levels, as a marker of biochemical alterations in the cells. H. pylori strain SS1, but not strain LC11 or LC20, significantly decreased claudin-4 protein levels in AGS cells (Fig. 7A and B).
FIG. 3.
H. pylori strain SS1 effects (at 12 h) on organization of SCBN tight-junctional proteins ZO-1 (A and B) and JAM-A (C and D). Photomicrographs are representative samples of control (A and C) or bacterially challenged (B and D) SCBN monolayers. Exposure of cells to H. pylori had no apparent effect on the staining pattern of ZO-1 or JAM-A. Western blotting of tight-junctional proteins confirmed the maintenance of ZO-1 (E) and JAM-A (F) protein levels in SCBN cells. Micrographs were taken at an original magnification of ×400. Western blot values are means ± standard error; n = 3 per group. P > 0.05 compared with control (CON).
FIG. 4.
H. pylori strain SS1 effects (at 12 h) on tight-junctional proteins claudin-4 (A and B), claudin-5 (C and D), and occludin (E and F). Photomicrographs are of control (A, C, and E) and bacterially challenged (B, D, and F) SCBN monolayers. Exposure of cells to H. pylori induced a dramatic redistribution of claudin-4 (B), claudin-5 (D), and occludin (F). Cellular changes include punctate (arrows) and diminished (arrowheads) staining of claudin-4, claudin-5, and occludin at the paracellular junction. Nuclei are counterstained with Hoechst stain. Photomicrographs were taken at an original magnification of ×400.
FIG. 5.
H. pylori strain SS1 changes the tight-junctional protein levels in SCBN cells (12 h). Western blotting of tight-junctional proteins confirmed the loss of claudin-4 (A) and claudin-5 (B). Occludin was lost from the membrane-associated fraction (D), while the change failed to reach statistical significance in the cytosolic fraction (C). Values are means ± standard error; n = 3 per group. *, P < 0.05 compared with control (CON).
FIG. 6.
Increased permeability coincides with claudin-4 and claudin-5 disruptions in epithelial monolayers. SCBN monolayers had increased permeability as measured by transepithelial 3,000-MW dextran flux after 6 and 8 h of bacterial exposure (A). Claudin-4 and claudin-5 show diminished staining after 4 and 6 h, respectively (B and C). Values are means ± standard error; n = 3 to 6 per group. *, P < 0.05 compared with control (time zero).
FIG. 7.
H. pylori SS1, but not LC11 or LC20, decreases claudin-4 protein levels in human gastric AGS cells (12 h). Densitometric analysis (A) and a representative Western blot labeled for claudin-4 (B) illustrate a strain-specific decrease in claudin-4 levels. Values are means ± standard error; n = 3 per group. *, P < 0.05 compared with control (CON).
H. pylori-induced loss of barrier function is MLCK dependent.
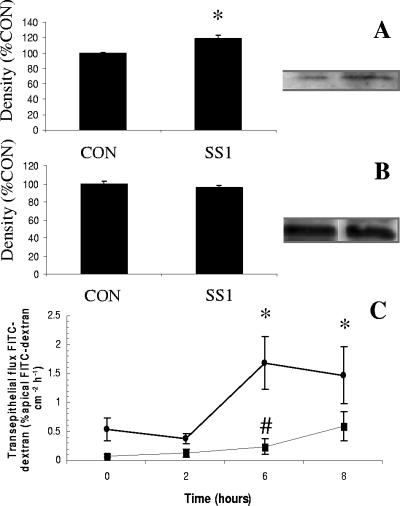
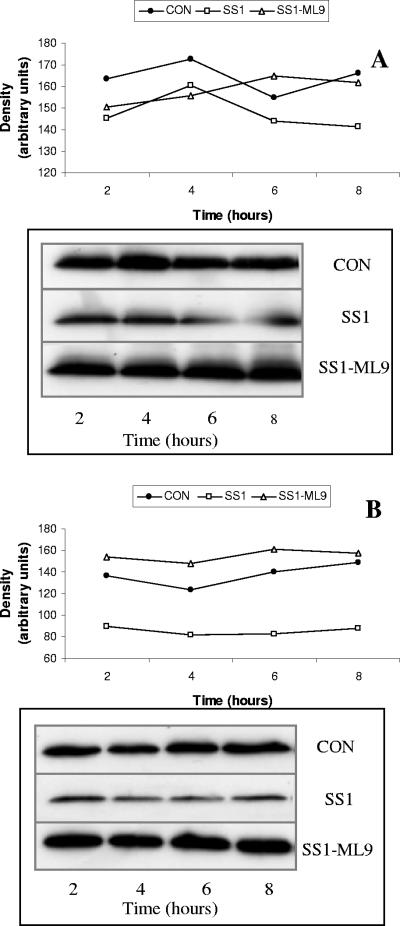
Exposure to H. pylori SS1 increased the phosphorylation of intraepithelial MLC 2 (Fig. 8A), while total MLC 2 levels remained unchanged (Fig. 8B). ML-9, a selective MLCK inhibitor, inhibited the H. pylori-induced increase in epithelial permeability (Fig. 8C). H. pylori-induced loss of claudin-4 and claudin-5 was also prevented by addition of ML-9 (Fig. 9A and B). ML-9 alone had no effect on claudin-4 and claudin-5 levels (data not shown).
