Abstract
CXC chemokines that lack the ELR motif, including interferon-inducible protein 10 [IP-10 (CXCL10)] and monokine induced by gamma interferon (IFN-γ) [MIG (CXCL9)], have been shown to mediate the generation of type 1 immune responses. In this study, we found that intrapulmonary administration of the gram-negative bacterium Klebsiella pneumoniae resulted in the local and systemic expression of IP-10, followed sequentially by MIG expression. MIG mRNA expression in the lungs of Klebsiella-infected mice required the endogenous production of IFN-γ, whereas IP-10 was expressed in both an IFN-γ-dependent and an IFN-γ-independent fashion. Antibody-mediated neutralization of IP-10 resulted in reduced bacterial clearance and decreased survival, whereas bacterial clearance was unaltered in mice treated with anti-MIG antibody. Impaired bacterial clearance in anti-IP-10 antibody-treated mice was associated with significant reductions in the number and/or activational status of NK and NK-T cells, CD4+ T cells, and γδ T cells, as well as a reduction in the expression of IFN-γ. Conversely, the transient transgenic expression of murine IP-10 using adenovirus-mediated gene transfer resulted in improved bacterial clearance when IP-10 adenovirus was given concomitant with intrapulmonary bacterial challenge. These results indicate that IP-10 is an important component of innate immunity against extracellular bacterial pathogens of the lung and may represent a candidate molecule for immunotherapy in the setting of severe respiratory tract infection.
Innate, or natural, immunity is the principal pathway for effective elimination of most bacterial pathogens of the lung (25). The two main phagocytic cells that constitute innate immunity in the lung are resident alveolar macrophages and recruited neutrophils (6, 20, 35, 36). Innate immune responses are enhanced in the presence of type 1 cytokines, including interleukin-12 and gamma interferon (IFN-γ) (14, 24, 30). Type 1 cytokine responses are absolutely required for the effective killing of intracellular pathogens and have also been shown to contribute to effective immunity against extracellular gram-positive and gram-negative bacteria (5, 8, 13, 24, 30, 32). In particular, innate responses against Streptococcus pneumoniae and Klebsiella pneumoniae are impaired in animals deficient in IFN-γ, whereas the exogenous administration or overexpression of IFN-γ can augment the clearance of bacteria from the lungs of immunocompetent mice (8, 19, 24, 30, 32). In most intracellular infections, IFN-γ is elaborated by T and NK cells in an antigen-specific fashion in response to dendritic cell-derived signals (4). IFN-γ can also be expressed early in bacterial infection in a non-antigen-specific fashion by lung macrophages, NK cells, and γδ T cells, either directly in response to microbial signals or in response to host-derived cytokines (8, 9, 23).
Chemokines are a large family of proteins that mediate the movement and activation of diverse groups of inflammatory cells (22). A subfamily of chemokines that plays a key role in promoting type 1 immune responses is the ELR− CXC chemokines. Specifically, the ELR− CXC chemokines, including interferon-inducible protein 10 [IP-10 (CXCL10)], monokine induced by gamma interferon [MIG (CXCL9)], and interferon-inducible T-cell chemoattractant (CXCL11), exert chemotactic effects on various mononuclear cell populations involved in type 1 immunity (11). In vitro, ELR− CXC chemokines stimulate the migration of monocytes, NK cells, Th1 T cells, and NK-T cells (16, 33). Additionally, these chemokines can activate NK cells and induce the production of IFN-γ from effector T cells (12, 33). In vivo, we and others have found that the intrapulmonary transient transgenic expression of mouse or human IP-10 in mice using adenoviral gene transfer resulted in the early accumulation and activation of NK and NK-T cells within the lung, followed by the delayed accumulation of CD4+ T cells (27, 40). The G protein-coupled receptor CXCR3 serves as the sole receptor for ELR− CXC chemokines. CXCR3 is expressed on activated T cells, predominantly of the Th1 phenotype, as well as on NK cells, NK-T cells, and a subset of circulating memory CD4+ and CD8+ T cells (21).
ELR− CXC chemokines have been closely linked with the generation of Th1-type inflammatory responses in vivo. For example, IP-10 and/or MIG is expressed in a wide variety of inflammatory disease states manifested by overzealous type 1 inflammation, including atherosclerosis, multiple sclerosis, rheumatoid arthritis, inflammatory bowel disease, sarcoidosis, and allograft rejection (1, 3, 28, 41). Moreover, both IP-10 and MIG have been shown to participate in the generation of the idiopathic pneumonia syndrome complicating experimental allogeneic bone marrow transplantation, with these molecules functioning in an additive fashion to promote lung injury in this model (15). Conversely, IP-10 has been shown to inhibit the development of allergic airway inflammation (39). These chemokines are also expressed in increased amounts in infections that require vigorous type 1 immunity, including antimicrobial responses against mouse hepatitis virus and intracellular microbial pathogens such as Toxoplasma gondii, Mycobacterium bovis, and Rickettsia conorii (2, 17, 18, 37). Importantly, mice deficient in IP-10 display impaired clearance of mouse hepatitis virus from the brain, which is associated with reduced CD4+ and CD8+ T-cell influx and IFN-γ production (10). Recently, IP-10, MIG, and interferon-inducible T-cell chemoattractant have been shown to be expressed in a murine model of intrapulmonary challenge with the gram-negative coccobacillus Bordetella bronchiseptica and reduced influx of T cells and NK cells and modestly impaired pulmonary clearance of B. bronchiseptica were observed in CXCR3-deficient mice relative to wild-type-infected controls (38). Interestingly, in addition to effects on recruitment and activation of various myeloid cell populations, members of the ELR− CXC chemokine family have been found to have direct bactericidal effects on several bacterial pathogens, including Escherichia coli and Listeria monocytogenes (7).
In this study, we found that both IP-10 and MIG are expressed during the evolution of murine Klebsiella pneumonia and that IP-10 but not MIG is an important component of type 1 immunity in lung antibacterial host defense. The beneficial effects of IP-10 on innate immunity in Klebsiella pneumonia are associated with enhanced IFN-γ production and augmented leukocyte antimicrobial responses.
MATERIALS AND METHODS
Reagents.
The recombinant murine IP-10 and MIG antibodies and biotinylated anti-murine IP-10 and MIG antibodies used in the enzyme-linked immunosorbent assays (ELISAs) were purchased from R&D Systems (Minneapolis, MN). Polyclonal rabbit anti-murine IP-10 antibody was generated as previously described and then purified prior to use (34). Monoclonal anti-murine MIG antibody (clone 2A6.9.9) used for in vivo neutralization studies was generated as previously described (41).
Mice.
Female, specific-pathogen-free, 6- to 8-week-old C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). For experiments involving IFN-γ knockout (IFN-γ−/−) animals, age- and sex-matched wild-type C57BL/6J and IFN-γ−/− female mice on a C57BL/6J background were purchased from the Jackson Laboratory. All animals were housed under specific- pathogen-free conditions within the University of Michigan Animal Care Facility (Ann Arbor, MI) until the day of sacrifice.
Bacterial preparation and intratracheal (i.t.) inoculation.
