Abstract
Staphylococcus aureus is a major cause of severe nosocomial and community-acquired infections. Phagocytes and humoral molecules, including complement, have been proposed to cooperate in host defense against gram-positive bacteria. Circumstantial evidence indicates a role for complement, but this has not been formally defined. Complement activation is initiated by the classical, alternative, or lectin pathway, with the latter requiring mannose-binding lectin (MBL, also known as mannose-binding protein). MBL is an oligomeric serum protein that recognizes carbohydrates decorating a broad range of infectious agents, including S. aureus. We previously reported that MBL null mice were highly susceptible to S. aureus infection, confirming that MBL plays a key role in first-line host defense. In this study, we evaluated the relative roles of C3 and MBL against S. aureus infection by generating MBL × C3 null mice to compare with C3 single null mice. C3 deficiency alone significantly reduced survival to 19% from 97% of wild-type mice (P < 0.0001). Surprisingly, an additional MBL deficiency reduced the survival further to 7% (P < 0.0001). However, the MBL deficiency alone had a smaller though significant effect on survival, which was 77% (P = 0.018 versus wild-type mice). These results confirm an essential function for complement in host resistance against S. aureus infection but also identify an MBL-dependent mechanism that is C3 independent.
Complement is thought to eliminate bacteria by a direct attack, forming a membrane attack complex and producing opsonins C3b and iC3b for opsonophagocytosis (4, 15, 44). Complement is activated by three pathways: the classical, alternative, and lectin pathways. The latter is initiated by mannose-binding lectin (MBL, also known as mannose-binding protein) and ficolin, both of which require MBL-associated serine protease (MASP) (22, 45), in particular MASP-2, which mimics the classical pathway of convertase cleaving C4 and then C2 followed by generation of C3 convertase (45). Although the complement pathway has been accepted as one of the most important mechanisms in host defense against infection (2, 14, 32, 34, 48), much of our knowledge has relied on indirect evidence from studies of complement component 3 (C3) that have utilized cobra venom factor (CVF). This strategy essentially achieves the depletion of complement through activation of the complement cascade (47) and therefore differs from genetic C3 deficiency. More recently, definitive studies using gene-targeted mice have been made possible. However, despite this availability, only a few studies have used gene-targeted mice to address the role of complement component (25, 33).
MBL is a pattern recognition molecule that can recognize the molecular patterns that decorate a wide range of microorganisms. Infectious agents that are recognized by MBL include certain gram-positive and gram-negative bacteria, yeast, parasites, mycobacteria, and viruses (6, 8). MBL as a part of complement can also activate the lectin pathway in an antibody-independent manner. Therefore, MBL has many functional properties that are reminiscent of an antibody, and in fact MBL is considered an opsonin (16, 28, 42, 46). A recent study suggested that the lectin complement pathway contributes largely to C3 deposition on Staphylococcus aureus (28). Furthermore, MBL alone is sufficient in the absence of complement to facilitate enhanced uptake of bacteria by phagocytes (28). MBL has many similarities with the first complement component C1q; both are structurally multimerized and are involved with apoptotic cell clearance (5, 13, 39). In our previous study, we demonstrated that direct attack via MBL, complement, or the combination with other soluble molecules was not effective whereas MBL-initiated opsonophagocytosis was effective in killing S. aureus (37). Indeed, MBL null mice were susceptible to S. aureus infection (37). However, that study did not address the relative roles of MBL and complement-dependent mechanisms.
In this study, we generated MBL × C3 null mice to answer that question. Although humans and new-world monkeys have a single MBL gene, two homologous forms of MBL, designated MBL-A and MBL-C, are present in rodents (17, 24). MBL-A and MBL-C are found predominantly in serum and bind MASPs to activate complement (11). Therefore, the MBL × C3 null mice are actually triple null in MBL-A, MBL-C, and C3. We found that C3 null mice were susceptible to S. aureus infection, similar to mice in which complement was depleted by pretreatment with CVF (35). Surprisingly, MBL × C3 null mice were more susceptible to infection than mice lacking only C3. This suggests a new distinctive host defense mechanism of MBL that is independent of complement.
MATERIALS AND METHODS
Generation of MBL × C3 null mice.
MBL null (MBL-A and MBL-C double null) and C3 null mice have been previously described (37). MBL null mice were crossed with C3 null mice (50) to create MBL × C3 null mice in this study. Experiments were carried out under protocols approved by the Subcommittee on Research Animal Care at Massachusetts General Hospital.
