Abstract
Pseudomonas aeruginosa can notably cause both acute and chronic infection. While several virulence factors are implicated in the acute phase of infection, advances in understanding bacterial pathogenesis suggest that chronic P. aeruginosa infection is related to biofilm formation. However, the relationship between these two forms of disease is not well understood. Accumulating evidence indicates that, during acute infection, P. aeruginosa enters epithelial cells, a process viewed as either a host-mediated defense response or a pathogenic mechanism to avoid host-mediated killing. We investigated the possibility that epithelial cell entry during early P. aeruginosa-epithelial cell contact favors bacterial survival and is linked to chronic infection. Using electron microscopy and confocal microscopy to analyze primary culture airway epithelial cells infected with P. aeruginosa, we found that epithelial cells developed pod-like clusters of intracellular bacteria with regional variation in protein expression. Extracellular gentamicin added to the medium after acute infection led to the persistence of intracellular P. aeruginosa for at least 3 days. Importantly, compared to bacterial culture under planktonic conditions, the intracellular bacteria were insensitive to growth inhibition or killing by antibiotics that were capable of intraepithelial cell penetration. These findings suggest that P. aeruginosa can use airway epithelial cells as a sanctuary for persistence and develop a reversible antibiotic resistance phenotype characteristic of biofilm physiology that can contribute to development of chronic infection.
Pseudomonas aeruginosa is remarkable in that it can cause both very acute and very chronic infections (34). Progress in understanding the pathogenesis of acute P. aeruginosa infections has implicated virulence factors including exotoxin A and type III secreted exotoxins (33, 39, 44). Understanding the pathogenesis of the chronic infections caused by P. aeruginosa is also progressing. Current concepts propose that biofilm formation is a key factor in chronic Pseudomonas airway infection in cystic fibrosis and bronchiectasis and chronic urinary tract and device-related infections (17, 29, 32, 40). However, much remains to be learned about how acute infections progress to a chronic phase.
Recent work suggests that many bacteria that are usually considered to have a primary pathogenic effect while extracellular also have the capacity to invade and perhaps reside in host cells during the early phase of infection. In the bladder, Escherichia coli has been found to persist within epithelia as “pods” in a mouse model of urinary tract infection (2). In this model, bacteria within the pods assumed a biofilm structure to resist host killing and to function as an intracellular “factory” for subsequent bacterial efflux into the bladder lumen, contributing to persistent infection. In the lung, discovery of intraepithelial Haemophilus influenzae in biopsy samples of airway epithelial cells from individuals with chronic obstructive lung disease harboring persistent antibiotic-resistant organisms implicates intracellular bacteria as an additional reservoir for infection (3, 26). Experimental studies using cell lines and animal models of acute lung infection showed that P. aeruginosa can move intracellularly soon after infection (16, 24, 31, 38, 47). Most recently, in vitro studies using airway epithelial cell lines demonstrated that these bacteria are capable of intraepithelial survival for up to 24 h without cytotoxicity (10). The observation that traditional extracellular organisms can be found intracellularly and the finding that E. coli within bladder cells forms biofilms raise the intriguing possibility that the intracellular bacteria may also play a role in persistent P. aeruginosa infections.
Here, we examined the events occurring after initial epithelial cell entry. Our data reveal that internalized bacteria have biofilm-like characteristics. Namely, following acute exposure, P. aeruginosa formed as clusters within airway cells and these epithelia tolerated the internalized bacteria for long periods. Importantly, the intracellular bacteria also developed a reversible antibiotic resistance phenotype. The findings indicate that epithelial entry of P. aeruginosa can favor persistence and may contribute to difficulties in the treatment of airway infection.
MATERIALS AND METHODS
Bacteria and culture.
P. aeruginosa strains used were PAO1 and PAO1-GFP (green fluorescent protein) (11) (both resistant to chloramphenicol, 50 μg/ml; kindly provided by M. Parsek, University of Iowa), PAK (kindly provided by S. Lory, Harvard University), and a mucoid clinical strain (clinical strain 1 [CS1]) isolated from an individual with cystic fibrosis by the Laboratory of Clinical Microbiology at Barnes-Jewish Hospital (St. Louis, MO). Strains were stored as a stock at −80°C. For each set of experiments, bacteria were streaked onto a Luria-Bertani (LB) agar medium plate and cultured for 18 to 22 h at 37°C. An individual colony was cultured in LB medium and then amplified in a larger volume to prepare aerated, log-phase bacteria by rotary shaking at 37°C until 1 × 109 to 2 × 109 CFU/ml was achieved as determined by spectrophotometry (optical density at 600 nm = 0.6). CFU of bacteria were quantified by plating serial dilutions on LB agar medium.
MTEC.
Primary culture mouse tracheal epithelial cells (MTEC) were established on membranes using air-liquid interface conditions as described previously (46). Cells were harvested from tracheas by pronase digestion, and epithelial cells were selected by differential adherence. To initiate cultures, 8 × 104 cells/cm2 were seeded on supported polyester semipermeable (0.4-μm pore) membranes (0.33 cm2; Transwell; Costar-Corning, Corning, NY) coated with type I rat tail collagen (Becton Dickinson) in 0.02 M acetic acid. Cells were grown in “basic medium” composed of Dulbecco modified Eagle medium-Ham's F-12 with 30 mM HEPES, 4 mM l-glutamine, 3.5 mM NaHCO3, amphotericin, and penicillin-streptomycin, supplemented with 10 μg/ml insulin, 10 μg/ml transferrin, 0.1 μg/ml cholera toxin, 25 ng/ml epithelial growth factor (Becton Dickinson, Bedford, MA), 30 μg/ml bovine pituitary extract, 0.01 mM retinoic acid, and 5% fetal bovine serum. Medium was maintained in upper and lower chambers until the transmembrane resistance was >1,000 Ω · cm2 corresponding to tight junction formation and sufficient to prevent medium from leaking into the apical compartment (46). Then the air-liquid interface condition was established for induction of differentiation by aspirating apical chamber medium and changing the medium in the lower compartment to serum-free basic medium supplemented with 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml epidermal growth factor, 30 μg/ml bovine pituitary extract, 1 mg/ml bovine serum albumin, and 0.01 μM retinoic acid. Cells used in studies were cultured in air-liquid interface conditions for at least 7 days, at which time ciliated and secretory cells were present.