K. pneumoniae strain 43816 serotype 2 (American Type Culture Collection, Manassas, VA) was used in our studies (13). K. pneumoniae was grown overnight in tryptic soy broth (Difco, Detroit, MI) at 37°C. The concentration of bacteria in broth was determined by measuring the absorbance at 600 nm and then plotting the optical density on a standard curve generated from known CFU values. The bacterial culture was then diluted to the desired concentration. Mice were anesthetized with an intraperitoneal (i.p.) ketamine and xylazine mixture. Next, the trachea was exposed, and 30 μl of inoculum was administered via a sterile 26-gauge needle. The skin incision was closed using surgical staples.
In vivo chemokine neutralization.
Two hours prior to i.t. K. pneumoniae challenge, mice were administered either purified polyclonal rabbit anti-murine IP-10 antibody (5 mg), monoclonal hamster anti-murine MIG antibody (100 μg), or purified mouse control immunoglobulin G (5 mg) i.p. For experiments extending past 48 h, mice received one-half doses of anti-cytokine or control antibody every 48 h. By using this protocol, we observed that treatment with anti-IP-10 antibody resulted in significant reductions in IP-10 to undetectable levels in the lungs of infected mice at 1 and 3 days after K. pneumoniae challenge. Similarly, we were unable to detect MIG in the lungs of infected mice treated with anti-MIG antibody.
Generation of recombinant adenovirus encoding murine IP-10.
The murine IP-10 (mIP-10) gene was cut by restriction enzymes Agel and Nhel from pBlast mIP-10 (InvivGen Co.), resulting in a 373-bp mIP-10 fragment (40). Plasmid pACCMV2 (4,769 bp) was obtained from the University of Michigan Vector Core and was digested by XbaI (931 bp) and XmaI (942 bp) to obtain a 4,758-bp backbone. We then ligated the mIP-10 gene and pACCMV2 backbone to get a 5.1-kp recombinant plasmid. A full-length E1, partial E3-deleted recombinant adenovirus was generated using in situ loxP recombinant between the shuttle vector (linearized with Nhel) and the cAd5-deltaE3.LoxP cosmid containing the Ad5 backbone (linearized with Cla1) in the presence of purified Cre recombinant. The resulting recombinant adenoviral DNA was then transfected into HER 911 cells by standard calcium phosphate precipitation methods. Recombinant clones were identified as plaques in soft agar culture and characterized by ELISA to verify the presence of IP-10 prior to further amplification. Large-scale, high-titer adenoviral purification, particle determination (particles/ml), and titer determination (PFU/ml) were performed using the University of Michigan Vector Core modification of established methods (40). Aliquots of AdcmvmIP10 (Ad IP-10) and Adcmvplpa (Ad CTL) were maintained at −80°C until immediately prior to use.
Intratracheal inoculation of Ad IP-10 or Ad CTL.
Mice were anesthetized by i.p. injection of a ketamine-xylazine mixture. The trachea was exposed, 30 μl of saline containing 109 PFU of Ad IP-10 or Ad CTL was administered via a sterile 26-gauge needle and the skin incision was closed via surgical staples.
Peripheral blood CFU determination.
Blood was collected in a heparinized syringe from the right ventricle at designated time points, serially diluted 1/2 with PBS, and plated on blood agar to determine blood CFU.
Whole-lung homogenization for CFU and cytokine analysis.
At designated time points, the mice were euthanized by CO2 inhalation. Before lung removal, the pulmonary vasculature was perfused by infusing 1 ml of phosphate-buffered saline (PBS) containing 5 mM EDTA into the right ventricle. Whole lungs were removed, taking care to dissect away lymph nodes. The lungs were then homogenized in 1 ml of PBS with protease inhibitor (Boehringer Mannheim, Indianapolis, IN). Homogenates were then serially diluted 1/5 in PBS and plated on blood agar to determine lung CFU. The remaining homogenates were sonicated and then centrifuged at 1,400 × g for 15 min. Supernatants were collected, passed through a 0.45-μm-pore-sized filter, and then stored at −20°C for the assessment of cytokine levels.
Total lung leukocyte preparation.
Lungs were removed from euthanized animals, and leukocytes were prepared as previously described (9, 23, 40). Briefly, lungs were minced with scissors to a fine slurry in 15 ml of digestion buffer (RPMI medium/10%fetal calf serum/1 mg/ml collagenase [Boehringer Mannheim Biochemical]/30 μg/ml DNase [Sigma]) per lung. Lung slurries were enzymatically digested for 30 min at 37°C. Any undigested fragments were further dispersed by drawing the solution up and down through the bore of a 10-ml syringe. The total lung cell suspension was pelleted, resuspended, and spun through a 40% Percoll gradient to enrich for leukocytes. Cell counts and viability were determined using trypan blue exclusion counting on a hemacytometer. Cytospin slides were prepared and stained with a modified Wright-Giemsa stain.
Multiparameter flow cytometric analyses.
Cells were isolated from lung digests as described above (9, 23, 40). For analyses of T-cell subsets, isolated leukocytes were stained with the following fluorescein isothiocyanate- or phycoerythrin-labeled antibodies: anti-γδ-T-cell receptor (TCR), anti-αβ-TCR, anti-CD8, anti-CD4, anti-DX5, and anti-CD69 (all reagents from PharMingen, San Diego, CA, unless otherwise noted). In addition, cells were stained with anti-CD45-tricolor (Caltag Laboratories, San Francisco, CA), allowing for the discrimination of leukocytes from nonleukocytes and thus eliminating any nonspecific binding of T-cell surface markers on nonleukocytes. T- and NK-cell subsets were analyzed by first gating on CD45-positive “lymphocyte-sized” leukocytes and then examined for FL1 and FL2 fluorescence expression. Cells were collected on a FACScan or FACScalibur cytometer (Becton Dickinson, San Jose, CA) by using CellQuest software (Becton Dickinson). Analyses of data were performed using the CellQuest software package.
Isolation and reverse transcription-PCR amplification of whole-lung mRNA.
Whole lung was harvested, immediately snap-frozen in liquid nitrogen, and stored at −70°C; then reverse transcription (RT)-PCR was performed as previously described (40). Briefly, total cellular RNA from the frozen lungs were isolated, reversed transcribed into cDNA, and then amplified using specific primers for mIP-10 and mMIG with β-actin serving as a control. The primers had the sequences 5′-ATC ATC CCT GCG AGC CTA TC-3′ and 5′-GAA CTG ACG AGC CTG AGC TA-3′ for IP-10; 5′-ACATTCTCGGACTTCACTCCA-3′ and 5′-CTAGGCAGGTTTGATCTCCGT-3′ for murine MIG; and 5′-ATG GAT GAC GAT ATC GCT C-3′ and 5′-GAT TCC ATA CCC AGG AGG G-3′ for β-actin. After amplification, the samples (20 μl) were separated on a 2% agarose gel containing ethidium bromide at 5 μl/100 ml (Sigma; 10 mg/ml), and bands were visualized and photographed using UV transillumination.
Real-time quantitative RT-PCR.
