Abstract
Neisseria meningitidis groups A (GAM) and W135 capsular polysaccharides (CPs) were bound to recombinant Staphylococcus aureus enterotoxin C1 (rSEC). The CPs were activated with 1-cyano-4-dimethylaminopyridinium tetrafluoroborate and then bound to adipic acid dihydrazide derivatives of rSEC. Syntheses were conducted with native GAM CP (GAMP), W135 CP (W135P), and ultrasonicated or hydrazine-treated W135P at various concentrations of reactants, pHs, and ionic strengths. The conjugates were characterized by compositional and serologic analyses, high-performance size-exclusion chromatography with multi-angle laser light scattering detection, and immunogenicity in 5- to 6-week-old mice. Conjugates injected subcutaneously in phosphate-buffered saline elicited immunoglobulin G (IgG) responses against their respective CPs and rSEC, whereas GAMP and W135P alone did not induce detectable CP antibodies. The O-acetyl content of W135P was low, and its removal had no adverse effect upon the conjugate's immunogenicity. Reduction of the molecular size of W135P by treatment with hydrazine improved the immunogenicity of W135P-rSEC. IgG anti-CP elicited by the conjugates showed complement-dependent bactericidal activity against their respective organisms, and IgG anti-rSEC neutralized the T-cell proliferative activity of native SEC. A bivalent formulation of GAMP-rSEC and W135P-rSEC elicited IgG anti-CP at comparable levels to those induced by the conjugates administered separately.
For more than 100 years meningococci have caused recurrent epidemics in the “meningitis belt” of sub-Saharan Africa. Almost all were caused by Neisseria meningitidis group A (GAM) and occurred about every 8 to 14 years, with attack rates approaching 1,000 cases per 100,000 population (20, 40). In 1996, in the largest epidemic recorded, there were more than 250,000 cases and 25,000 deaths (37). In 1999, a severe GAM epidemic in Burkina Faso (∼13 million population) involved about 40,000 cases (∼0.33% of the population). Mass vaccination with bivalent A+C polysaccharide vaccine in individuals >2 years of age was instituted. In 2001, approximately 14,000 meningitis cases caused mostly by N. meningitidis group W135, occurred in Burkina Faso, Cameroon, and Niger (12,31, 39). Soon thereafter, cases of W135 meningococcal meningitis were identified in pilgrims to theHajj in Saudi Arabia and in Europe, North America, and Australia (1, 9, 23, 36, 38, 40). This was the second recorded epidemic caused by this serogroup in West Africa since an outbreak of W135 meningitis was reported in Dakar and in Niamey in 1981 (7,13).
We previously reported on several GAM CP (GAMP) conjugates using bovine serum albumin (BSA) as a carrier and evaluated their immunogenicity in mice (18). The synthesis used CDAP (1-cyano-4-dimethylaminopyridinium tetrafluoroborate)-activated polysaccharide bound to adipic acid dihydrazide (AH)-derivatized protein. Based on this, we prepared GAMP conjugates with a potentially medically useful protein: recombinant staphylococcal enterotoxin C1 (rSEC). Taking into account the recent epidemic in West Africa, we also prepared W135 CP (W135P) conjugates by using the same carrier and formulated a bivalent groups A and W135 conjugate. Antibody responses elicited by these conjugates to the CPs and rSEC were evaluated in mice.
MATERIALS AND METHODS
Reagents.