Inoculation of MTEC with P. aeruginosa.
One day prior to bacterial inoculation, the medium of MTEC was replaced with infection medium composed of serum-free basic medium without antibiotics. Cholera toxin was removed from the medium to allow normal ceramide trafficking in P. aeruginosa-infected cells (19). Bacteria were resuspended in the infection medium as 106 to 107 CFU/150 μl that was applied to the apical compartment of an 0.33-cm2 Transwell membrane insert.
EM.
Cells on membranes were prepared for scanning electron microscopy (EM) as previously described (25). Briefly, samples were fixed with 2.5% glutaraldehyde, stained with 1.25% osmium tetroxide, sputter coated with gold, and then visualized on a Hitachi S-450 microscope (Tokyo, Japan).
Immunohistochemistry and confocal microscopy.
Cells on supported membranes were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.4, for 10 min at 25°C and immunostained as described previously (46). Rabbit anti-P. aeruginosa flagellin (FlgA; kindly provided by A. Prince, Columbia University) and mouse anti-P. aeruginosa outer coat protein F (OprFMA4; kindly provided by R. E. W. Hancock, University of British Columbia) were detected by fluorescent dye-labeled secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). Filamentous actin was detected using rhodamine-labeled phalloidin (Molecular Probes, Eugene, OR) incubated with fixed samples for 30 min at 25°C. Membranes containing cells were mounted on glass slides using medium (Vectashield; Vector Laboratories, Burlingame, CA). Microscopy was performed using a Zeiss laser scanning confocal system with LSM-510 software (Zeiss, Thornwood, NY). Images were composed using Photoshop and Illustrator software (Adobe Systems, San Jose, CA).
Recovery of intracellular bacteria.
The gentamicin survival assay was used to detect intracellular bacteria based on the inability of this agent to penetrate epithelial cells to achieve sufficient concentrations to kill intracellular bacteria (13, 24, 31). Following incubation with bacteria, MTEC on supported membranes were washed twice for 5 min with sterile PBS, incubated with 50 μg/ml gentamicin for 30 min, and washed twice again. The membranes were then cut out of the plastic support and transferred to a 1.5-ml plastic tube. There the epithelial cells were lysed by incubation with lysis buffer (0.25% Triton X-100 in PBS) for 3 min and then diluted and plated onto antibiotic-free agar medium and incubated (18 h, 37°C) for subsequent determination of intracellular CFU. No bacteria were recovered from apical surface washes of epithelia following treatment with gentamicin for 30 min and up to 4 h. Thus, to minimize time for possible intracellular proliferation, gentamicin treatment for 30 min was used for studies.
Bacterial killing assays.
The antibiotics ceftazidime (Eli Lilly, Indianapolis, IN) and ciprofloxacin (Bayer Corp., Pittsburgh, PA), which are known to accumulate intracellularly and are employed for the treatment of clinical P. aeruginosa infections, were used for in vitro bacterial killing assays (4, 9). The MICs of these antibiotics were determined using Etest assays (AB Biodisk, Solna, Sweden). Planktonic-phase bacterial killing was assayed following rotary shaking of bacteria at 37°C in the presence of antibiotics by quantification of CFU on agar medium. Intracellular bacterial killing assays were performed in MTEC incubated with P. aeruginosa, treated with 50 μg/ml gentamicin for 30 min to kill extracellular bacteria, washed, and incubated with ceftazidime or ciprofloxacin in the upper and lower chambers of the membrane support for the indicated time. Cells on the membranes were then lysed, and CFU of surviving intracellular bacteria were determined as described above.
Intracellular fluoroquinolone assay.
Accumulation of intracellular ciprofloxacin in MTEC was measured based on the methods of Mortimer and Piddock (27). Ciprofloxacin fluorescence was measured over a range of concentrations using excitation at 279 nm and emission at 447 nm detected by fluorometric spectrophotometry (Spectra Max Gemini; Molecular Devices, Sunnyvale, CA). Cell volume per insert was determined based on cell number, height, and radius obtained from scanning and transmission EM and an assumed 50% intracellular water-soluble compartment in each cell (V = πr2h · 0.50 = 0.82 μl/insert), a method used by others (8). Ciprofloxacin in serum free-medium was applied to the apical surface of MTEC, and they were incubated at 37°C for 4 h. Cell-associated fluoroquinolone fluorescence was measured in triplicate samples after MTEC were washed four times and collected in 100 ml of lysis buffer. The concentration of ciprofloxacin was calculated at 4 h for MTEC samples compared to the standards (also diluted in lysis buffer).
Statistical analysis.
CFU of bacteria were analyzed using a one-way analysis of variance (ANOVA). If significance was achieved by one-way analysis, post-ANOVA comparison of means was performed using Scheffe's F test. Data from samples evaluated by a single measure of transepithelial cell resistance were analyzed using the Kruskal-Wallis test and subjected to the Wilcoxon rank sum test. Studies using repeat measure of transepithelial cell resistance were analyzed using the Friedman two-way ANOVA. The level of significance for all analyses was <0.05.
RESULTS
The effect of P. aeruginosa on integrity of primary cultured mouse tracheal epithelial cell layers.
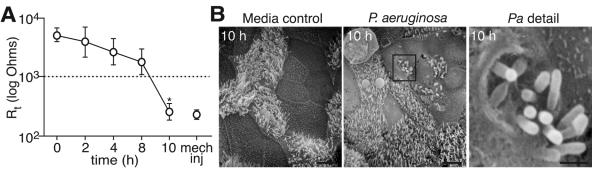
To determine responses of differentiated airway epithelial cells to P. aeruginosa, mouse tracheal epithelial cells (2 × 105/well) cultured using air-liquid interface conditions were exposed to 106 to 107 CFU of strain PAO1 for 0 to 10 h, similar to conditions used for prior studies of P. aeruginosa pathogenesis in other cell types (7, 24, 31). As a measure of cell viability, we monitored the transepithelial cell resistance during infection. Although this level of transmembrane resistance is higher than in vivo (20), the maintenance of high resistance observed during the initial 8 h of infection indicated intactness of the cell layer (Fig. 1A). After 10 h, some cells lifted off the membrane as described previously (24, 31), and a fall in transmembrane resistance was observed. When imaged by scanning EM at 10 h, bacteria were observed that appeared to be associated with a defect in the apical membrane of epithelial cells (Fig. 1B, detail in right panel). These cells containing bacteria were part of the intact layer of epithelia rather than sloughed cells. These findings suggested that bacteria may enter, persist within, and/or exit the epithelial cell during acute infection, and therefore we designed a series of studies to investigate this pathway.