Measurement of gene expression was performed utilizing the ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA) as previously described (40). Briefly, primers and probes for β-actin, IP-10, MIG, and IFN-γ were designed using Shortcut to Primer Express software (Applied Biosystems). The primers, placed in different exons, were tested not to amplify genomic DNA. Primer and probe nucleotide sequences for mIP-10 were as follows: forward primer 5′-CCA GTG AGA ATG AGG GCC ATA-3′ and reverse primer 5′-CTC AAC ACG TGG GCA GGA T-3′; forward primer 5′-TGA AGT CCG CTG TTC TTT TCC-3′ and reverse primer 5′-GGG TTC CTC GAA CTC CAC ACT-3′; TaqMan probe 5′(6-carboxyfluorescein [FAM])-TTT GGG CAT CAT CTT CCT GGA-(6-carboxytetramethylrhodamine [TAMRA])3′for mMIG; forward primer 5′-CTG CGG CCT AGC TCT GAG A-3′ and reverse primer 5′-CAG CCA GAA ACA GCC ATG AG-3′; TaqMan probe 5′ (FAM)-CAC ACT GCA TCT TGG CTT TGC AGC TC-(TAMRA)3′; and TaqMan probe 5′(FAM)-CTT GAA ATC ATC CCT GCG AGC C-(TAMRA)3′ for mIFN-γ. Primer and probe nucleotide sequences for mβ-actin were as follows: forward primer 5′-CCG TGA AAA GAT GAC CCA GAT C-3′, reverse primer 5′-CAC AGC CTG GAT GGC TAC GT-3′, and TaqMan probe 5′(FAM)-TTT GAG ACC TTC AAC ACC CCA GCC A-(TAMRA)-3′. Specific thermal cycling parameters used with the TaqMan one-step RT-PCR master mix reagent kit included 30 min at 48°C, 10 min at 95°C, 40 cycles involving denaturation at 95°C for 15 s, and annealing/extension at 60°C for 1 min. Relative quantitation of IP-10 or MIG mRNA levels was plotted as change (n-fold) relative to untreated control. All experiments were performed in duplicate.
Murine cytokine ELISA.
Murine IP-10 and MIG were quantitated using a modification of a double-ligand method as previously described (40). Standards were one-half-log dilutions of murine recombinant cytokines from 1 pg/ml to 100 ng/ml. This ELISA method consistently detected murine IP-10 or MIG concentrations above 50 pg/ml. The ELISAs did not cross-react with other cytokines tested.
Statistical analysis.
Survival curves were compared using the log-rank test. For other data, statistical significance was determined using the unpaired t test. All calculations were performed using the Prism 3.0 software program for Windows (GraphPad Software, San Diego, CA). All mean data shown are expressed as means plus or minus standard errors of the means.
RESULTS
Induction of IP-10 and MIG mRNA after i.t. administration of K. pneumoniae.
To determine the effect of i.t. K. pneumoniae administration on the expression of IP-10 and MIG mRNA levels, C57B/6J mice were administered K. pneumoniae (5 × 103 CFU). Lungs, liver, and spleen were harvested at 12, 24, 48, and 72 h after i.t. administration. K. pneumoniae administration and chemokine mRNA levels were determined by RT-PCR (Fig. 1A, top panel) and real-time quantitative PCR (bottom panel). As shown in Fig. 1A, there was a time-dependent increase in the expression of IP-10 and MIG mRNA in the lung, with maximal expression occurring at 48 h for IP-10 (>100-fold increase over the uninfected control) and 72 h for MIG (70-fold increase over the uninfected control). In addition, we observed substantial, albeit somewhat smaller, increases in the expression of IP-10 and MIG mRNA in liver (Fig. 1B) and spleen (Fig. 1C), with temporal patterns of expression similar to those observed in the lung.
FIG. 1.
Expression of IP-10 and MIG mRNA in lung, liver, and spleen after i.t. administration of K. pneumoniae. Animals were administered 5 × 103 CFU K. pneumoniae i.t., and then organs were harvested at various times after bacterial administration. mRNA levels in lung, liver, and spleen homogenates are shown in panels A, B, and C, respectively. The top of each panel shows results of RT-PCR, whereas the bottom of each panel shows results of real-time PCR. For RT-PCR, 30 cycles were performed for IP-10 or MIG, while 25 cycles were performed for β-actin. For real-time PCR, fold increases represent that over untreated lung. n was three organs per each time point that was combined (RT-PCR) or averaged (real-time PCR). kp, Klebsiella-infected mice.
Induction of IP-10 and MIG protein after i.t. administration of K. pneumoniae.
We next assessed the production of IP-10 and MIG protein in whole lung after i.t. K. pneumoniae administration. As shown in Fig. 2, i.t. K. pneumoniae challenge resulted in an increase in IP-10 protein expression by 24 h, a maximal sevenfold increase in IP-10 levels at 48 h, and continued production out to 96 h. K. pneumoniae administration resulted in a somewhat delayed expression of MIG, with a maximal fivefold increase in MIG protein levels at 48 h, compared to that observed in the untreated control lung. MIG levels remained elevated at 96 h after i.t. K. pneumoniae administration.
FIG. 2.
Effect of i.t. K. pneumoniae administration on IP-10 and MIG protein levels in lung homogenates. Animals were administered 5 × 103 CFU K. pneumoniae i.t., then lungs were harvested at various times after bacterial administration. *, P was <0.05 compared to that for the uninfected control. Experimental n was four to seven animals per group per time point.
Contribution of IFN-γ to the induction of IP-10 and MIG in murine Klebsiella pneumonia.
To determine if the expression of MIG and/or IP-10 mRNA and protein was dependent on the endogenous production of IFN-γ in Klebsiella pneumonia, we assessed the expression of MIG and IP-10 mRNA by quantitative real-time PCR (Fig. 3A) and protein analysis (Fig. 3B) in whole lungs of wild-type and IFN-γ-deficient mice after i.t. K. pneumoniae administration. As shown in Fig. 3, intrapulmonary bacterial administration resulted in substantial upregulation of MIG mRNA in wild-type animals at 48 h, whereas no induction of MIG message was detected in IFN-γ-deficient animals. In contrast, a modest but significant induction (14.3-fold induction) of IP-10 mRNA was observed in IFN-γ knockout animals infected with Klebsiella relative to uninfected animals. However, the expression of IP-10 was substantially reduced from that observed in wild-type mice challenged with K. pneumoniae. Consistent with the mRNA data, we observed a small but appreciable induction of IP-10 protein in the lungs of IFN-γ knockout animals 72 h after i.t. challenge with Klebsiella (Fig. 3B) (P, <0.05), whereas no increase in MIG was noted in IFN-γ knockout mice at either 48 or 72 h after K. pneumoniae administration. These observations indicate that the expression of both MIG and IP-10 in the lungs of Klebsiella-infected animals is largely dependent upon IFN-γ, although other signals can serve to induce IP-10, but not MIG, in pneumonia independently of IFN-γ.
FIG. 3.
Expression of IP-10 and MIG mRNA and protein in lungs of wild-type (wt) and IFN-γ−/− mice challenged with K. pneumoniae. For mRNA analysis, lungs were harvested 48 h after bacterial administration and chemokine mRNA levels were determined by quantitative real-time PCR (A). For protein analysis, lungs were harvested 48 and 72 h after bacterial administration and chemokine protein levels were determined by ELISA. For real-time PCR four lungs per group per time point were combined and averaged and represent fold increase over uninfected control. For protein analysis, n was six lungs per group per time point and data presented are a composite of two separate experiments. *, P was <0.05 compared to that for the uninfected control. **, P was <0.01 compared to that for the uninfected control or infected IFN-γ knockout (ko) mice. kp, Klebsiella-infected mice.