FIG. 8.
H. pylori-induced barrier dysfunction is MLCK dependent. Western blots revealed an increase in the level of phosphorylated MLC (A) in the presence of H. pylori (12 h), whereas myosin light-chain 2 levels were unchanged (B). The selective MLCK inhibitor ML-9 (▪) prevented the H. pylori-induced increase (•) in epithelial permeability (C). Values are means ± standard error; n = 3 to 6 per group. *, P < 0.05 compared with control (CON); #, P < 0.05 compared with H. pylori SS1.
FIG. 9.
H. pylori-induced loss of claudin-4 and claudin-5 is MLCK dependent. The selective MLCK inhibitor ML-9 prevented the H. pylori-induced loss of claudin-4 (A) and claudin-5 (B) in SCBN epithelial cells. Densitometry values are plotted over time of bacterial exposure, and the original Western blots are shown in the insets in panels A and B. CON, control.
DISCUSSION
Findings from the present study indicate that H. pylori may disrupt tight-junctional occludin, claudin-4, and claudin-5 and increase epithelial permeability by activating MLCK in a CagA- and VacA-independent fashion and in the presence or absence of host inflammatory cells.
Studies using in vitro systems such as confluent canine kidney (1, 42), human colonic adenocarcinoma (53), and murine mammary cells (41) suggest that H. pylori can increase epithelial permeability. Indeed, the lack of nontransformed confluent human gastric cell lines with functional tight junctions has forced scientists to use models of epithelial cells belonging to phenotypes that are not normally infected by H. pylori. The SCBN cell line was used in this study because, although of canine genotype, it is a nontransformed intestinal epithelial cell line that forms well-organized tight junctions, and because it exhibits well-characterized permeability alterations in response to microbial or inflammatory stimuli (6, 7, 48). While H. pylori exerts remarkable tropism for human gastric epithelial cells in vivo (13), the bacteria used in the present study did form tight attachments to SCBN cells (not shown). Also, the gastric adenocarcinoma cell line AGS does not form confluent monolayers, but it does express a number of tight-junctional proteins at the periphery of individual cells (1). A combination of in vivo and in vitro approaches was used in this study to examine H. pylori-epithelial cell interactions that may be of significance to the clinical outcome of infection. Studies involving human subjects have argued for (44) and against (2) the ability of the bacterium to disrupt tight junctions of the gastric epithelium. In the host, the transmigration of inflammatory cells across the epithelium in response to infection likely contributes to the increase in permeability (38) and the degree of H. pylori-induced gastritis has been correlated with barrier disruption (19). However, while establishing that H. pylori can transiently disrupt gastric barrier function in vivo, the present study also demonstrates that the increase in permeability does not necessarily require inflammatory cells. Intestinal pathogens such as Vibrio cholerae, Salmonella, Escherichia coli, rotavirus, Shigella, and Giardia have been found to directly alter tight-junctional permeability, a change suggested to contribute to disease symptoms (11, 14, 16, 46, 48, 57). However, whereas these pathogens generally cause a self-limiting diarrheal disease, H. pylori chronically infects the human stomach. Recent studies have shown that H. pylori may allow the transepithelial entry of food antigens, and the infection has been associated with the development of food allergies in some patients (8, 15, 33, 34). Future research will determine if strain-specific gastric barrier disruptions may explain, at least in part, the variable clinical outcomes seen in response to H. pylori infection.