FIG. 1.
Effect of P. aeruginosa on integrity of primary cultured mouse tracheal epithelial cell layers. A. Transepithelial resistance (Rt) of MTEC incubated with P. aeruginosa. PAO1 (106 to 107 CFU) was applied to the apical surface of MTEC (2 × 105 cells) for up to 10 h. Values are the means ± standard deviations (n = 3 to 9 samples/time). The dashed line represents the lower limit of Rt corresponding to maintenance of tight junctions (46). Mechanical injury (mech inj) induced by scraping an uninfected cell layer is shown as a reference. A significant difference at 10 h (P < 0.05) compared to other times is indicated (*). B. Scanning EM of cells obtained 10 h after inoculation with control medium or P. aeruginosa (Pa) as in panel A. The boxed region (center panel) showing bacteria either entering or emerging from the apical membrane of cells is enlarged in the right panel. Bars = 5 μm (left and center panels); 1 μm (right panel).
Time-dependent changes in intracellular abundance of P. aeruginosa.
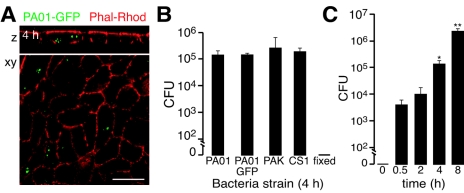
Most studies of P. aeruginosa internalization have been performed in cell lines, so as an initial characterization of the MTEC model we used GFP-labeled bacteria detected by laser scanning confocal microscopy. Intracellular PAO1 labeled with GFP (PAO1-GFP) was incubated with MTEC which were washed and then treated with gentamicin to kill extracellular bacteria. Confocal micrographs showed that the GFP signal occurred as clusters within cells (Fig. 2A). To further characterize intracellular survival of P. aeruginosa, we used the gentamicin survival assay to kill only extracellular bacteria and recovered bacteria after epithelial cell lysis (13, 24, 31). Intracellular P. aeruginosa abundance, evaluated after incubation with bacteria for 4 h followed by gentamicin treatment, was observed to occur at similar amounts for PAO1, PAO1-GFP, PAK (another laboratory strain), and a mucoid clinical isolate called CS1 (from Barnes-Jewish Hospital, St. Louis, MO), indicating that the phenomenon was not uniquely strain specific (Fig. 2B). We next sought to characterize the temporal nature of internalization of bacteria during the acute incubation. The abundance of intracellular bacteria recovered after 30-min to 8-h periods of incubation with P. aeruginosa increased over time (Fig. 2C). We could not accurately measure intracellular bacteria at 10 h because some epithelial cells lifted off the membrane at this time due to a cytotoxic effect of intra- and/or extracellular bacteria.
FIG. 2.
Time-dependent changes in intracellular abundance of P. aeruginosa. A. Representative laser scanning confocal photomicrograph (xy) and z-axis reconstruction of MTEC stained with rhodamine-labeled phalloidin (red) to identify filamentous actin, obtained 4 h after incubation with PAO1-GFP and then gentamicin to kill extracellular bacteria. Bar = 10 μm. B. Gentamicin survival assay of MTEC incubated for 4 h with indicated strains of P. aeruginosa (CS1, clinical strain). PAO1-GFP treated with paraformaldehyde prior to assay is indicated (“fixed”). MTEC were incubated with bacteria and then treated with gentamicin, lysed, and cultured on solid medium to determine surviving intracellular bacteria (CFU/well). Shown is the mean ± standard deviation of triplicate samples from a representative experiment. C. Gentamicin survival assay of P. aeruginosa PAO1-GFP strain following 0.5 to 8 h of incubation with MTEC performed as for panel B. Shown are means ± standard deviations of replicate samples from at least three experiments. A significant difference (P < 0.05) from 0.5 h and 2 h is at 4 h (*) and from 0.5 and 4 h is at 8 h (**).
P. aeruginosa forms intracellular pods.
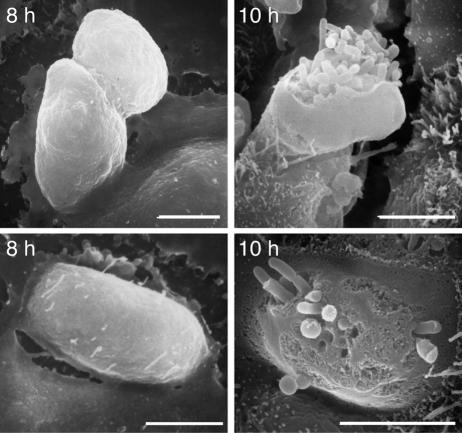
To begin to assess bacterium-epithelial cell interactions during loss of cell layer integrity, we used EM to compare differences in cell morphology between 8 and 10 h. Scanning electron photomicrographs obtained following incubation of MTEC with PAO1 for 8 h revealed multiple pod-like cells and few extracellular bacteria. However, after 10 h, open epithelial cells filled with bacteria were present (Fig. 3). This pod-like appearance was similar to that first described in airway epithelial cells following ex vivo infection of monkey trachea and in vivo infection of mouse lung (38). It was also reminiscent of the pods of E. coli that formed biofilms within the large epithelial cells lining the bladder (2), leading us to consider that the intracellular P. aeruginosa may similarly have biofilm-like characteristics.
FIG. 3.