Effect of anti-murine IP-10 or anti-murine MIG antibody administration on bacterial clearance in murine Klebsiella pneumonia.
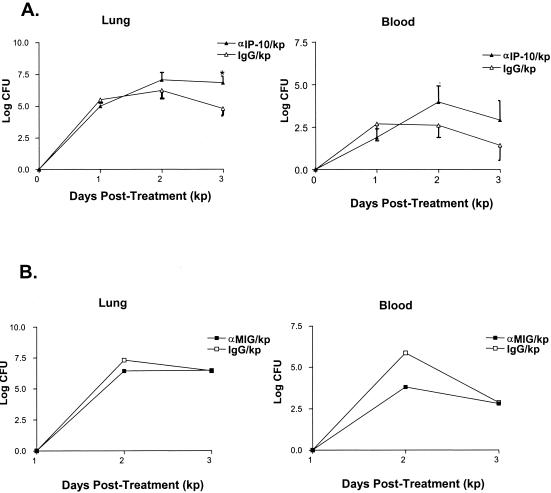
Experiments were performed to determine whether IP-10 or MIG was required for bacterial clearance in animals with Klebsiella pneumonia. In these experiments, animals were administered either rabbit polyclonal anti-murine IP-10 or control IgG (5 mg) or monoclonal anti-murine MIG antibody (clone 2A6.9.9) or control IgG (100 μg) 2 h prior to K. pneumoniae inoculation (5 × 103 CFU), and then one-half of the original antibody dose was administered 48 h later. Lungs and blood were harvested on days 1, 2, and 3 after bacterial challenge for the assessment of K. pneumoniae CFU. As shown in Fig. 4A, the administration of anti-IP-10 antibody resulted in approximately 10- and 100-fold increases in the number of K. pneumoniae CFU isolated from lung homogenates recovered from animals 2 and 3 days after Klebsiella administration, respectively, relative to animals receiving control IgG (P, <0.05). Similarly, i.p. administration of anti-IP-10 antibody resulted in an increase in bacterial burden in blood, with approximately 24- and 31-fold increases in the number of K. pneumoniae CFU observed at 2 and 3 days after Klebsiella administration, respectively, relative to animals receiving control IgG (Fig. 5B). To confirm these results, we performed additional studies using a hamster anti-murine IP-10 antibody that has been shown to effectively neutralize IP-10 in a systemic murine Toxoplasma gondii model (17). Like results obtained using the polyclonal antibody, we found that the i.p. administration of hamster anti-IP-10 antibody resulted in a similar increase in lung and blood K. pneumoniae CFU relative to animals receiving control antibody (data not shown).
FIG. 4.
Effect of IP-10 or MIG neutralization on bacterial clearance in Klebsiella-infected mice. Animals were administered either purified rabbit control IgG, purified rabbit anti-murine IP-10 antibody (5 mg) i.p. or monoclonal hamster anti-murine IP-10 antibody (100 μg) 2 h before and 2.5 mg every 48 h after the administration of K. pneumoniae (5 × 103 CFU). Panel A represents effects of anti-IP-10 on lung (left) and blood (right) K. pneumoniae CFU at 1, 2, and 3 days after infectious challenge expressed on a log10 scale. Panel B represents the effect of MIG neutralization on lung (left) and blood (right) K. pneumoniae CFU at 2 and 3 days after infectious challenge expressed on a log10 scale. *, P was <0.05 compared to that for mice receiving control IgG. Experimental n was five to eight animals per time point, and data presented are a composite of two separate experiments. kp, Klebsiella-infected mice.
FIG. 5.
Effect of IP-10 neutralization on survival in Klebsiella-infected mice. Animals were administered either purified rabbit control IgG or purified rabbit anti-murine IP-10 antibody (5 mg) i.p. 2 h before and a one-half dose every 48 h after the administration of K. pneumoniae (5 × 103 CFU). *, P was <0.05 compared to that of mice receiving control IgG. Experimental n was 20 to 22 animals per group, and data presented are a composite of two separate experiments. kp, Klebsiella-infected mice.
In contrast to the impaired clearance of bacteria observed with antibody-mediated IP-10 neutralization, we observed no difference in lung or blood K. pneumoniae CFU in animals pretreated with monoclonal anti-murine MIG antibody (100 μg) at 2 and 3 days after bacterial challenge relative to animals receiving control IgG (Fig. 4B). The failure of the anti-murine MIG antibody to alter clearance was not attributable to an inability to effectively neutralize MIG, as the administration of this antibody substantially reduced MIG levels in lungs of Klebsiella-infected animals and has been shown to be effective in other animal model systems (41).
Effect of anti-murine IP-10 antibody administration on survival in murine Klebsiella pneumonia.
Having determined that the neutralization of IP-10, but not MIG, impaired the clearance of bacteria from the lung, we next assessed the effect of anti-IP-10 antibody administration on survival in mice infected with K. pneumoniae. In these experiments, animals were administered anti-IP-10 antibody or control IgG (5 mg) i.p. and then 2.5 mg antibody every 48 h throughout the duration of the study. Two hours following the initial antibody administration, animals received K. pneumoniae (5 × 103 CFU) i.t., and then effects on survival were determined. As shown in Fig. 5, the neutralization of IP-10 significantly decreased survival, as <20% of anti-IP-10-treated animals survived long-term (more than 10 days postinoculation), whereas 60% of infected animals receiving control IgG survived (P, <0.05).
Effect of anti-IP-10 antibody administration on lung leukocyte influx in murine Klebsiella pneumonia.
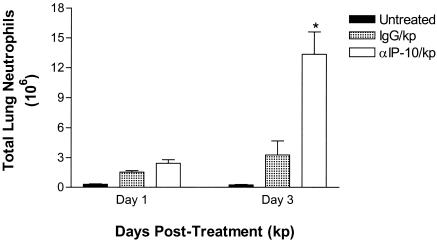
To determine if the detrimental effects of IP-10 neutralization on bacterial clearance and survival were attributable to alterations in lung leukocyte influx, numbers of lung leukocytes were quantitated in control IgG- and anti-IP-10-treated animals. Animals were administered control or anti-IP-10 antibodies 2 h prior to and 48 h after K. pneumoniae inoculation (5 × 103 CFU), and then lungs were harvested at 24 and 72 h postinfection and total leukocyte populations were quantitated by lung digestion. The i.t. administration of K. pneumoniae resulted in a maximal 2.5-fold increase in total numbers of lung leukocytes at 72 h (data not shown), with the greatest increase observed in the number of neutrophils and a smaller increase observed in mononuclear cells (mononuclear phagocytes plus lymphocytes). Interestingly, the administration of anti-IP-10 antibody resulted in a significant increase in the number of neutrophils in the lung, most notable at 72 h after infectious challenge (P was <0.05 compared to that for the control IgG group) (Fig. 6). No change in total number of mononuclear cells was noted at either time point (data not shown). Also, no change in either neutrophil or mononuclear cell influx was observed in animals pretreated with anti-MIG antibody prior to i.t. bacterial challenge (data not shown).