S. aureus infection.
All mice were between 6 and 12 weeks old, from generation F8 and F9, and were maintained on a mixed background of 129/C57BL/6J. Age- and gender-matched mice were used in each experiment. S. aureus CP5+ was used as previously described (37). Mice were inoculated intravenously (i.v.) in the tail vein with 107 CFU/0.2 ml saline/mouse. This dose was derived from dose-response experiments to distinguish differences between C3 null mice and MBL × C3 null mice. For reconstitution experiments, 75 μg of recombinant human MBL (rhMBL; a gift from NatImmune A/S, Copenhagen, Denmark) in 0.2 ml saline/mouse was injected intraperitoneally (i.p.) 1 h prior to the S. aureus inoculation and the day following inoculation (37). For reconstitution of C3, plasma from MBL null mice was prepared and pooled freshly on the day of experiment, and 0.4 ml of the pooled plasma was injected i.p. with the same protocol as the rhMBL.
Assays.
Bacterial loads in blood and organs were measured as described previously (37). Plasma or supernatant of homogenized tissues was used for cytokine and chemokine assays. Tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), macrophage inflammatory protein 2 (MIP-2), and MCP-1 were measured by enzyme-linked immunosorbent assay kits (R&D System, Minneapolis, MN) according to the manufacturer's instructions. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST), creatinine, and blood urea nitrogen were measured at the Chemistry Laboratory (director, James G. Flood), Massachusetts General Hospital.
Statistical analysis.
Survival data were assessed using Kaplan-Meier analysis (JMP5 software; SAS Institute, Cary, North Carolina). Data regarding bacterial loads, soluble factors, and enzymes were analyzed by t test in Statview or JMP5 (SAS Institute, Cary, North Carolina).
RESULTS
C3 null mice are highly susceptible to S. aureus infection.
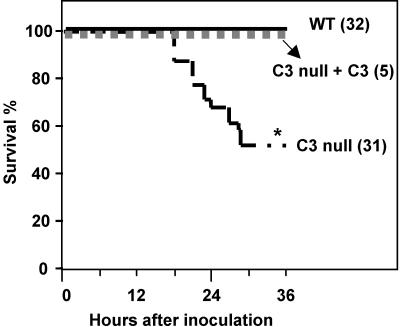
We infected wild-type (WT) and C3 null mice with S. aureus intravenously in a model of bacteremia. Fifteen out of 31 (52%) C3 null mice died, whereas all WT mice survived at 36 h (P < 0.0001) (Fig. 1). The phenotype was reversed by injections of C3-sufficient plasma that was prepared from MBL null mice as described in Materials and Methods. This provided evidence that the plasma of MBL null mice was C3 sufficient and functionally replaced C3 in vivo. These results confirmed that C3 null mice were susceptible to S. aureus infection, as previously observed in mice that were C3 depleted by injection of CVF (3), and that the phenotype was reversed by reconstitution with C3, demonstrating that the susceptibility is C3 dependent.
FIG. 1.
Increased mortality in C3 null mice. WT and C3 null mice were inoculated i.v. with 107 CFU/mouse, and the survival percentage was monitored. Parentheses after genotypes indicate numbers of mice in each group. *, P < 0.05.
Bacteremia and systemic inflammation.
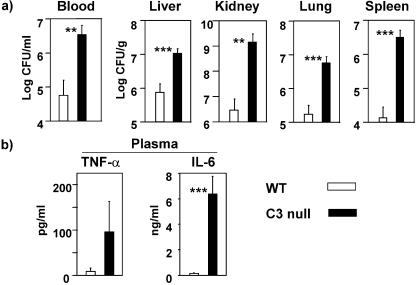
The previous study with CVF-treated, C3-depleted mice did not investigate the cause of death (3). We examined the state of infection as the cause of death from the bacterial infections and found that death could be attributed to complications from bacteremia, septicemia due to uncontrollable inflammatory response, and organ failure in C3-deficient animals as previously reported (1, 10, 43). We determined bacterial loads in blood, liver, kidney, lung, and spleen at 16 h, since C3 null mice began to die approximately 18 h postinoculation (Fig. 1). All assays were performed on samples from 16 h postinoculation for this reason. There were 1 to 2.5 log orders more bacteria in the blood and organs of C3 null mice than in those of WT mice (Fig. 2a). These results suggested that the increased mortality was caused by uncontrollably increased bacterial load in C3 null mice. There is a well-established correlation between mortality and increased production of proinflammatory cytokines, such as TNF-α and IL-6 (1, 10, 43). IL-6 in plasma was increased by 45-fold in C3 null mice compared with WT mice, while the increase in plasma TNF-α was similar in magnitude but with great variation (Fig. 2b). These data suggested that C3 null mice had increased circulating proinflammatory cytokines consistent with a systemic inflammatory response accompanied by bacteremia.