P. aeruginosa forms intracellular pods. Scanning electron photomicrographs of MTEC obtained 8 and 10 h after incubation with strain PAO1. Cells were incubated as in Fig. 1. Images are from two independent preparations (top and bottom). Bars = 5 μm.
Persistence of intracellular P. aeruginosa.
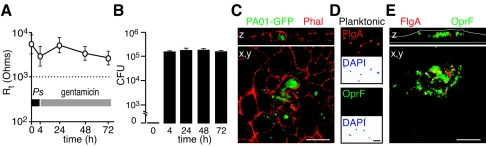
An important characteristic of bacteria that assume biofilm physiology is the ability to survive in a confined location (29). To determine if P. aeruginosa could persist within epithelial cells in an intact epithelial layer (for more than 8 h), we incubated MTEC with PAO1-GFP for 4 h and then added fresh gentamicin-containing medium daily (in upper and lower chambers) for up to 72 h. This treatment achieved killing of extracellular bacteria as demonstrated by a failure to recover bacteria from the apical or basolateral medium over the 72-h period. Assessment by light microscopy and scanning EM showed that cell layers remained intact during constant gentamicin treatment. To confirm the microscopic inspection, we again monitored cell layer integrity by measurement of transepithelial resistance. The maintenance of high resistance over the 72-h period also demonstrated the viability of the epithelial cell layer (Fig. 4A). Evaluation by confocal microscopy showed that, after 24-h treatment with gentamicin, dense intracellular clusters of PAO1-GFP could be found in complexes of epithelia and were encased by septa of remodeled actin (Fig. 4B). The abundance of recovered intracellular bacteria was similar when gentamicin incubation was extended from 24 to 72 h (Fig. 4C). The prolonged persistence of intracellular P. aeruginosa suggested that bacteria could be organizing into communities that favored intracellular survival and are tolerated by an intact epithelium.
FIG. 4.
Persistence and heterogeneity of gene expression of intracellular P. aeruginosa. A. Transepithelial cell resistance (Rt) of MTEC incubated with PAO1-GFP for 4 h, treated with fresh medium containing gentamicin daily for up to 72 h. The dashed line represents the level of Rt associated with maintenance of epithelial cell tight junctions (46). Values are the means ± standard deviations of replicate samples from three independent preparations. B. Gentamicin survival assay of recovered intracellular bacteria (CFU/well) from MTEC incubated with PAO1-GFP as in panel A. Values are the means ± standard deviations of triplicate samples from three independent experiments. C. Laser scanning confocal photomicrograph (x, y) and z-axis reconstruction of MTEC stained with rhodamine-labeled phalloidin (red), obtained after incubation with PAO1-GFP for 4 h followed by gentamicin for 24 h. Bar = 10 μm. D. Immunofluorescence photomicrograph of P. aeruginosa strain CS1 cultured using planktonic conditions, applied to a glass slide, and then immunostained for FlgA (red) and/or OprF (green). Accompanying DAPI (4′,6′-diamidino-2-phenylindole)-stained images are below (background was inverted to white for contrast). Bar = 1 μm. E. Confocal photomicrograph (x, y) and z-axis reconstruction of MTEC obtained after incubation with CS1 immunostained for FlgA (red) and OprF (green). White lines indicate Transwell membrane and apical cell border. Bar = 10 μm.
Heterogeneity of P. aeruginosa gene expression in intracellular bacteria within pods.
Biofilms generated using in vitro systems display regional variation in gene expression within the bacterial communities (35). Heterogeneous bacterial gene expression was also noted in bladder epithelial cells of mice infected with E. coli (2). To test for this possibility in our model, the mucoid clinical strain CS1 was incubated with MTEC for 4 h and then maintained for 24 h in gentamicin prior to assay for expression of FlgA, a protein that is decreased in biofilm communities under some conditions (43), or outer membrane protein OprF, reported to be upregulated in biofilms (45). As a control, gene expression was compared to CS1 cultured using planktonic conditions. Under planktonic conditions bacilli expressed FlgA but not OprF (Fig. 4D). However, when intracellular, there was heterogeneity of FlgA and OprF expression within the bacterial communities of the pods (Fig. 4E). Thus, these intracellular bacterial pods had acquired a shift in gene expression as seen in the E. coli-infected bladder cells and shared some features of P. aeruginosa biofilms generated in vitro.
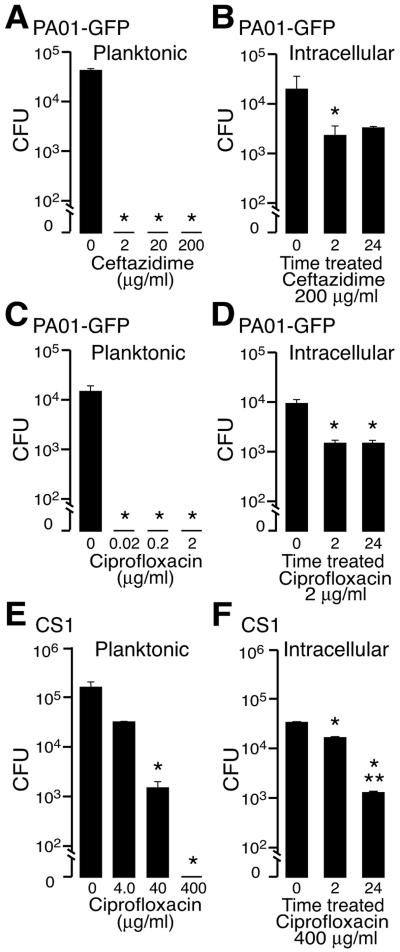
Intracellular bacterial clusters exhibit impaired antibiotic killing characteristic of biofilms.