FIG. 6.
Effect of IP-10 neutralization on lung neutrophil influx after i.t. K. pneumoniae administration. Animals were administered either purified rabbit control IgG or purified rabbit anti-murine IP-10 antibody (5 mg) i.p. 2 h before and a one-half of the dose 48 h after the administration of K. pneumoniae (5 × 103 CFU). *, P was <0.05 compared to that for mice receiving control IgG. Experimental n was five to seven animals per group, and data presented are a composite of two separate experiments. kp, Klebsiella-infected mice.
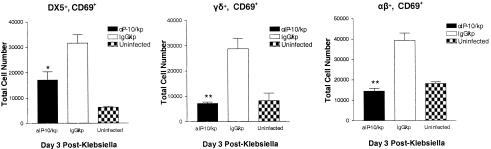
To determine whether anti-IP-10 antibody administration altered the influx and/or activation of selected T-cell and NK-cell populations, C57BL/6 mice were administered control IgG or anti-IP-10 antibody i.p., followed 2 h later by the i.t. administration of K. pneumoniae (5 × 103 CFU), and then the presence of specific T- and NK-cell populations in lungs was determined at 24 and 72 h after bacterial administration by flow cytometry. Minimal changes in numbers of total NK, NK-T, CD4+, and CD8+ T cells were noted in the lungs of Klebsiella-infected mice at 24 h relative to uninfected animals, and the numbers of these cells were not altered by the administration of anti-IP-10 antibody at this time point (data not shown). At 72 h, however, K. pneumoniae administration resulted in an increase in both NK (DX5+ αβ/γδ TCR−) and NK-T cells (DX5+ αβ/γδ TCR+), which were decreased by approximately 50% and >70% in Klebsiella-infected animals pretreated with anti-IP-10 antibody, respectively (Table 1). Similarly, neutralization of IP-10 resulted in a decrease in the numbers of CD8+ T cells (P, <0.05) and a trend toward a decrease in numbers of CD4+ T cells (P = 0.06) relative to Klebsiella-infected animals receiving control IgG. There was also a significant reduction in the total number of γδ T cells (P, <0.05), but the change in the total number of αβ T cells did not reach the level of statistical significance. To determine effects of anti-IP-10 antibody on the accumulation of activated T and NK cells, we assessed cell surface expression of the activational marker CD69. As shown in Fig. 7, treatment with anti-IP-10 antibody resulted in a marked decrease in the number of activated NK plus NK-T cells (DX5+ CD69+), αβ T cells (αβ TCR+ CD69+), and γδ T cells (γδ TCR+ CD69+) relative to K. pneumoniae-infected animals receiving control IgG (P was <0.01 for all groups).
TABLE 1.
Effect of IP-10 neutralization of accumulation of mononuclear cells in lung 72 h after i.t. K. pneumoniae administration
| Cell type | Treatment typea
|
||
|---|---|---|---|
| Uninfected | Kp/IgG | Kp/αIP-10 | |
| CD4+ | 21.66 ± 5.91 | 47.16 ± 6.54 | 34.56 ± 3.45 |
| CD8+ | 7.34 ± 0.46 | 11.42 ± 1.24 | 8.96 ± 0.41b |
| αβ TCR+ | 34.44 ± 5.46 | 50.32 ± 11.7 | 39.18 ± 4.01b |
| γδ TCR+ | 0.93 ± 0.16 | 1.76 ± 0.41 | 1.03 ± 0.09b |
| DX5+ | 9.29 ± 0.54 | 14.34 ± 1.52 | 11.84 ± 1.10b |
| DX5+ αβ/γδ TCR+ | 1.76 ± 0.30 | 5.44 ± 0.84 | 2.51 ± 0.24b |
Cells are expressed as cell number × 104. n was three to six per group, and data presented are a composite of two separate experiments. Kp, Klebsiella-infected mice.
P was <0.05 relative to that for infected mice receiving control IgG.
FIG. 7.
Effect of IP-10 neutralization on the accumulation of activated (CD69+) mononuclear cells in lung after i.t. K. pneumoniae administration. Animals were administered either purified rabbit control IgG or purified rabbit anti-murine IP-10 antibody (5 mg) i.p. 2 h before and 2.5 mg 48 h after the administration of K. pneumoniae (5 × 103 CFU), and then lungs were harvested 72 h after bacterial challenge. Shown are absolute numbers of DX5+ (left), γδ TCR+ (middle), and αβ TCR+ (right) cells coexpressing the activational marker CD69. *, P was <0.05 compared to that for mice receiving control IgG. **, P was <0.01 compared to that for mice receiving control IgG. Experimental n was six animals per group, and data presented are a composite of two separate experiments. kp, Klebsiella-infected mice.
Effect of anti-IP-10 antibody administration on the intracellular accumulation of bacteria within lung leukocytes isolated from mice with Klebsiella pneumonia.
Lung macrophages and neutrophils rapidly kill bacteria that have been internalized. Consequently, bacteria are rarely identified within phagolysosomes under normal conditions. To determine the effects of IP-10 neutralization on the accumulation of intracellular bacteria within lung phagocytic populations ex vivo, animals received control or anti-IP-10 antibody followed by the administration of K. pneumoniae i.t. Leukocytes were then recovered from dispersed lungs assessed at 48 and 72 h after bacterial challenge for the presence of intracellular bacteria as determined by Wright-Giemsa staining of cytospins. Leukocyte populations present in the lungs of infected control mice at 48 to 72 h consisted predominantly of alveolar macrophages and neutrophils (Fig. 8). Few internalized bacteria were detected within either alveolar macrophages (0.1 ± 0.05 bacteria/cell) or neutrophils (0.1 ± 0.07 bacteria/cell) recovered from the lungs of Klebsiella-infected mice receiving control IgG (Fig. 8B). In contrast, we found an increase in the number of neutrophils in dispersed lungs of anti-IP-10-treated mice and large quantities of bacteria were present within phagolysosomes of both neutrophils (6.5 ± 1.6 bacteria/cell; P was <0.01 relative to that for IgG control cells) (Fig. 8A) and alveolar macrophages (13.7 ± 3.2 bacteria/cell; P was <0.01 relative to that for IgG control cells). These findings indicate that in the absence of IP-10, there is a striking accumulation of bacteria within phagolysosomes, which could occur as a result of a greater burden of organisms within the airspace or, more likely, as a consequence of impaired ability to kill ingested organisms.
FIG. 8.
Effect of anti-IP-10 antibody administration on the intracellular accumulation of bacteria within leukocytes isolated from the lungs of mice with Klebsiella pneumonia. Animals were administered either purified rabbit control IgG or purified rabbit anti-murine IP-10 antibody (5 mg) i.p. 2 h before and 2.5 mg 48 h after administration of K. pneumoniae (5 × 103 CFU), and then lungs were harvested 72 h after bacterial challenge. Panel A is a representative cytospin of dispersed lung leukocytes from anti-IP-10-treated mice, whereas panel B is a representative cytospin from control IgG-treated mice. The closed arrow shows intracellular K. pneumoniae within alveolar macrophages, and the open arrow shows intracellular K. pneumoniae with neutrophils. Representative of five to six separate experiments. kp, Klebsiella-infected mice.