Recent experiments found that apical stimuli, such as microbial adhesion or proteinase-activated receptor agonists, could increase epithelial permeability in a caspase 3-dependent fashion (6, 7). As H. pylori is known to increase epithelial cell apoptosis (25), the current study also assessed the effects of caspase 3 inhibition on bacterially induced increases in epithelial permeability. The present findings suggest that the H. pylori-induced loss of barrier function was mediated at the level of the tight junction, independent of caspase 3 activation. In contrast to a recent study (1), the disruptions occurred in the absence of overt abnormalities in ZO-1, JAM-A, or monolayer structure under light microscopy. The findings are consistent with the hypothesis that H. pylori may disrupt tight-junctional structure and function in a strain-dependent fashion.
Findings from recent studies suggest that H. pylori may increase epithelial permeability in a CagA- and/or VacA-dependent fashion (1, 41, 42). Unexpectedly, the data from this study implicate a heat-sensitive, membrane-bound bacterial factor, other than CagA and VacA, in the disruption of the tight junction. Although H. pylori SS1 was originally described as being both CagA and VacA positive (27), subsequent studies reported low levels of vacuolating cytotoxin activity and the inability of the bacterium to translocate CagA into host cells and induce an interleukin-8 response (10, 43). In the present study, H. pylori caused a transient increase in gastric permeability in vivo and a marked tight-junctional disruption in vitro. Further investigations will determine whether this apparent discrepancy may reflect the different capacities of epithelial cells to recover from bacterial challenges in vivo and in vitro and/or the variable expression of virulence factors in response to the distinct selective pressures encountered in vivo and in vitro. Previous studies have demonstrated that H. pylori can regulate the expression of adhesin molecules in response to a changing gastric environment (3, 20). Adhesin molecules allow H. pylori to form tight attachments to gastric epithelial cells and subsequently alter the outcome of infection by increasing the inflammatory response (3, 20). More research is needed to determine whether outer membrane proteins of H. pylori not only facilitate binding to host cells but also signal to modulate paracellular permeability.
Tight junctions of the gastrointestinal epithelium limit the movement of materials through the paracellular space and define the polarity of the cell. Enteric pathogens can increase epithelial permeability by altering the protein composition of the tight junction. For example, enteropathogenic E. coli increases epithelial permeability by dephosphorylating occludin, a process associated with the shift of occludin away from the tight junction and into the cytosol (50). Consistent with this observation, the present study also found that H. pylori has the ability to dissociate occludin from the tight junction. However, another report demonstrating that cells deficient in occludin could still form functional tight-junctional strands has established that transmembrane proteins different from occludin were of central importance in maintaining the epithelial barrier (45). This study and others established that claudins are critical structural and functional components of the tight junction (22, 51). Tight junctions serve as a scaffold for signaling molecules involved in controlling cell division and differentiation (32, 47). During carcinogenesis, cell adhesion and polarity are often lost, and a role for the down-regulation of specific claudins in these events has been recently suggested (26). Indeed, claudin-4 expression is inversely correlated with the metastatic potential of pancreatic cancer cells (35, 36). In the present study, H. pylori disrupted claudin-4 and claudin-5 at the level of the tight junction and reduced the overall levels of these proteins in intestinal epithelial and gastric adenocarcinoma cells, changes that correlated with the increase in epithelial permeability. H. pylori is considered to be a class 1 carcinogen, a risk associated with the ability of certain strains to inject CagA into the cell, causing a growth factor-like response (23, 49). Further research is needed to assess whether the loss of tight-junctional protein claudin-4 and/or claudin-5 may contribute to this phenotypic change and increase the metastatic potential of gastric cancer cells. Indeed, a recent report suggests that reduced expression of claudin-4 correlates with poor differentiation in gastric cancer adenocarcinoma (28).