Biofilm bacteria notably have antibiotic resistance that reverses when the biofilms are disrupted and the cells are cultured in planktonic conditions (1, 42). To determine if intraepithelial bacterial clusters exhibited this biofilm characteristic, we treated epithelial layers infected with PAO1-GFP or CS1 with antibiotics that are capable of intracellular penetration. Ceftazidime and ciprofloxacin were assayed for killing because these antibiotics are used to treat clinical Pseudomonas infections and achieve high levels in tissue and ciprofloxacin in particular can be concentrated in airway cells above delivered concentrations within 1 h (4, 8, 9). In the planktonic state, 104 CFU of PAO1-GFP (the approximate number of bacteria found inside cells at 4 h) was completely killed by 0.02 μg/ml of ceftazidime and 0.2 μg/ml of ciprofloxacin after a 2-h treatment. However, these antibiotic concentrations were much less effective against epithelial layers that had been infected with PAO1-GFP for 4 h (to allow intracellular entry). Even a 100-fold concentration above that required for complete killing of bacteria cultured planktonically did not eradicate the intracellular bacteria (Fig. 5B). Similar studies performed using the clinical isolate CS1 also revealed incomplete antibiotic killing of intracellular bacteria compared to planktonic bacteria (Fig. 5E and F). Ciprofloxacin measured by fluorescent spectroscopy in MTEC after 4 h of incubation showed that 100 mg/ml and 20 mg/ml achieved intracellular concentrations of 87.1 ± 1.78 (standard deviation) mg/ml and 17.3 ± 0.62 (standard deviation) mg/ml, respectively, consistent with the rapid uptake and intracellular concentration of this drug previously described (8). However, even after incubation of infected MTEC with antibiotics for 24 h, intracellular killing of either strain was not enhanced (Fig. 5B, D, and F).
FIG. 5.
Efficacy of intracellular P. aeruginosa killing by antibiotics. A. Survival of P. aeruginosa PAO1-GFP (104 CFU) cultured using planktonic conditions in the presence of control medium or ceftazidime for an additional 2 h. B. Recovery of intracellular PAO1-GFP from MTEC, 2 and 24 h after incubation with ceftazidime. Intracellular bacteria were established by incubation of MTEC with P. aeruginosa for 4 h, washed, treated with gentamicin for 30 min as in Fig. 2, and then incubated with control medium or ceftazidime for 2 h or 24 h, followed by cell lysis and culture on solid medium. C. Survival of planktonic PAO1-GFP treated with ciprofloxacin as in panel A. D. Recovery of intracellular PAO1-GFP from MTEC infected as in panel B, after incubation with ciprofloxacin. E. Survival of strain CS1 cultured planktonically and treated with ciprofloxacin as in panel C. F. Recovery of intracellular CS1 from MTEC infected as in panel C and treated with ciprofloxacin. Values represent the means ± standard deviations of three to six samples from a representative experiment of at least three different preparations. A significant difference (P < 0.05) from untreated bacteria is indicated (*), as is a significant difference at 2 h compared to 24 h (**).
Reversibility of the intracellular bacterial antibiotic resistance phenotype.
To determine if internalized bacteria had acquired antibiotic insensitivity due to mutation or due to their mode of growth inside epithelia, the intracellular bacterial structures were disrupted in lysis buffer and the bacteria were grown planktonically. Planktonic growth restored antibiotic sensitivity to both PAO1-GFP and CS1 strains. The antibiotic sensitivity of the recovered bacteria was identical to that of naïve organisms (bacteria never internalized), demonstrating 100% killing. These data suggest that genetic mutations were not responsible for changes in antibiotic sensitivity and are consistent with the biofilm-like morphology of the intraepithelial cell clusters.
Fate of bacteria in epithelial cells.
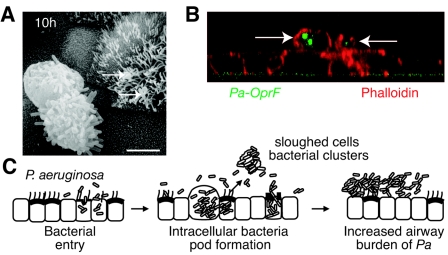
Individuals with chronic airway diseases have P. aeruginosa biofilms within a complex of sloughed cells and mucus (32, 34). To follow the fate of bacteria in the in vitro model, we examined cells and debris that were retained on the apical (luminal) side of the cell culture preparation when not treated with antibiotics or after antibiotics were withdrawn following prolonged exposure to gentamicin. As noted, P. aeruginosa exposure caused cell death at 10 h and resulted in the ejection of bacterial clusters and epithelial cell pods of intracellular bacteria (with OprF expression) onto the cell culture layer (Fig. 6A and B). These observations are consistent with several potential bacterial fates including clearance (in vivo), intraluminal infection, dissemination, or contribution to intraluminal biofilm communities within the airways.
FIG. 6.
Fate of intraepithelial P. aeruginosa and model. A. Scanning electron photomicrograph of sloughed pod of airway epithelial cell containing bacteria on the MTEC layer obtained 10 h after incubation with PAO1. Arrows indicate other bacteria adherent to cilia. Bar = 5 μm. B. Confocal scanning photomicrograph (z-axis reconstruction) of MTEC incubated with P. aeruginosa clinical sample (CS1) for 4 h and then with gentamicin for 24 h, demonstrating sloughed cells filled with bacteria (arrows) on top of the MTEC layer. Cells were immunostained with antibody to OprF detected with fluorescein isothiocyanate (green) to identify P. aeruginosa (Pa) and rhodamine-phalloidin (red). C. Model of bacterial internalization and release into the airway lumen. Following entry into airway epithelial cells, P. aeruginosa bacteria proliferate to form pods. Bacteria within cells form biofilm-like communities. Subsequent cell death results in ejection of epithelia filled with bacteria into the airway, where bacteria may contribute to airway biofilm formation and persistent P. aeruginosa infection in the lung.
DISCUSSION
Biofilms have been implicated in several types of chronic P. aeruginosa infections occurring in individuals with cystic fibrosis and other forms of bronchiectasis (22, 29, 32). This is consistent with the established view that P. aeruginosa is primarily an extracellular pathogen. However, several recent studies performed in various epithelial cell and animal models have demonstrated that P. aeruginosa can also enter host cells early in the bacterium-epithelial cell interaction and in vivo appear as intracellular clusters (16, 31, 38, 47). A relationship between intracellular P. aeruginosa and development of biofilm has not been reported, but the recent description of the biofilm properties of intracellular E. coli raises the possibility that other organisms use a similar pathogenic mechanism. Our studies showed that P. aeruginosa also acquires biofilm-like features after entering epithelial cells. The bacteria formed dense, pod-like aggregates within epithelia similar to those seen in mouse P. aeruginosa lung and E. coli bladder infection models (2, 38). Importantly, we found that the in vitro intracellular bacteria demonstrated marked resistance to antibiotic killing that was reversed when bacteria were cultured planktonically. Furthermore, P. aeruginosa survival was tolerated within intraepithelial structures for relatively prolonged periods. The findings led us to conclude that epithelial entry of P. aeruginosa may contribute to difficulties in bacterial eradication associated with acute or chronic airway infection.