Effect of anti-IP-10 antibody administration on the expression of IFN-γ mRNA in murine Klebsiella pneumonia.
Because our data suggest that neutrophils and macrophages isolated from anti-IP-10-treated animals displayed impaired killing of ingested organisms and IP-10 has been shown to directly stimulate IFN-γ production from T cells and mediate the accumulation of IFN-γ-producing cells, we next assessed the effect of IP-10 neutralization on the expression of IFN-γ mRNA in the lungs of Klebsiella-infected mice. In these studies, mice were administered either control IgG or rabbit anti-mouse IP-10 antibody i.p. and challenged with K. pneumoniae, and then IFN-γ mRNA levels were determined in lung homogenates at various time points by real-time quantitative PCR. As shown in Fig. 9, there was a significant induction of IFN-γ message (27-fold increase) in lung homogenate of Klebsiella-infected mice, peaking at 48 h after bacterial administration and decreasing by 72 h. In comparison, peak IFN-γ mRNA expression was significantly blunted in the lungs of infected animals receiving anti-IP-10 antibody (P was <0.05 relative to that for infected control animals).
FIG. 9.
Effect of IP-10 neutralization on expression of IFN-γ mRNA in Klebsiella-infected mice. Animals were administered either purified rabbit control IgG or purified rabbit anti-murine IP-10 antibody (5 mg) i.p. 2 h before and 2.5 mg 48 h after administration of K. pneumoniae (5 × 103 CFU). IFN-γ mRNA levels in lung homogenates were quantitated by real-time PCR and expressed as mean fold increases over untreated lung. n was four per time point. kp, Klebsiella-infected mice.
Effect of adenovirus-mediated transgenic expression of IP-10 on bacterial clearance in murine Klebsiella pneumonia.
Having shown that antibody-mediated neutralization of IP-10 resulted in impaired lung antibacterial host defense, we next evaluated the effect of intrapulmonary transient transgenic expression of IP-10 on clearance of K. pneumoniae from the lung. To accomplish this, we inserted the murine IP-10 or a control gene into a human E1, partial E3-deleted recombinant adenovirus. The i.t. administration of 109 PFU of recombinant IP-10-containing adenovirus (Ad IP-10) resulted in a fivefold increase in IP-10 in lung homogenates, maximal at 24 h postadministration (40). Importantly, the administration of Ad IP-10 (109 PFU) i.t. concomitant with K. pneumoniae (5 × 103 CFU) resulted in a nearly 100-fold reduction in bacterial CFU in lung harvest 3 days later, compared to that of infected animals receiving control adenovirus i.t. (Fig. 10, P, <0.05). Additionally, adenovirus-mediated expression of IP-10 resulted in 30-fold and more than 100-fold reductions in blood CFU at day 2 (P, 0.05) and day 3 (P, 0.10) after bacterial administration, respectively.
FIG. 10.
Effect of i.t. Ad IP-10 or Ad CTL administration on lung and blood CFU in Klebsiella-infected mice. Animals were treated with 109 PFU Ad IP-10 or Ad CTL i.t. concomitant with K. pneumoniae (5 × 103 CFU), and then bacterial CFU in lung (top) and blood (bottom) were determined at various days after K. pneumoniae infection. CFU values are expressed on a log10 scale. *, P was <0.05 compared to that for mice treated with Ad CTL. Experimental n was five to seven animals per time point, and data presented are a composite of two separate experiments. kp, Klebsiella-infected mice.
DISCUSSION
Our studies indicate that the intrapulmonary challenge with K. pneumoniae results in marked induction of IP-10 and MIG expression and that IP-10, but not the MIG, contributes substantially to host innate responses against this virulent bacterial pathogen. IP-10 and/or MIG has previously been shown to contribute to host defense against several intracellular microbial pathogens and viruses (2, 17, 18, 37). In the current study, we found that IP-10 is an important component of lung innate immunity against extracellular gram-negative bacteria that are highly relevant respiratory pathogens in humans.
We found that i.t. bacterial administration resulted in the early expression of IP-10 mRNA in lung, with a somewhat delayed expression (approximately 24 h later) of the MIG message. Furthermore, we observed that the expression of MIG in Klebsiella pneumonia required the endogenous production of IFN-γ, whereas IP-10 expression was detected in IFN-γ-deficient mice challenged with K. pneumoniae, albeit considerably diminished compared to that found in wild-type animals. Our findings are consistent with that of Neumann and associates, who observed that the production of both MIG and IP-10 was largely IFN-γ dependent in a staphylococcal enterotoxin B model of lung inflammation (26). In addition, intrapulmonary bacterial administration resulted in considerable systemic expression of both IP-10 and MIG in a time frame similar to that observed in lung. The early induction of IP-10 may occur as a direct result of bacterial exposure or perhaps in response to host-derived genes, including TNF-α and heat shock protein, expressed early in the innate response (reference 11 and unpublished observations). In contrast, MIG expression is likely delayed relative to IP-10 due to the absolute requirement for IFN-γ, which is not maximally expressed in Klebsiella pneumonia until at least 48 h after bacterial challenge. Early expression of IFN-γ in the lung during pneumonia is derived from several cellular sources, including NK cells, γδ-T cells, and perhaps pulmonary macrophages (8, 9, 23).
Mechanisms by which IP-10 promotes effective innate responses in the lung have been incompletely defined. However, our findings indicate that neutralization of IP-10 results in significant reductions in the number or activational status of IFN-γ-producing cells in the lung, including NK cells, NK-T cells, CD4+ T cells, and γδ-T cells. Moreover, antibody-mediated neutralization of IP-10 resulted in a significant decrease in the intrapulmonary expression of IFN-γ mRNA in pneumonia. This reduction in IFN-γ could result from impaired recruitment of IFN-γ-producing cells or, alternatively, may be attributable to loss of direct stimulatory effects of IP-10 on IFN-γ production from effector T cells (12). Our in vivo findings are consistent with known in vitro chemotactic effects of IP-10 on specific NK, T, and NK-T cell populations (11, 12, 33). We and others have also shown that the transient transgenic expression of IP-10 within the lung results in the accumulation of NK cells, NK-T cells, and CD4+ T cells, as well as an increase in the number of activated T cells and NK cells, as manifested by CD69 expression and intracellular IFN-γ expression (27, 40). Given that IFN-γ is a key activator of both neutrophil and alveolar macrophage antimicrobial function (19, 29, 31), reduced IFN-γ responses readily explain the impaired killing of internalized bacteria, as observed in neutrophils and alveolar macrophages isolated ex vivo from anti-IP-10-treated mice.
Interestingly, we found an increased number of neutrophils within the lungs of infected animals receiving anti-IP-10 antibody. Neither IP-10 nor other ELR− CXC chemokines exert direct chemotactic effects on neutrophils in vitro. However, the transient transgenic expression of mouse or human IP-10 in rodents can induce lung neutrophilia in vivo, and this effect occurs in a T-cell-dependent fashion (27, 40). The most plausible explanation for enhanced neutrophil influx is that this response is being driven by the increased bacterial burden in lungs of anti-IP-10-treated mice. These findings would also argue against a direct stimulatory effect of IP-10 on neutrophil migration.