The tight junction is a dynamic barrier regulated by a number of physiological and pathophysiological stimuli (31, 55). A number of signaling pathways converge on MLCK, the enzyme responsible for phosphorylating MLC and increasing paracellular permeability. Activated protein kinase C inhibits MLCK (54), and H. pylori has previously been found to disrupt the epithelium in a process inhibited by protein kinase C activators (53). In this study, a specific inhibitor of MLCK (24) could block the increase in permeability and the disruptions of claudin-4 and claudin-5 seen in response to H. pylori exposure. This novel finding suggests that MLCK is involved in the regulation of tight-junctional claudin expression and that H. pylori is able to subvert normal cell signaling pathways to phosphorylate MLC and increase paracellular permeability.
Together, the present observations indicate that the pathophysiological mechanisms of H. pylori-induced permeability changes require direct host-microbe interactions. While inflammatory cells may play a role in vivo, the present study also identifies a direct strain-specific effect of the bacterium on epithelial barrier function. Preliminary investigations into mechanisms responsible for this phenomenon suggest that certain strains of H. pylori may express an outer membrane protein that upon contact with an unidentified apical cell surface component activates MLCK; phosphorylates MLC; disrupts occludin, claudin-4, and claudin-5; and ultimately increases paracellular permeability. These findings describe a novel mechanism whereby a gastric pathogen subverts host-signaling pathways to disrupt epithelial claudin proteins, a change with recently identified carcinogenic potential. Future studies will further define the role played by strain-dependent disruptions in epithelial permeability in the clinical outcome of H. pylori infection.
Acknowledgments
This study was funded by grants from the Natural Sciences and Engineering Research Council of Canada, the Canadian Institutes of Health Research, and the Alberta Heritage Foundation for Medical Research.
We gratefully acknowledge Kim Tran for technical assistance.
Editor: F. C. Fang
REFERENCES
- 1.Amieva, M. R., R. Vogetmann, A. Covacci, L. S. Tompkins, W. J. Nelson, and S. Falkow. 2003. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 300:1430-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borch, K., C. Sjostedt, U. Hannestad, J. D. Soderholm, L. Franzen, and S. Mardh. 1998. Asymptomatic Helicobacter pylori gastritis is associated with increased sucrose permeability. Dig. Dis. Sci. 43:749-753. [DOI] [PubMed] [Google Scholar]
- 3.Boren, T., P. Falk, K. A. Roth, G. Larson, and S. Normark. 1993. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science 262:1892-1895. [DOI] [PubMed] [Google Scholar]
- 4.Buresi, M. C., E. Schleihauf, N. Vergnolle, A. Buret, J. L. Wallace, M. D. Hollenberg, and W. K. MacNaughton. 2001. Protease-activated receptor-1 stimulates Ca(2+)-dependent Cl(−) secretion in human intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 281:G323-G332. [DOI] [PubMed] [Google Scholar]
- 5.Censini, S., M. Stein, and A. Covacci. 2001. Cellular responses induced after contact with Helicobacter pylori. Curr. Opin. Microbiol. 4:41-46. [DOI] [PubMed] [Google Scholar]
- 6.Chin, A. C., D. A. Teoh, K. G.-E. Scott, J. B. Meddings, W. K. Macnaughton, and A. G. Buret. 2002. Strain-dependent induction of enterocyte apoptosis by Giardia lamblia disrupts epithelial barrier function in a caspase-3-dependent manner. Infect. Immun. 70:3673-3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chin, A. C., N. Vergnolle, W. K. MacNaughton, J. L. Wallace, M. D. Hollenberg, and A. G. Buret. 2003. Proteinase-activated receptor 1 activation induces epithelial apoptosis and increases intestinal permeability. Proc. Natl. Acad. Sci. USA 100:11104-11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corrado, G., I. Luzzi, S. Lucarelli, T. Frediani, C. Pacchiarotti, M. Cavaliere, P. Rea, and E. Cardi. 1998. Positive association between Helicobacter pylori infection and food allergy in children. Scand. J. Gastroenterol. 33:1135-1139. [DOI] [PubMed] [Google Scholar]