Despite the increasing implication of biofilms as a cause of human infections, there are not firm criteria to define biofilm growth in vivo. Biofilms are a growth mode, rather than a fixed property of bacteria, and, as a complex community, are highly heterogeneous in behavior (6, 35, 36, 41). Identifying biofilm growth is complicated by the fact that, in many human infections, biofilm and planktonic bacteria may coexist and, when organisms isolated from in vivo sites are cultured ex vivo, the biofilm phenotype rapidly reverts. In view of these challenges, we have applied a proposed set of criteria to define biofilm-like properties in our model of intracellular P. aeruginosa (17, 29, 41). These include, first, finding infecting bacteria in cell clusters (as we observed) or microcolonies associated with a surface. Second, biofilms are normally confined to a particular location and involve less host injury than the acute infections caused by free-living bacteria, consistent with the survival of epithelial cells in the presence of extracellular gentamicin that we demonstrated. Third, biofilms show marked antibiotic resistance in vivo despite susceptibility to killing in the planktonic state. Finally, bacterial biofilms are typically encased in an extracellular polymeric matrix. The intraepithelial P. aeruginosa clusters described in our study manifested most of these characteristics including a biofilm morphology, reversible antibiotic resistance, and moderated host injury. But we did not see definite evidence for an extracellular polymeric matrix surrounding the clusters, in part due to the inability of transmission EM to identify clearly identifiable clusters of intracellular bacteria. Reasons for this may include the relative infrequency of the large intracellular formations, the ease with which cells filled with bacteria (which may be undergoing apoptosis) wash off the membrane during rigorous EM sample preparation, or a change in bacterial size or shape making intracellular bacteria difficult to identify (5). However, we were able to identify many of the other features of biofilms in the intracellular P. aeruginosa.
Specific changes in P. aeruginosa gene expression that could define intracellular biofilms are also unknown, since most studies of biofilms are performed using in vitro reactors under a variety of conditions. We cannot exclude the possibility that the changes in intracellular gene expression that we observed were induced by epithelial cell factors rather than a biofilm environment per se. Related to this issue is the identification of specific bacterial genes necessary for creation of intracellular biofilms. It is likely that bacterial functions required for biofilm formation vary in different environmental conditions. For example, mutations in the P. aeruginosa quorum-sensing system have pronounced effects on biofilm development in some conditions but minimal effects in others (21, 37). Testing genetically deficient bacteria for intracellular biofilm formation is also difficult in the MTEC system since many strains deficient in candidate “biofilm genes” also have defects in adherence, motility, and/or epithelial cell invasion (15, 18, 28).
Prior studies of intracellular P. aeruginosa have focused on bacterial host factors that are required for binding and entry rather than the fate of the intracellular bacteria. Those findings indicate a requirement for the bacterial ligand flagellin (14, 15) and putative roles for airway epithelial cell molecules asialo-GM1, CFTR, and CD95 as receptors for adherence and entry (12, 19, 30). Epithelial cytoskeletal rearrangement and ceramide activation of sphingolipid-rich raft proteins further facilitate P. aeruginosa entry (19, 23, 47). While these epithelial cell factors may be initially required for development of intracellular biofilms, the expression of genes (epithelial and bacterial) that favor host cell survival may also be important.
After entry, one paradigm of P. aeruginosa pathogenesis is that programmed cell death rapidly commences, leading to ejection of the epithelial cell filled with bacteria into the lumen followed by prompt removal by professional phagocytes (7, 19, 30). It is likely that, in our model, this mode of cell death was operative but without the subsequent immune cell phagocytosis in the MTEC system. While epithelial cell internalization may favor clearance by phagocytosis in mice with normal host defense, individuals with genetic or acquired deficiencies in airway clearance may retain intracellular pods filled with bacteria and increase the lung burden of bacteria, ultimately favoring biofilm formation.
The role of intracellular bacteria in the human airway is not well defined either in healthy hosts or in those with impaired clearance. To date, intracellular P. aeruginosa has not been identified in clinical samples. Only recently has H. influenzae, another respiratory pathogen typified as extracellular, been localized within airway epithelial cells (3, 26). It is possible that intracellular P. aeruginosa is particularly difficult to find in human samples for several reasons. First, if epithelial cells containing bacteria are rapidly ejected from the epithelium and phagocytosed, then fewer intracellular bacteria will be found. Second, diagnostic or other histopathologic studies of infection are typically performed only in individuals with chronic, rather than acute, infection, possibility resulting in evaluation of populations of bacteria with behaviors different (i.e., noninvasive) from those in the acute infection (36, 37, 43). Animal model data suggest that internalization of bacteria occurs rapidly following acute infection (38, 47), and to our knowledge, clinical samples from patients acutely infected with P. aeruginosa have not been systematically examined for intracellular bacteria.
In summary, our data suggest a model (Fig. 6C) for early pathogenesis of P. aeruginosa whereby epithelial cells provide a sanctuary for bacterial proliferation into biofilm-like communities. These intracellular bacteria may contribute to the common clinical finding of impaired antibiotic killing. Persistence of these intracellular bacteria and sloughing of epithelial cells containing these bacteria may contribute to a larger burden of luminal biofilm, making bacterial eradication by antibiotics difficult and favoring bacterial survival in the host airway.
Acknowledgments
We thank B. Iglewski, S. Lory, M. Parsek, A. Prince, and R. Hancock for reagents; M. Veith for assistance with electron microscopy; R. Sasich for intracellular fluoroquinolone assays; and M. Walter, C. Cannon, J. Palermo, and S. Hultgren for helpful discussions.