We observed an important role of IP-10, but not MIG, in lung antibacterial host defense. The specificity of chemokine responses is somewhat surprising, given that in vitro chemotactic and activating effects are similar and these chemokines signal through a common receptor (11, 12, 21). Direct comparisons of IP-10 and MIG in type 1 immune responses, particularly in infection, have not been reported. Others have noted disparate regulation of IP-10 and MIG in other inflammatory conditions, including infection (37). These observations support the notion that specific ELR− CXC chemokines are functionally distinct, and there is not complete redundancy in biological effects in vivo.
A possible mechanism that could account for the lack of anti-MIG antibody effects is a compensatory increase in IP-10 in Klebsiella-infected animals in which MIG has been neutralized. However, we failed to observe changes in IP-10 in animals receiving anti-MIG antibodies relative to infected mice receiving control IgG (data not shown).
Our findings differ somewhat from that reported by Widney and associates (38). Specifically, these investigators found that mice that were deficient in CXCR3 had impaired lymphocyte and NK-cell influx in response to intrapulmonary challenge with the gram-negative coccobacillus B. bronchiseptica but displayed only modest defects in bacterial clearance compared to that of wild-type controls. The disparity in our findings may be partially attributable to differences in the virulence of the bacterial pathogen used, as K. pneumoniae is an encapsulated, highly virulent pathogen that requires maximal neutrophil and alveolar macrophage effector responses, whereas B. bronchiseptica is considerably less virulent and the nature of protective responses against this pathogen has been only partially defined (14). Also, the aforementioned study utilized mice which had a life-long deficiency in CXCR3 and, as a result, may have developed alternative pathways to compensate for the absence of this receptor. Moreover, by blocking the effects of the entire ELR− CXC chemokine family, disparate and perhaps opposing effects of various family members might considerably confound the phenotype expressed.
The intrapulmonary transient transgenic expression of IP-10 resulted in a significant improvement in bacterial clearance in mice with Klebsiella pneumonia. The mechanism of this improved clearance has not yet been defined. However, we have previously shown that adenovirus-mediated IP-10 expression resulted in augmented accumulation of IFN-γ-producing cells, including NK, NK-T, and CD4+ T cells (40). These results are consistent with the protective effects on pulmonary innate immunity when other type 1 cytokines, including interleukin-12 and IFN-γ, are compartmentally expressed in lung in murine models of pneumonia due to both extracellular and intracellular bacterial pathogens (8, 13, 19). These findings support a potential role for IP-10 as a candidate for immunoadjuvant therapy, especially in conditions of impaired type 1 immune responses.
Acknowledgments
This work was supported by NIH grants PO50 HL60289 and HL57243 (T.J.S.).
Editor: J. N. Weiser
REFERENCES
- 1.Agostini, C., F. Calabrese, F. Rea, M. Facco, A. Tosoni, M. Loy, G. Binotti, M. Valente, L. Trentin, and G. Semenzato. 2001. CXCR3 and its ligand CXCL10 are expressed by inflammatory cells infiltrating lung allografts and mediate chemotaxis of T cells at sites of rejection. Am. J. Pathol. 158:1703-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arai, K., Z-X. Liu, T. Lane, and G. Dennert. 2002. IP-10 and Mig facilitate accumulation of T cells in the virus-infected liver. Cell. Immunol. 219:48-56. [DOI] [PubMed] [Google Scholar]
- 3.Balashov, K., J. Rottman, H. Weiner, and A. Hancock. 1999. CCR5+ and CXCR3+ T cells are increased in multiple sclerosis and their ligands MIP-1α and IP-10 are expressed in demyelinating brain lesions. Proc. Natl. Acad. Sci. USA 66:6873-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 5.Brieland, J. K., D. G. Remick, M. L. LeGendre, N. C. Engleberg, and J. C. Fantone. 1998. In vivo regulation of replicative Legionella pneumophila lung infection by endogenous interleukin-12. Infect. Immun. 66:65-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broug-Holub, E., G. B. Toews, J. F. van Iwaarden, R. M. Strieter, S. L. Kunkel, R. Paine, and T. J. Standiford. 1997. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect. Immun. 65:1139-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole, A. M., T. Ganz, A. M. Liese, M. D. Burdick, L. Liu, and R. M. Strieter. 2001. Cutting edge: IFN-inducible ELR- CXC chemokines display defensins-like antimicrobial activity. J. Immunol. 167:623-627. [DOI] [PubMed] [Google Scholar]
- 8.Deng, J. C., K. Tateda, X. Zeng, and T. J. Standiford. 2001. Transient transgenic expression of gamma interferon promotes Legionella pneumophila clearance in immunocompetent hosts. Infect. Immun. 69:6382-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng, J. C., X. Zeng, M. W. Newstead, T. A. Moore, W. C. Tsai, V. J. Thannickal, and T. J. Standiford. 2004. STAT4 is a critical mediator of early innate immune responses against pulmonary Klebsiella infection. J. Immunol. 173:4075-4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dufour, J. H., M. Dziejman, M. T. Liu, J.H. Leung, T. E. Lane, and A. D. Luster. 2002. IFN-γ-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J. Immunol. 168:3195-3204. [DOI] [PubMed] [Google Scholar]
- 11.Farber, J. M. 1997. Mig and IP-10: CXC chemokines that target lymphocytes. J. Leuk. Biol. 61:246-257. [PubMed] [Google Scholar]
- 12.Gaugur, V., F. E. R. Simons, and K. T. Hayglass. 1998. Human IP-10 selectively promotes dominance of polyclonally activated and environmentally antigen-driven IFN-γ over IL-4 responses. FASEB J. 12:705-711. [DOI] [PubMed] [Google Scholar]
- 13.Greenberger, M. J., S. L. Kunkel, R. M. Strieter, N. W. Lukacs, J. Bramson, J. Gauldie, F. L. Graham, M. Hitt, J. M. Danforth, and T. J. Standiford. 1996. IL-12 gene therapy protects mice in lethal Klebsiella pneumonia. J. Immunol. 157:3006-3012. [PubMed] [Google Scholar]
- 14.Harvill, E. T., P. A. Cotter, M. H. Yuk, and J. F. Miller. 1999. Probing the function of Bordetella bronchiseptica adenylate cyclase toxin by manipulating host immunity. Infect. Immun. 67:1493-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hildebrandt, G. C., L. A. Corrion, K. M. Olkiewicz, B. Lu, K. Lowler, U. A. Duffner, B. B. Moore, W. A. Kuziel, C. Liu, K. R. Cooke. 2004. Blockade of CXCR3 receptor:ligand interactions reduces leukocyte recruitment to the lung and the severity of experimental idiopathic pneumonia syndrome. J. Immunol. 173:2050-2059. [DOI] [PubMed] [Google Scholar]