- 9.Cover, T. L., and M. J. Blaser. 1999. Helicobacter pylori factors associated with disease. Gastroenterology 117:257-260. [DOI] [PubMed] [Google Scholar]
- 10.Day, A. S., N. L. Jones, Z. Policova, H. A. Jennings, E. K. Yau, P. Shannon, A. W. Neumann, and P. M. Sherman. 2001. Characterization of virulence factors of mouse-adapted Helicobacter pylori strain SS1 and effects on gastric hydrophobicity. Dig. Dis. Sci. 46:1943-1951. [DOI] [PubMed] [Google Scholar]
- 11.Dickman, K. G., S. J. Hempson, J. Anderson, S. Lippe, L. Zhao, R. Burakoff, and R. D. Shaw. 2000. Rotavirus alters paracellular permeability and energy metabolism in Caco-2 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 279:G757-G766. [DOI] [PubMed] [Google Scholar]
- 12.Dixon, M. F., R. M. Genta, J. H. Yardley, and P. Correa. 1996. Classification and grading of gastritis. The updated Sydney system. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 20:1161-1181. [DOI] [PubMed] [Google Scholar]
- 13.Falk, P., K. A. Roth, T. Boren, T. U. Westblom, and J. I. Gordon. 1993. An in vitro adherence assay reveals that Helicobacter pylori exhibits cell lineage-specific tropism in the human gastric epithelium. Proc. Natl. Acad. Sci. USA 90:2035-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fasano, A., S. Uzzau, C. Fiore, and K. Margaretten. 1997. The enterotoxic effect of zonula occludens toxin on rabbit small intestine involves the paracellular pathway. Gastroenterology 112:839-846. [DOI] [PubMed] [Google Scholar]
- 15.Figura, N., A. Perrone, C. Gennari, G. Orlandini, L. Bianciardi, R. Giannace, D. Vaira, M. Vagliasinti, and P. Rottoli. 1999. Food allergy and Helicobacter pylori infection. Ital. J. Gastroenterol. Hepatol. 31:186-191. [PubMed] [Google Scholar]
- 16.Finlay, B. B., and S. Falkow. 1990. Salmonella interactions with polarized human intestinal Caco-2 epithelial cells. J. Infect. Dis. 162:1096-1106. [DOI] [PubMed] [Google Scholar]
- 17.Furuse, M., K. Fujita, T. Hiiragi, K. Fujimoto, and S. Tsukita. 1998. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 141:1539-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furuse, M., T. Hirase, M. Itoh, A. Nagafuchi, S. Yonemura, and S. Tsukita. 1993. Occludin: a novel integral membrane protein localizing at tight junctions. J. Cell Biol. 123:1777-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodgame, R. W., H. M. Malaty, H. M. El-Zimaity, and D. Y. Graham. 1997. Decrease in gastric permeability to sucrose following cure of Helicobacter pylori infection. Helicobacter 2:44-47. [DOI] [PubMed] [Google Scholar]
- 20.Guruge, J. L., P. G. Falk, R. G. Lorenz, M. Dans, H. P. Wirth, M. J. Blaser, D. E. Berg, and J. I. Gordon. 1998. Epithelial attachment alters the outcome of Helicobacter pylori infection. Proc. Natl. Acad. Sci. USA 95:3925-3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hazell, S. L., A. Lee, L. Brady, and W. Hennessy. 1986. Campylobacter pyloridis and gastritis: association with intercellular spaces and adaptation to an environment of mucus as important factors in the colonization of the gastric epithelium. J. Infect. Dis. 153:658-663. [DOI] [PubMed] [Google Scholar]
- 22.Heiskala, M., P. A. Peterson, and Y. Yang. 2001. The roles of claudin superfamily proteins in paracellular transport. Traffic 2:93-98. [DOI] [PubMed] [Google Scholar]
- 23.Higashi, H., R. Tsutsumi, S. Muto, T. Sugiyama, T. Azuma, M. Asaka, and M. Hatakeyama. 2002. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science 295:683-686. [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa, T., T. Chijiwa, M. Hagiwara, S. Mamiya, M. Saitoh, and H. Hidaka. 1988. ML-9 inhibits the vascular contraction via the inhibition of myosin light chain phosphorylation. Mol. Pharmacol. 33:598-603. [PubMed] [Google Scholar]