FIG. 2.
Increased bacteremia and systemic inflammatory response in C3 null mice. (a) Bacterial loads in blood and organs. Bacterial loads in blood and organ homogenates were determined at 16 h, as described in Materials and Methods. (b) TNF-α and IL-6 in plasma. Both were measured in plasma at 16 h as described in Materials and Methods. Bars indicate means ± standard errors. Ten mice in each group were used. **, P < 0.005; ***, P < 0.001.
Local inflammation and organ failure.
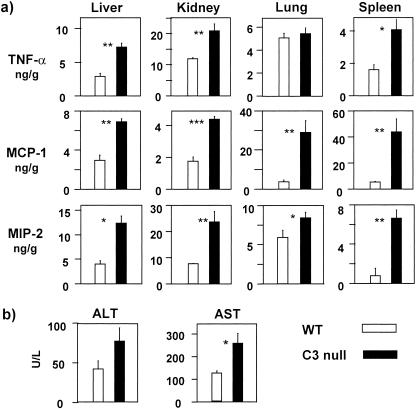
We hypothesized that C3 null mice would be prone to tissue damage induced by cytokines and chemokines, reflecting local inflammatory responses to the local infection. We analyzed chemokines, including MCP-1 and MIP-2, in addition to TNF-α as inflammatory markers in liver, kidney, lung, and spleen. The chemokines and TNF-α in all organs tested were significantly elevated in C3 null mice compared with WT mice, with the exception of lung (P = 0.0002 to 0.047) (Fig. 3a). The data indicated that organs of C3 null mice were inflamed. This was correlated with increased serum AST and ALT in C3 null mice compared with WT mice (Fig. 3b), while serum levels of blood urea nitrogen and creatinine remained similar (data not shown), despite elevations of all inflammatory markers (Fig. 3a). These results suggested that C3 null mice developed inflammatory responses throughout the body, including likely liver damage.
FIG. 3.
Elevated inflammation in organs of C3 null mice. (a) TNF-α, MCP-1, and MIP-2 were measured in organ homogenates at 16 h as described in Materials and Methods. (b) Elevated level of AST in C3 null mice. ALT and AST in blood were determined at 16 h as described in Materials and Methods. Bars indicate means ± standard errors. Four mice were used in each group. *, P < 0.05; **, P < 0.01; ***, P < 0.005.
MBL × C3 null mice are more susceptible to infection than C3 single null mice.
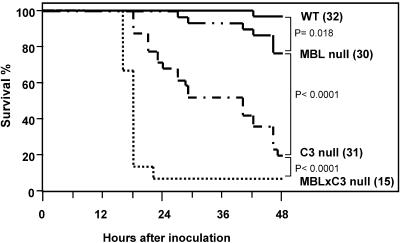
We previously reported that MBL null mice were highly susceptible to S. aureus infection, but we could not address the relative roles of MBL and complement in that study (37). In this investigation, we addressed that question by using transgenic mice that lack MBL × C3. MBL null mice were crossed with C3 null mice in order to generate MBL × C3 null mice. MBL × C3 null mice were fertile and demonstrated no obvious growth defects or abnormalities (data not shown). WT, MBL null, C3 null, and MBL × C3 null mice were inoculated i.v. with 107 CFU of S. aureus/mouse as described in Materials and Methods. Seven percent (1/14) of MBL × C3 null mice survived at 22 h following inoculation, compared with 65% (20/31) of C3 null mice at the same time and 19% (6/31) by 48 h (P < 0.001) (Fig. 4). MBL × C3 null mice died synchronously, as their survival curve precipitously declined compared with a gradually declining curve of C3 null mice (Fig. 4). The 77% (23/30) survival of MBL null mice was significantly lower than the 97% (31/32) survival of WT mice (P = 0.018) (Fig. 4), similar to previous observations with a higher inoculation dose (37). However, the 77% survival of MBL null mice was significantly greater than the survival of C3 null mice (Fig. 4) (P < 0.0001). These results indicate that the susceptibility to S. aureus infection was ranked, with MBL × C3 null mice being the most susceptible followed by C3 null and then MBL null mice.
FIG. 4.