- 16.Johnston, B., C. H. Kim, D. Soler, M. Emoto, and E. C. Butcher. 2003. Differential chemokine responses and homing patterns of murine TCRαβ NKT cell subsets. J. Immunol. 171:2960-2969. [DOI] [PubMed] [Google Scholar]
- 17.Khan, I. A., J. A. MacLean, F. S. Lee, L. Casciotti, E. DeHaan, J. D. Schwartzman, and A. D. Luster. 2000. IP-10 is critical for effector T cell trafficking and host survival in Toxoplasma gondii infection. Immunity 12:483-494. [DOI] [PubMed] [Google Scholar]
- 18.Lande, R. E., T. Giacomini, M. E. Grassi, E. Remoli, M. Iona, I. Mietinnen, I. Julkunen, and E. M. Coccia. 2003. IFN-αβ released by Mycobacterium tuberculosis-infected human dendritic cells induces the expression of CXCL10: selective recruitment of NK and activated T cells. J. Immunol. 170:1174-1182. [DOI] [PubMed] [Google Scholar]
- 19.Lei, D., J. R. Lancaster, M. S. Joshi, S. Nelson, D. Stoltz, G. J. Bagby, G. Odom, J. E. Shellito, and J. K. Kolls. 1997. Activation of alveolar macrophages and lung host defenses using transfer of the interferon-gamma gene. Am. J. Physiol. 272:L852-L859. [DOI] [PubMed] [Google Scholar]
- 20.Lipscomb, M. F., J. M. Onofrio, and E. J. Nash. 1983. A morphological study of the role of phagocytes in the clearance of Staphylococcus aureus from the lung. J. Reticuloend. Soc. 33:429-442. [PubMed] [Google Scholar]
- 21.Loetscher, M. B., P. Gerber, S. A. Loetscher, L. Jones, I. Piali, M. Clark-Lewis, M. Baggiolini, and B. Moser. 1996. Chemokine receptor specific for IP-10 and Mig: structure, function and expression in activated T-lymphocytes. J. Exp. Med. 184:963-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luster, A. D. 1998. Chemokines-chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 338:436-445. [DOI] [PubMed] [Google Scholar]
- 23.Moore, T. A., B. B. Moore, M. W. Newstead, and T. J. Standiford. 2000. γδ-T cells are critical for survival and early proinflammatory cytokine gene expression during murine Klebsiella pneumonia. J. Immunol. 165:2643-2650. [DOI] [PubMed] [Google Scholar]
- 24.Moore, T. A., M. L. Perry, A. G. Getsoian, M. W. Newstead, and T. J. Standiford. 2002. Divergent role of IFN-γ in a murine model of pulmonary versus systemic Klebsiella pneumoniae infection. Infect Immun. 70:6310-6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson, S., C. M. Mason, J. Kolls, and W. R. Summer. 1995. Pathophysiology of pneumonia. Clin. Chest Med. 16:1-12. [PubMed] [Google Scholar]
- 26.Neumann, B., K. Emmanuilidis, M. Stadler, and B. Holzmann. 1998. Distinct functions of interferon-gamma for chemokine expression in models of lung inflammation. Immunology 95:512-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer, K., P. C. R. Emtage, R. M. Streiter, and J. Gauldie. 1999. Transient gene transfer of non-ELR chemokines to rodent lung induces mononuclear cell accumulation and activation. J. Interferon Cytokine Res. 19:1381-1390. [DOI] [PubMed] [Google Scholar]
- 28.Patel, D., J. P. Zachariah, and L. P. Whichard. 2001. CXCR3 and CCR5 ligands in rheumatoid arthritis synovium. Clin. Immunol. 98:39-46. [DOI] [PubMed] [Google Scholar]
- 29.Roilides, E., K. Uhlig, D. Venzon, P. A. Pizzo, and T. J. Walsh. 1993. Enhancement of oxidative response and damage caused by human neutrophils to Aspergillus fumigatus hyphae by granulocyte colony-stimulating factor and gamma interferon. Infect. Immun. 61:1185-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubins, J. B., and C. Pomeroy. 1997. Role of gamma interferon in the pathogenesis of bacteremic pneumococcal pneumonia. Infect. Immun. 65:2975-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skerrett, S. J., and T. R. Martin. 1992. Recombinant murine interferon-gamma reversibly activates rat alveolar macrophages to kill Legionella pneumophila. J. Infect. Dis. 166:1354-1361. [DOI] [PubMed] [Google Scholar]
- 32.Skerrett, S. J., and T. R. Martin. 1994. Intratracheal interferon-gamma augments pulmonary defenses in experimental legionellosis. Am. J. Respir. Crit. Care Med. 149:50-58. [DOI] [PubMed] [Google Scholar]
- 33.Taub, D. D., T. J. Sayers, C. R. D. Carter, and J. R. Ortaldo. 1995. Alpha and beta chemokines induce NK cell migration and enhanced NK cell cytolytic activity via cellular degradation. J. Immunol. 177:1809-1814. [PubMed] [Google Scholar]
- 34.Thomas, M. S., S. L. Kunkel, and N. W. Lukacs. 2002. Differential role of IFN-γ-inducible protein 10kDa in a cockroach antigen-induced model of allergic airway hyperreactivity: systemic versus local effects. J. Immunol. 169:7045-7053. [DOI] [PubMed] [Google Scholar]
- 35.Toews, G. B., G. N. Gross, and A. K. Pierce. 1980. The relationship of inoculum size to lung bacterial clearance and phagocytic cell response in mice. Am. Rev. Respir. Dis. 120:559-566. [DOI] [PubMed] [Google Scholar]
- 36.Tsai, W. C., R. M. Strieter, B. Mehrad, M. W. Newstead, X. Zeng, T. J. Standiford. 2000. CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect. Immun. 68:4289-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valbuena, G., W. Brandford, and D. H. Walker. 2003. Expression analysis of the T-cell targeting chemokines CXCL9 and CXCL 10 in mice and humans with endothelial infections caused by Rickettsiae of the spotted fever group. Am. J. Pathol. 163:1357-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Widney, D. P., Y. Hu, A. K. Foremen-Wykert, K. C. Bui, T. T. Nguyen, B. Lu, G. C. Miller, J. B. Smith. 2005. CXCR3 and its ligands participate in the host response to Bordetella bronchiseptica infection of the mouse respiratory tract but are not required for clearance of bacteria from the lung. Infect. Immun. 73:485-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiley, R. E., K. Palmer, B. U. Gajewska, M. R. Stampfli, D. Alvarez, A. J. Coyle, J. C. Gutierrez-Ramos, and M. Jordana. 2001. Expression of the Th1 chemokine IFN-γ-inducible protein 10 in the airway alters mucosal allergic sensitization in mice. J. Immunol. 166:2750-2759. [DOI] [PubMed] [Google Scholar]
- 40.Zeng, X., T. A. Moore, M. W. Newstead. J. C. Deng, N. W. Lukacs, and T. J. Standiford. 2005. IP-10 mediates selective mononuclear cell accumulation and activation in response to intrapulmonary transgenic expression and during adenovirus-induced pulmonary inflammation. J. Interferon Cytokine Res. 25:103-112. [DOI] [PubMed] [Google Scholar]
- 41.Zhao, D. X.-M., Y. Hu, G. C. Miller, A. D. Luster, R. N. Mitchell, and P. Libby. 2002. Differential expression of the IFN-γ-inducible CXCR3-binding chemokines, IFN-inducible protein 10, monokine induced by IFN, and IFN-inducible T cell α chemoattractant in human cardiac allografts: association with cardiac allograft vasculopathy and acute rejection. J. Immunol. 169:1556-1560. [DOI] [PubMed] [Google Scholar]