- 25.Jones, N. L., A. S. Day, H. A. Jennings, and P. M. Sherman. 1999. Helicobacter pylori induces gastric epithelial cell apoptosis in association with increased Fas receptor expression. Infect. Immun. 67:4237-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kominsky, S. L., P. Argani, D. Korz, E. Evron, V. Raman, E. Garrett, A. Rein, G. Sauter, O. P. Kallioniemi, and S. Sukumar. 2003. Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene 22:2021-2033. [DOI] [PubMed] [Google Scholar]
- 27.Lee, A., J. O'Rourke, M. Corazon De Ungria, B. Robertson, G. Daskalapoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 28.Lee, S. K., J. Moon, S. W. Park, S. Y. Song, J. B. Chung, and J. K. Kang. 2005. Loss of the tight junction protein claudin 4 correlates with histological growth-pattern and differentiation in advanced gastric adenocarcinoma. Oncol. Rep. 13:193-199. [PubMed] [Google Scholar]
- 29.Liu, Y., A. Nusrat, F. J. Schnell, T. A. Reaves, S. Walsh, M. Pochet, and C. A. Parkos. 2000. Human junction adhesion molecule regulates tight junction resealing in epithelia. J. Cell Sci. 113:2363-2374. [DOI] [PubMed] [Google Scholar]
- 30.Madara, J. L. 1988. Tight junction dynamics: is paracellular transport regulated? Cell 53:497-498. [DOI] [PubMed] [Google Scholar]
- 31.Madara, J. L., and J. R. Pappenheimer. 1987. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J. Membr. Biol. 100:149-164. [DOI] [PubMed] [Google Scholar]
- 32.Matter, K., and M. S. Balda. 2003. Signalling to and from tight junctions. Nat. Rev. Mol. Cell Biol. 4:225-236. [DOI] [PubMed] [Google Scholar]
- 33.Matysiak-Budnik, T., B. Coffin, A. Lavergne-Slove, J. M. Sabate, F. Megraud, and M. Heyman. 2004. Helicobacter pylori increases the epithelial permeability to a food antigen in human gastric biopsies. Am. J. Gastroenterol. 99:225-232. [DOI] [PubMed] [Google Scholar]
- 34.Matysiak-Budnik, T., G. van Niel, F. Mégraud, K. Mayo, C. Bevilacqua, V. Gaboriau-Routhiau, M.-C. Moreau, and M. Heyman. 2003. Gastric Helicobacter infection inhibits development of oral tolerance to food antigens in mice. Infect. Immun. 71:5219-5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michl, P., C. Barth, M. Buchholz, M. M. Lerch, M. Rolke, K. H. Holzmann, A. Menke, H. Fensterer, K. Giehl, M. Lohr, G. Leder, T. Iwamura, G. Adler, and T. M. Gress. 2003. Claudin-4 expression decreases invasiveness and metastatic potential of pancreatic cancer. Cancer Res. 63:6265-6271. [PubMed] [Google Scholar]
- 36.Michl, P., M. Buchholz, M. Rolke, S. Kunsch, M. Lohr, B. McClane, S. Tsukita, G. Leder, G. Adler, and T. M. Gress. 2001. Claudin-4: a new target for pancreatic cancer treatment using Clostridium perfringens enterotoxin. Gastroenterology 121:678-684. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell, D. J., H. Q. Huynh, P. J. M. Ceponis, N. L. Jones, and P. M. Sherman. 2004. Helicobacter pylori disrupts STAT1-mediated gamma interferon-induced signal transduction in epithelial cells. Infect. Immun. 72:537-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nash, S., J. Stafford, and J. L. Madara. 1987. Effects of polymorphonuclear leukocyte transmigration on the barrier function of cultured intestinal epithelial monolayers. J. Clin. Investig. 80:1104-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Odenbreit, S., J. Puls, B. Sedlmaier, E. Gerland, W. Fischer, and R. Haas. 2000. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287:1497-1500. [DOI] [PubMed] [Google Scholar]
- 40.Pang, G., A. Buret, E. O'Loughlin, A. Smith, R. Batey, and R. Clancy. 1996. Immunologic, functional, and morphological characterization of three new human small intestinal epithelial cell lines. Gastroenterology 111:8-18. [DOI] [PubMed] [Google Scholar]