MBL × C3 null mice were more susceptible than C3 single null mice. WT, MBL null, C3 null, and MBL × C3 null mice were inoculated i.v. with S. aureus, and survival was monitored up to 48 h. Parentheses after genotypes indicate numbers of mice in each group.
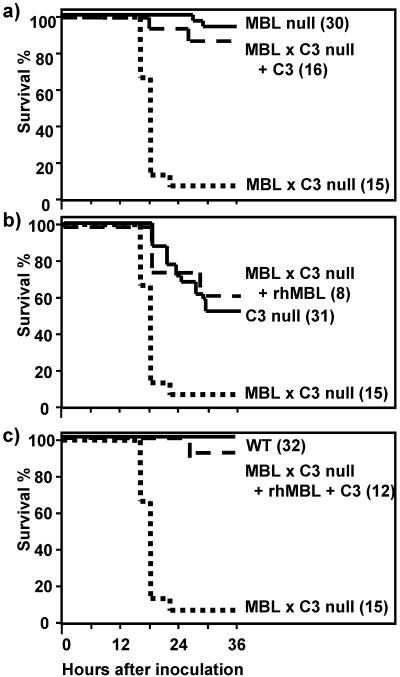
In order to confirm whether the susceptible phenotype was due to a lack of C3, MBL, and/or both, MBL × C3 null mice were injected i.p. with rhMBL and/or C3-sufficient plasma of MBL null mice as described above. The replacement of C3, rhMBL, or both is anticipated to restore phenotypes to MBL null, C3 null, or WT mice, respectively. The reconstitution experiments were terminated at 36 h, since the half-life of murine C3 was expected to be short (30). The half-life of rhMBL was 14 to 20 h as previously reported (37). The C3 replacement restored the survival of MBL × C3 null mice close to that of MBL null mice (Fig. 5a). Likewise, administration of rhMBL improved the survival of MBL × C3 null mice to the level of C3 null mice (Fig. 5b). Finally, the administration of both C3 and rhMBL restored the survival curve of MBL × C3 null mice back to the level of WT mice (Fig. 5c). These results confirmed two things: (i) the low survival of MBL × C3 null mice was due to lack of both C3 and MBL, and (ii) the difference in survival between MBL × C3 null mice and C3 null mice was attributed to an MBL-dependent mechanism(s).
FIG. 5.
Significantly high mortality of MBL × C3 null mice was due to lack of MBL × C3. MBL × C3 null mice were reconstituted with C3-sufficient plasma from MBL null mice (C3) (a), rhMBL (b), or both C3 and rhMBL (c) as described in Materials and Methods. Survival was monitored for 36 h. Parentheses after genotypes indicate numbers of mice in each group.
DISCUSSION
In this study, we demonstrated that mice genetically deficient in C3 were indeed susceptible to S. aureus infection. Survival of C3 null mice was 19% at 48 h following infection, while all WT mice survived (Fig. 4). The susceptible phenotype was reversed by administration of C3-sufficient plasma (Fig. 1), confirming that the high mortality was due to lack of C3. This phenotype was predicted from the previous study using mice that were complement depleted by CVF pretreatment (3). CVF is similar to C3b in activating C3/C5, resulting in activation of complement C5 to C9. CVF-treated, C3-depleted mice (47) therefore differ from C3 gene-targeted mice, i.e., C3 null mice lack C3, while all other lower complement components are intact. C3 null mice have been reported to be susceptible to infections with group B streptococcus, Pseudomonas aeruginosa, and acute septic peritonitis (25, 33, 50). In all cases, high mortality was associated with bacteremia, and in the latter two cases, high mortality was associated with increased induction of TNF-α (25, 33). In our study, the high mortality of C3 null mice was also associated with high-grade bacteremia, as evidenced by the significantly elevated bacterial loads in blood, liver, kidney, lung, and spleen (Fig. 2a). This elevated bacteremia was also associated with increased inflammatory responses throughout the body based on the results that cytokines and chemokines, such as IL-6, TNF-α, MIP-2, and MCP-1, were significantly increased in blood and organs (Fig. 2 and 3). These results are in agreement with the report by Mueller-Ortiz et al. that TNF-α, IL-6, and MIP-2 were increased in bronchoalveolar lavage specimens of C3 null mice following P. aeruginosa infection (25). The increased inflammatory response and bacteremia were also associated with liver damage, indicated by elevated liver enzymes AST and ALT (Fig. 3b). These observations are supported by previous studies in which elevated bacteremia and inflammatory responses were associated with organ damage (21, 31, 43). Taken together, these results demonstrate that C3 null mice are susceptible to S. aureus infection due to increased bacteremia with elevated inflammatory responses and organ damage.