This work was supported by awards from the National Institutes of Health (S.L.B.) and the American Lung Association (S.L.B.).
Editor: J. T. Barbieri
REFERENCES
- 1.Aaron, S. D., W. Ferris, K. Ramotar, K. Vandemheen, F. Chan, and R. Saginur. 2002. Single and combination antibiotic susceptibilities of planktonic, adherent, and biofilm-grown Pseudomonas aeruginosa isolates cultured from sputa of adults with cystic fibrosis. J. Clin. Microbiol. 40:4172-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. [DOI] [PubMed] [Google Scholar]
- 3.Bandi, V., M. A. Apicella, E. Mason, T. F. Murphy, A. Siddiqi, R. L. Atmar, and S. B. Greenberg. 2001. Nontypeable Haemophilus influenzae in the lower respiratory tract of patients with chronic bronchitis. Am. J. Respir. Crit. Care Med. 164:2114-2119. [DOI] [PubMed] [Google Scholar]
- 4.Birmingham, M. C., R. Guarino, A. Heller, J. H. Wilton, A. Shah, L. Hejmanowski, D. E. Nix, and J. J. Schentag. 1999. Ciprofloxacin concentrations in lung tissue following a single 400 mg intravenous dose. J. Antimicrob. Chemother. 43(Suppl. A):43-48. [DOI] [PubMed] [Google Scholar]
- 5.Boles, B. R., M. Thoendel, and P. K. Singh. 2004. Self-generated diversity produces “insurance effects” in biofilm communities. Proc. Natl. Acad. Sci. USA 101:16630-16635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borriello, G., E. Werner, F. Roe, A. M. Kim, G. D. Ehrlich, and P. S. Stewart. 2004. Oxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilms. Antimicrob. Agents Chemother. 48:2659-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannon, C. L., M. P. Kowalski, K. S. Stopak, and G. B. Pier. 2003. Pseudomonas aeruginosa-induced apoptosis is defective in respiratory epithelial cells expressing mutant cystic fibrosis transmembrane conductance regulator. Am. J. Respir. Cell Mol. Biol. 29:188-197. [DOI] [PubMed] [Google Scholar]
- 8.Cavet, M. E., M. West, and N. L. Simmons. 1997. Transepithelial transport of the fluoroquinolone ciprofloxacin by human airway epithelial Calu-3 cells. Antimicrob. Agents Chemother. 41:2693-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cazzola, M., M. Gabriella Matera, M. Polverino, G. Santangelo, I. De Franchis, and F. Rossi. 1995. Pulmonary penetration of ceftazidime. J. Chemother. 7:50-54. [DOI] [PubMed] [Google Scholar]
- 10.Darling, K. E., A. Dewar, and T. J. Evans. 2004. Role of the cystic fibrosis transmembrane conductance regulator in internalization of Pseudomonas aeruginosa by polarized respiratory epithelial cells. Cell Microbiol. 6:521-533. [DOI] [PubMed] [Google Scholar]
- 11.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 12.de Bentzmann, S., P. Roger, F. Dupuit, O. Bajolet-Laudinat, C. Fuchey, M. C. Plotkowski, and E. Puchelle. 1996. Asialo-GM1 is a receptor for Pseudomonas aeruginosa adherence to regenerating respiratory epithelial cells. Infect. Immun. 64:1582-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elsinghorst, E. A. 1994. Measurement of invasion by gentamicin resistance. Methods Enzymol. 236:405-420. [DOI] [PubMed] [Google Scholar]
- 14.Feldman, M., R. Bryan, S. Rajan, L. Scheffler, S. Brunnert, H. Tang, and A. Prince. 1998. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect. Immun. 66:43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleiszig, S. M., S. K. Arora, R. Van, and R. Ramphal. 2001. FlhA, a component of the flagellum assembly apparatus of Pseudomonas aeruginosa, plays a role in internalization by corneal epithelial cells. Infect. Immun. 69:4931-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleiszig, S. M., T. S. Zaidi, M. J. Preston, M. Grout, D. J. Evans, and G. B. Pier. 1996. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect. Immun. 64:2288-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fux, C. A., J. W. Costerton, P. S. Stewart, and P. Stoodley. 2005. Survival strategies of infectious biofilms. Trends Microbiol. 13:34-40. [DOI] [PubMed] [Google Scholar]
- 18.Glessner, A., R. S. Smith, B. H. Iglewski, and J. B. Robinson. 1999. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of twitching motility. J. Bacteriol. 181:1623-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grassme, H., V. Jendrossek, A. Riehle, G. von Kurthy, J. Berger, H. Schwarz, M. Weller, R. Kolesnick, and E. Gulbins. 2003. Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat. Med. 9:322-330. [DOI] [PubMed] [Google Scholar]
- 20.Grubb, B. R., A. M. Paradiso, and R. C. Boucher. 1994. Anomalies in ion transport in CF mouse tracheal epithelium. Am. J. Physiol. 267:C293-C300. [DOI] [PubMed] [Google Scholar]
- 21.Heydorn, A., B. Ersboll, J. Kato, M. Hentzer, M. R. Parsek, T. Tolker-Nielsen, M. Givskov, and S. Molin. 2002. Statistical analysis of Pseudomonas aeruginosa biofilm development: impact of mutations in genes involved in twitching motility, cell-to-cell signaling, and stationary-phase sigma factor expression. Appl. Environ. Microbiol. 68:2008-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoiby, N., H. Krogh Johansen, C. Moser, Z. Song, O. Ciofu, and A. Kharazmi. 2001. Pseudomonas aeruginosa and the in vitro and in vivo biofilm mode of growth. Microbes Infect. 3:23-35. [DOI] [PubMed] [Google Scholar]
- 23.Kazmierczak, B. I., T. S. Jou, K. Mostov, and J. N. Engel. 2001. Rho GTPase activity modulates Pseudomonas aeruginosa internalization by epithelial cells. Cell Microbiol. 3:85-98. [DOI] [PubMed] [Google Scholar]