- 41.Papini, E., B. Satin, N. Norais, M. de Bernard, J. L. Telford, R. Rappuoli, and C. Montecucco. 1998. Selective increase of the permeability of polarized epithelial cell monolayers by Helicobacter pylori vacuolating toxin. J. Clin. Investig. 102:813-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pelicic, V., J. M. Reyrat, L. Sartori, C. Pagliaccia, R. Rappuoli, J. L. Telford, C. Montecucco, and E. Papini. 1999. Helicobacter pylori VacA cytotoxin associated with the bacteria increases epithelial permeability independently of its vacuolating activity. Microbiology 145:2043-2050. [DOI] [PubMed] [Google Scholar]
- 43.Philpott, D. J., D. Belaid, P. Troubadour, J.-M. Thiberge, J. Tankovic, A. Labigne, and R. L. Ferrero. 2002. Reduced activation of inflammatory responses in host cells by mouse-adapted Helicobacter pylori isolates. Cell Microbiol. 4:285-296. [DOI] [PubMed] [Google Scholar]
- 44.Rabassa, A. A., R. Goodgame, F. M. Sutton, C. N. Ou, C. Rognerud, and D. Y. Graham. 1996. Effects of aspirin and Helicobacter pylori on the gastroduodenal mucosal permeability to sucrose. Gut 39:159-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saitou, M., K. Fujimoto, Y. Doi, M. Itoh, T. Fujimoto, M. Furuse, H. Takano, T. Noda, and S. Tsukita. 1998. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J. Cell Biol. 141:397-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakaguchi, T., H. Kohler, X. Gu, B. A. McCormick, and H. C. Reinecker. 2002. Shigella flexneri regulates tight junction-associated proteins in human intestinal epithelial cells. Cell Microbiol. 4:367-381. [DOI] [PubMed] [Google Scholar]
- 47.Schneeberger, E. E., and R. D. Lynch. 2004. The tight junction: a multifunctional complex. Am. J. Physiol. Cell Physiol. 286:C1213-C1228. [DOI] [PubMed] [Google Scholar]
- 48.Scott, K. G. E., J. B. Meddings, D. R. Kirk, S. P. Lees-Miller, and A. G. Buret. 2002. Intestinal infection with Giardia spp. reduces epithelial barrier function in a myosin light chain kinase-dependent fashion. Gastroenterology 123:1179-1190. [DOI] [PubMed] [Google Scholar]
- 49.Segal, E. D., J. Cha, J. Lo, S. Falkow, and L. S. Tompkins. 1999. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc. Natl. Acad. Sci. USA 96:14559-14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simonovic, I., J. Rosenberg, A. Koutsouris, and G. Hecht. 2000. Enteropathogenic Escherichia coli dephosphorylates and dissociates occludin from intestinal epithelial tight junctions. Cell. Microbiol. 2:305-315. [DOI] [PubMed] [Google Scholar]
- 51.Sonoda, N., M. Furuse, H. Sasaki, S. Yonemura, J. Katahira, Y. Horiguchi, and S. Tsukita. 1999. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: evidence for direct involvement of claudins in tight junction barrier. J. Cell Biol. 147:195-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sutherland, L. R., M. Verhoef, J. L. Wallace, G. Van Rosendaal, R. Crutcher, and J. B. Meddings. 1994. A simple, non-invasive marker of gastric damage: sucrose permeability. Lancet 343:998-1000. [DOI] [PubMed] [Google Scholar]
- 53.Terrés, A. M., J. M. Pajares, A. M. Hopkins, A. Murphy, A. Moran, A. W. Baird, and D. Kelleher. 1998. Helicobacter pylori disrupts epithelial barrier function in a process inhibited by protein kinase C activators. Infect. Immun. 66:2943-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turner, J. R., J. M. Angle, E. D. Black, J. L. Joyal, D. B. Sacks, and J. L. Madara. 1999. PKC-dependent regulation of transepithelial resistance: roles of MLC and MLC kinase. Am. J. Physiol. 277:C554-C562. [DOI] [PubMed] [Google Scholar]
- 55.Turner, J. R., B. K. Rill, S. L. Carlson, D. Carnes, R. Kerner, R. J. Mrsny, and J. L. Madara. 1997. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am. J. Physiol. 273:C1378-C1385. [DOI] [PubMed] [Google Scholar]
- 56.Warren, J. R., and B. Marshall. 1983. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet i:1273-1275. [PubMed] [Google Scholar]
- 57.Yuhan, R., A. Koutsouris, S. D. Savkovic, and G. Hecht. 1997. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology 113:1873-1882. [DOI] [PubMed] [Google Scholar]