To further probe the relative roles of C3 and MBL, we also generated MBL × C3 null mice in this study. All complement pathways, classical, alternative, and lectin, require C3. Animals that lack C3 are therefore devoid of all lower complement products, including the membrane attack complex and opsonins C3b and iC3b. A lack of opsonization by C3 fragments has a strong impact on survival, as C3 deficiency greatly reduced survival (Fig. 4). A portion of the C3 fragments in C3 null mice is a product of activated lectin pathway, as the C3 fragments are generated by the three complement pathways. In order to examine the efficiency of generating the C3 fragments by each pathway, mice lacking C1q (classical), factor D (alternative), or MBL (lectin) will have to be compared. Surprisingly, MBL × C3 null mice were significantly more susceptible to S. aureus infection than were C3 null mice (Fig. 4). This difference is MBL dependent, as MBL replacement of MBL × C3 null mice reversed the phenotype to the level of C3 null mice (Fig. 5b). This result suggests that MBL has a distinctive role that is C3 independent, at least against S. aureus. MBL was initially recognized as an opsonin (42) and later identified as the initiator of the lectin complement pathway with MASPs (22, 45); however, it has not been clear whether MBL simply amplifies the complement cascade or has its own role that is dissociated from the complement pathway. This study supports the idea that MBL has its own function that is C3 independent and that includes a direct opsonin. The mechanism of this activity is yet to be investigated in detail. There is another possibility: MBL may function in parallel to complement. This effect could be either synergistic or additive, since the effect of MBL deficiency seemed to increase that of C3 deficiency (Fig. 4). At any rate, the C3-independent activity of MBL most likely operates via collectin receptors whose candidates are CD91/calreticulin (29), C1q/MBL receptor (27), and/or CD21/CD35 (CR1) receptor (9), while the opsonic function requires the complement receptor, since the effector molecule is expected to be the C3b and iC3b opsonins (4, 7, 18, 28). MBL also may regulate other receptors, either directly by facilitating accessibility of ligands or indirectly by binding to the collectin receptors that may further modulate other receptors. Further in vitro studies will inform us how MBL regulates inflammatory responses. In vitro observations demonstrated that MBL could bind and opsonize bacteria as well as the yeast cell wall product mannan (16).
Clinical studies correlated an MBL-dependent opsonic defect in human serum with a phenotype of recurrent infection (42). These patients were found to have one of three amino acid substitutions due to single nucleotide polymorphisms in exon 1 of the MBL gene that disrupts the collagen helix (40). More detailed analysis of the MBL gene has revealed at least seven distinct MBL haplotypes in humans, four of which (LYPB, LYQC, HYPD, and LXPA) dictate low serum levels (20, 38). Interestingly, there is a high rate of these haplotypes in various human populations, with a range of heterozygosity from 15% in Caucasians to 30% in certain African populations (19, 41). Importantly, MBL is able to distinguish species-self as well as altered-self, in the form of apoptotic cells, from non-self (26, 29, 39). The specificity that allows the distinction of surfaces of virally infected cells and transformed cells from normal host cells depends on both fine recognition of molecular patterns and a macropattern (12). The macropattern appears to be dictated by the spatial orientation of the carbohydrate-binding domains and the differences in geometry of the sugars that adorn microorganisms compared to host glycoproteins (36, 49).
In this study, we have shown that C3 null mice are susceptible but MBL × C3 null mice are significantly more susceptible to S. aureus infection. Thus, MBL appears to have a unique role in vivo, as we postulated from an in vitro study (16). The emergence of multiresistant strains of S. aureus (23) has complicated the treatment of infection caused by the pathogen. MBL, having the ability to limit this organism by mechanisms different from those of antibiotics, may therefore provide a new therapeutic adjunct to be used in combination with existing antibiotic treatment. In conclusion, this study provides the in vivo evidence that C3 and MBL have a dynamic interaction in combating S. aureus infection and that MBL has the independent effector function.
Acknowledgments
This work was supported by grant NIH RO1AI42788.
We thank Iain Fraser, Mykol Larvie, and Lynda Stuart for critical reading of the manuscript and helpful discussion. We also thank Michael C. Carroll for providing breeding pairs of C3 null mice. We also thank NatImmune S/A, Denmark, for providing rhMBL.