- 24.Lee, A., D. Chow, B. Haus, W. Tseng, D. Evans, S. Fleiszig, G. Chandy, and T. Machen. 1999. Airway epithelial tight junctions and binding and cytotoxicity of Pseudomonas aeruginosa. Am. J. Physiol. 277:L204-L217. [DOI] [PubMed] [Google Scholar]
- 25.Look, D. C., M. J. Walter, M. R. Williamson, L. Pang, Y. You, J. N. Sreshta, J. E. Johnson, D. S. Zander, and S. L. Brody. 2001. Effects of paramyxoviral infection on airway epithelial cell Foxj1 expression, ciliogenesis, and mucociliary function. Am. J. Pathol. 159:2055-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moller, L. V., W. Timens, W. van der Bij, K. Kooi, B. de Wever, J. Dankert, and L. van Alphen. 1998. Haemophilus influenzae in lung explants of patients with end-stage pulmonary disease. Am. J. Respir. Crit. Care Med. 157:950-956. [DOI] [PubMed] [Google Scholar]
- 27.Mortimer, P. G., and L. J. Piddock. 1991. A comparison of methods used for measuring the accumulation of quinolones by Enterobacteriaceae, Pseudomonas aeruginosa and Staphylococcus aureus. J. Antimicrob. Chemother. 28:639-653. [DOI] [PubMed] [Google Scholar]
- 28.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295-304. [DOI] [PubMed] [Google Scholar]
- 29.Parsek, M. R., and P. K. Singh. 2003. Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 57:677-701. [DOI] [PubMed] [Google Scholar]
- 30.Pier, G. B. 2000. Role of the cystic fibrosis transmembrane conductance regulator in innate immunity to Pseudomonas aeruginosa infections. Proc. Natl. Acad. Sci. USA 97:8822-8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plotkowski, M. C., S. de Bentzmann, S. H. Pereira, J. M. Zahm, O. Bajolet-Laudinat, P. Roger, and E. Puchelle. 1999. Pseudomonas aeruginosa internalization by human epithelial respiratory cells depends on cell differentiation, polarity, and junctional complex integrity. Am. J. Respir. Cell Mol. Biol. 20:880-890. [DOI] [PubMed] [Google Scholar]
- 32.Prince, A. S. 2002. Biofilms, antimicrobial resistance, and airway infection. N. Engl. J. Med. 347:1110-1111. [DOI] [PubMed] [Google Scholar]
- 33.Roy-Burman, A., R. H. Savel, S. Racine, B. L. Swanson, N. S. Revadigar, J. Fujimoto, T. Sawa, D. W. Frank, and J. P. Wiener-Kronish. 2001. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J. Infect. Dis. 183:1767-1774. [DOI] [PubMed] [Google Scholar]
- 34.Sadikot, R. T., T. S. Blackwell, J. W. Christman, and A. S. Prince. 2005. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am. J. Respir. Crit. Care Med. 171:1209-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sauer, K. 2003. The genomics and proteomics of biofilm formation. Genome Biol. 4:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sauer, K., A. K. Camper, G. D. Ehrlich, J. W. Costerton, and D. G. Davies. 2002. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 184:1140-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaber, J. A., N. L. Carty, N. A. McDonald, E. D. Graham, R. Cheluvappa, J. A. Griswold, and A. N. Hamood. 2004. Analysis of quorum sensing-deficient clinical isolates of Pseudomonas aeruginosa. J. Med. Microbiol. 53:841-853. [DOI] [PubMed] [Google Scholar]
- 38.Schroeder, T. H., N. Reiniger, G. Meluleni, M. Grout, F. T. Coleman, and G. B. Pier. 2001. Transgenic cystic fibrosis mice exhibit reduced early clearance of Pseudomonas aeruginosa from the respiratory tract. J. Immunol. 166:7410-7418. [DOI] [PubMed] [Google Scholar]
- 39.Shaver, C. M., and A. R. Hauser. 2004. Relative contributions of Pseudomonas aeruginosa ExoU, ExoS, and ExoT to virulence in the lung. Infect. Immun. 72:6969-6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh, P. K., A. L. Schaefer, M. R. Parsek, T. O. Moninger, M. J. Welsh, and E. P. Greenberg. 2000. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407:762-764. [DOI] [PubMed] [Google Scholar]
- 41.Stoodley, P., K. Sauer, D. G. Davies, and J. W. Costerton. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56:187-209. [DOI] [PubMed] [Google Scholar]
- 42.Walters, M. C., III, F. Roe, A. Bugnicourt, M. J. Franklin, and P. S. Stewart. 2003. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob. Agents Chemother. 47:317-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whiteley, M., M. G. Bangera, R. E. Bumgarner, M. R. Parsek, G. M. Teitzel, S. Lory, and E. P. Greenberg. 2001. Gene expression in Pseudomonas aeruginosa biofilms. Nature 413:860-864. [DOI] [PubMed] [Google Scholar]
- 44.Wick, M. J., A. N. Hamood, and B. H. Iglewski. 1990. Analysis of the structure-function relationship of Pseudomonas aeruginosa exotoxin A. Mol. Microbiol. 4:527-535. [DOI] [PubMed] [Google Scholar]
- 45.Yoon, S. S., R. F. Hennigan, G. M. Hilliard, U. A. Ochsner, K. Parvatiyar, M. C. Kamani, H. L. Allen, T. R. DeKievit, P. R. Gardner, U. Schwab, J. J. Rowe, B. H. Iglewski, T. R. McDermott, R. P. Mason, D. J. Wozniak, R. E. Hancock, M. R. Parsek, T. L. Noah, R. C. Boucher, and D. J. Hassett. 2002. Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev. Cell 3:593-603. [DOI] [PubMed] [Google Scholar]
- 46.You, Y., E. J. Richer, T. Huang, and S. L. Brody. 2002. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am. J. Physiol. Lung Cell Mol. Physiol. 283:L1315-L1321. [DOI] [PubMed] [Google Scholar]
- 47.Zaas, D. W., M. J. Duncan, G. Li, J. R. Wright, and S. N. Abraham. 2005. Pseudomonas invasion of type I pneumocytes is dependent on the expression and phosphorylation of caveolin-2. J. Biol. Chem. 280:4864-4872. [DOI] [PubMed] [Google Scholar]