Editor: J. B. Bliska
REFERENCES
- 1.Boes, M., A. P. Prodeus, T. Schmidt, M. C. Carroll, and J. Chen. 1998. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J. Exp. Med. 188:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corvini, M., C. Randolph, and S. I. Aronin. 2004. Complement C7 deficiency presenting as recurrent aseptic meningitis. Ann. Allergy Asthma Immunol. 93:200-205. [DOI] [PubMed] [Google Scholar]
- 3.Cunnion, K. M., D. K. Benjamin, Jr., C. G. Hester, and M. M. Frank. 2004. Role of complement receptors 1 and 2 (CD35 and CD21), C3, C4, and C5 in survival by mice of Staphylococcus aureus bacteremia. J. Lab. Clin. Med. 143:358-365. [DOI] [PubMed] [Google Scholar]
- 4.Cunnion, K. M., P. S. Hair, and E. S. Buescher. 2004. Cleavage of complement C3b to iC3b on the surface of Staphylococcus aureus is mediated by serum complement factor I. Infect. Immun. 72:2858-2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drickamer, K., M. S. Dordal, and L. Reynolds. 1986. Mannose-binding proteins isolated from rat liver contain carbohydrate-recognition domains linked to collagenous tails. Complete primary structures and homology with pulmonary surfactant apoprotein. J. Biol. Chem. 261:6878-6887. [PubMed] [Google Scholar]
- 6.Epstein, J., Q. Eichbaum, S. Sheriff, and R. A. Ezekowitz. 1996. The collectins in innate immunity. Curr. Opin. Immunol. 8:29-35. [DOI] [PubMed] [Google Scholar]
- 7.Fearon, D. T. 1980. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J. Exp. Med. 152:20-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser, I. P., H. Koziel, and R. A. Ezekowitz. 1998. The serum mannose-binding protein and the macrophage mannose receptor are pattern recognition molecules that link innate and adaptive immunity. Semin. Immunol. 10:363-372. [DOI] [PubMed] [Google Scholar]
- 9.Ghiran, I., S. F. Barbashov, L. B. Klickstein, S. W. Tas, J. C. Jensenius, and A. Nicholson-Weller. 2000. Complement receptor 1/CD35 is a receptor for mannan-binding lectin. J. Exp. Med. 192:1797-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groeneveld, A. B., A. N. Tacx, A. W. Bossink, G. J. van Mierlo, and C. E. Hack. 2003. Circulating inflammatory mediators predict shock and mortality in febrile patients with microbial infection. Clin. Immunol. 106:106-115. [DOI] [PubMed] [Google Scholar]
- 11.Hansen, S., S. Thiel, A. Willis, U. Holmskov, and J. C. Jensenius. 2000. Purification and characterization of two mannan-binding lectins from mouse serum. J. Immunol. 164:2610-2618. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann, J. A., F. C. Kafatos, C. A. Janeway, and R. A. Ezekowitz. 1999. Phylogenetic perspectives in innate immunity. Science 284:1313-1318. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda, K., T. Sannoh, N. Kawasaki, T. Kawasaki, and I. Yamashina. 1987. Serum lectin with known structure activates complement through the classical pathway. J. Biol. Chem. 262:7451-7454. [PubMed] [Google Scholar]
- 14.Jack, D. L., N. J. Klein, and M. W. Turner. 2001. Mannose-binding lectin: targeting the microbial world for complement attack and opsonophagocytosis. Immunol. Rev. 180:86-99. [DOI] [PubMed] [Google Scholar]
- 15.Joiner, K. A., E. J. Brown, and M. M. Frank. 1984. Complement and bacteria: chemistry and biology in host defense. Annu. Rev. Immunol. 2:461-491. [DOI] [PubMed] [Google Scholar]
- 16.Kuhlman, M., K. Joiner, and R. A. Ezekowitz. 1989. The human mannose-binding protein functions as an opsonin. J. Exp. Med. 169:1733-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laursen, S. B., T. S. Dalgaard, S. Thiel, B. L. Lim, T. V. Jensen, H. R. Juul-Madsen, A. Takahashi, T. Hamana, M. Kawakami, and J. C. Jensenius. 1998. Cloning and sequencing of a cDNA encoding chicken mannan-binding lectin (MBL) and comparison with mammalian analogues. Immunology 93:421-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, L. Y., X. Liang, M. Hook, and E. L. Brown. 2004. Identification and characterization of the C3 binding domain of the Staphylococcus aureus extracellular fibrinogen-binding protein (Efb). J. Biol. Chem. 279:50710-50716. [DOI] [PubMed] [Google Scholar]
- 19.Madsen, H. O., P. Garred, S. Thiel, J. A. Kurtzhals, L. U. Lamm, L. P. Ryder, and A. Svejgaard. 1995. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J. Immunol. 155:3013-3020. [PubMed] [Google Scholar]
- 20.Madsen, H. O., M. L. Satz, B. Hogh, A. Svejgaard, and P. Garred. 1998. Different molecular events result in low protein levels of mannan-binding lectin in populations from southeast Africa and South America. J. Immunol. 161:3169-3175. [PubMed] [Google Scholar]
- 21.Matsukawa, A., M. H. Kaplan, C. M. Hogaboam, N. W. Lukacs, and S. L. Kunkel. 2001. Pivotal role of signal transducer and activator of transcription (Stat)4 and Stat6 in the innate immune response during sepsis. J. Exp. Med. 193:679-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsushita, M., and T. Fujita. 1992. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J. Exp. Med. 176:1497-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melzer, M., S. J. Eykyn, W. R. Gransden, and S. Chinn. 2003. Is methicillin-resistant Staphylococcus aureus more virulent than methicillin-susceptible S. aureus? A comparative cohort study of British patients with nosocomial infection and bacteremia. Clin. Infect. Dis. 37:1453-1460. [DOI] [PubMed] [Google Scholar]
- 24.Mogues, T., T. Ota, A. I. Tauber, and K. N. Sastry. 1996. Characterization of two mannose-binding protein cDNAs from rhesus monkey (Macaca mulatta): structure and evolutionary implications. Glycobiology 6:543-550. [DOI] [PubMed] [Google Scholar]
- 25.Mueller-Ortiz, S. L., S. M. Drouin, and R. A. Wetsel. 2004. The alternative activation pathway and complement component C3 are critical for a protective immune response against Pseudomonas aeruginosa in a murine model of pneumonia. Infect. Immun. 72:2899-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nauta, A. J., N. Raaschou-Jensen, A. Roos, M. R. Daha, H. O. Madsen, M. C. Borrias-Essers, L. P. Ryder, C. Koch, and P. Garred. 2003. Mannose-binding lectin engagement with late apoptotic and necrotic cells. Eur. J. Immunol. 33:2853-2863. [DOI] [PubMed] [Google Scholar]
- 27.Nepomuceno, R. R., A. H. Henschen-Edman, W. H. Burgess, and A. J. Tenner. 1997. cDNA cloning and primary structure analysis of C1qR(P), the human C1q/MBL/SPA receptor that mediates enhanced phagocytosis in vitro. Immunity 6:119-129. [DOI] [PubMed] [Google Scholar]
- 28.Neth, O., D. L. Jack, M. Johnson, N. J. Klein, and M. W. Turner. 2002. Enhancement of complement activation and opsonophagocytosis by complexes of mannose-binding lectin with mannose-binding lectin-associated serine protease after binding to Staphylococcus aureus. J. Immunol. 169:4430-4436. [DOI] [PubMed] [Google Scholar]
- 29.Ogden, C. A., A. deCathelineau, P. R. Hoffmann, D. Bratton, B. Ghebrehiwet, V. A. Fadok, and P. M. Henson. 2001. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J. Exp. Med. 194:781-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peake, P. W., J. A. Charlesworth, and B. A. Pussell. 1991. Activation of rabbit C3: studies of the generation of cleavage products in vitro and of their metabolism in vivo. Complement Inflamm. 8:261-270. [DOI] [PubMed] [Google Scholar]
- 31.Peyton, C., and W. G. C. Shrotri. 1999. Handbook of animal models of infection. Academic Press, New York, N.Y.
- 32.Picard, C., A. Puel, J. Bustamante, C. L. Ku, and J. L. Casanova. 2003. Primary immunodeficiencies associated with pneumococcal disease. Curr. Opin. Allergy Clin. Immunol. 3:451-459. [DOI] [PubMed] [Google Scholar]
- 33.Prodeus, A. P., X. Zhou, M. Maurer, S. J. Galli, and M. C. Carroll. 1997. Impaired mast cell-dependent natural immunity in complement C3-deficient mice. Nature 390:172-175. [DOI] [PubMed] [Google Scholar]
- 34.Ross, S. C., and P. Densen. 1984. Complement deficiency states and infection: epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency. Medicine (Baltimore) 63:243-273. [PubMed] [Google Scholar]
- 35.Sakiniene, E., T. Bremell, and A. Tarkowski. 1999. Complement depletion aggravates Staphylococcus aureus septicaemia and septic arthritis. Clin. Exp. Immunol. 115:95-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheriff, S., C. Y. Chang, and R. A. Ezekowitz. 1994. Human mannose-binding protein carbohydrate recognition domain trimerizes through a triple alpha-helical coiled-coil. Nat. Struct. Biol. 1:789-794. [DOI] [PubMed] [Google Scholar]
- 37.Shi, L., K. Takahashi, J. Dundee, S. Shahroor-Karni, S. Thiel, J. C. Jensenius, F. Gad, M. R. Hamblin, K. N. Sastry, and R. A. Ezekowitz. 2004. Mannose-binding lectin-deficient mice are susceptible to infection with Staphylococcus aureus. J. Exp. Med. 199:1379-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steffensen, R., S. Thiel, K. Varming, C. Jersild, and J. C. Jensenius. 2000. Detection of structural gene mutations and promoter polymorphisms in the mannan-binding lectin (MBL) gene by polymerase chain reaction with sequence-specific primers. J. Immunol. Methods 241:33-42. [DOI] [PubMed] [Google Scholar]
- 39.Stuart, L. M., K. Takahashi, L. Shi, J. Savill, and R. A. Ezekowitz. 2005. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J. Immunol. 174:3220-3226. [DOI] [PubMed] [Google Scholar]
- 40.Sumiya, M., M. Super, P. Tabona, R. J. Levinsky, T. Arai, M. W. Turner, and J. A. Summerfield. 1991. Molecular basis of opsonic defect in immunodeficient children. Lancet 337:1569-1570. [DOI] [PubMed] [Google Scholar]
- 41.Super, M., S. D. Gillies, S. Foley, K. Sastry, J. E. Schweinle, V. J. Silverman, and R. A. Ezekowitz. 1992. Distinct and overlapping functions of allelic forms of human mannose binding protein. Nat. Genet. 2:50-55. [DOI] [PubMed] [Google Scholar]
- 42.Super, M., S. Thiel, J. Lu, R. J. Levinsky, and M. W. Turner. 1989. Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet 2:1236-1239. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi, K., J. Gordon, H. Liu, K. N. Sastry, J. E. Epstein, M. Motwani, I. Laursen, S. Thiel, J. C. Jensenius, M. Carroll, and R. A. Ezekowitz. 2002. Lack of mannose-binding lectin-A enhances survival in a mouse model of acute septic peritonitis. Microbes Infect. 4:773-784. [DOI] [PubMed] [Google Scholar]
- 44.Taylor, P. W. 1992. Complement-mediated killing of susceptible gram-negative bacteria: an elusive mechanism. Exp. Clin. Immunogenet. 9:48-56. [PubMed] [Google Scholar]
- 45.Thiel, S., T. Vorup-Jensen, C. M. Stover, W. Schwaeble, S. B. Laursen, K. Poulsen, A. C. Willis, P. Eggleton, S. Hansen, U. Holmskov, K. B. Reid, and J. C. Jensenius. 1997. A second serine protease associated with mannan-binding lectin that activates complement. Nature 386:506-510. [DOI] [PubMed] [Google Scholar]
- 46.Valdimarsson, H., T. Vikingsdottir, P. Bang, S. Saevarsdottir, J. E. Gudjonsson, O. Oskarsson, M. Christiansen, L. Blou, I. Laursen, and C. Koch. 2004. Human plasma-derived mannose-binding lectin: a phase I safety and pharmacokinetic study. Scand. J. Immunol. 59:97-102. [DOI] [PubMed] [Google Scholar]
- 47.Vogel, C. W., and H. J. Muller-Eberhard. 1982. The cobra venom factor-dependent C3 convertase of human complement. A kinetic and thermodynamic analysis of a protease acting on its natural high molecular weight substrate. J. Biol. Chem. 257:8292-8299. [PubMed] [Google Scholar]
- 48.Walport, M. J. 2001. Complement. First of two parts. N. Engl. J. Med. 344:1058-1066. [DOI] [PubMed] [Google Scholar]
- 49.Weis, W. I., M. E. Taylor, and K. Drickamer. 1998. The C-type lectin superfamily in the immune system. Immunol. Rev. 163:19-34. [DOI] [PubMed] [Google Scholar]
- 50.Wessels, M. R., P. Butko, M. Ma, H. B. Warren, A. L. Lage, and M. C. Carroll. 1995. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc. Natl. Acad. Sci. USA 92:11490-11494. [DOI] [PMC free article] [PubMed] [Google Scholar]