Adipic acid dihydrazide, l-ethyl-3-(3-dimethylaminopropyl) carbodiimide, CDAP, Brij 35, anhydrous hydrazine, and hexadecyltrimethylammonium bromide (Cetavlon) were obtained from Sigma Chemical Co. St. Louis, MO. Triethylamine was from Pierce, Rockford, IL; acetonitrile was from T. J. Baker, Inc., Philipsburg, NJ; and alkaline phosphatase-labeled goat anti-mouse immunoglobulin G (IgG) was from KPL, Inc., Gaithersburg, MD. Baby rabbit serum was obtained from Pel-Freez Clinical Systems, LLC, Brown Deer, WI; equine hyperimmune GAM serum (H49) and W135 serum (H54) were as described previously (2, 34); and hyperimmune anti-SEC serum was prepared as described previously (6). Hyperimmune GAMP murine serum (14-1-A, 2D7-B5B5), used as a reference in enzyme-linked immunosorbent assay (ELISA), was obtained from Wendell Zollinger, Water Reed Army Institute of Research, Washington, DC. Pyrogen-free water (PFW) was from Ultrapure Water Systems (HYDRC), Durham, NC; ethanol was from Pharmco, Brookfield, CT; and [3H]thymidine was from Moravek, Brea, CA. Filtermate Harvester and the TopCount NXT scintillation and luminescence counter were from Packard BioScience, Meriden, CT.
Sepharose CL-4B, CL-6B, and Sephadex G-50 were obtained from Pharmacia AB, Uppsala, Sweden; dialysis membranes (molecular weight cutoff, 3,000 to 10,000) were from Spectra-Por, Laguna Hills, CA; ultrafiltration membranes (YM10) were from Amicon, Bedford, MA; and 14% Tris-glycine gel was from Invitrogen (Carlsbad, CA).
Bacteria and polysaccharides.
GAM (F8238) and W135 (BB701) strains of N. meningitidis were provided by Carl Frasch, CBER, U.S. Food and Drug Administration. GAMP was supplied by the Chiron Corp. (Emeryville, CA). The cultivation of W135 and purification of W135P were done according to World Health Organization (WHO) requirements (described in references 41 and 42) with some modifications: W135P was purified by Sepharose CL-6B gel filtration after phenol extration or by Sepharose CL-6B gel filtration without the process of phenol extraction.
The CP (150 mg/10 ml of 0.2 M NaCl) was loaded onto a Sepharose CL-6B (5-by-90-cm) column and eluted with 0.2 M NaCl. The eluate was monitored by determining the absorbances at 206 and 280 nm. Fractions that showed absorbance at 206 nm only were pooled. The pooled fractions were brought to 75% of ethanol, and the pellet was centrifuged, dialyzed against PFW, and freeze-dried. The powder was dissolved in 0.1 M CaCl2 and ultracentrifuged, and then the supernatant was dialyzed against PFW and freeze-dried.
rSEC.
rSEC is a mutated S. aureus enterotoxin C1 (SEC1) with a deletion between positions 93 and 110, which forms a disulfide loop in the native protein. rSEC has neither the emetic nor the T-cell proliferation activities of the native toxin. The recombinant rSEC was expressed in S. aureus strain RN4220, cultivated, and purified as described previously (5, 10).
rSEC was characterized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), double immunodiffusion against rabbit anti-SEC, toxicity in rabbits, and in vitro T-cell proliferation (10).
Analytic.
Protein, nucleic acid, phosphorus, CP concentration, AH derivatization of protein, and the partitian coefficient of column chromatography (Kd) were measured as described previously (18). O-acetyl (OAc) content was analyzed by the Hestrin reaction (17), and the 7-OAc and 9-OAc contents of W135P were measured by NMR. Double immunodiffusion was performed in 0.8% agarose-0.2 M NaCl with H49, H54, or rabbit anti-SEC sera.
Ultrasonication and hydrazine treatment W135P [W135P(S), W135P(H)].
W135P, dissolved in 10 ml of 0.2 M NaCl at 5 mg/ml, was ultrasonicated for 60 min Sonic Dismembrator (Fisher model no. 300) sonicator with a tapered microprobe (0.3-mm diameter) under a stream of N2 at room temperature and then precipitated by 75% alcohol, dialyzed, and freeze-dried (30). The same concentration and volume of W135P was treated with 10 ml of anhydrous hydrazine at 5 mg/ml at ca. 40 to 50°C for 4 h, precipitated by 75% alcohol, dialyzed, and freeze-dried (15).
Derivatization of rSEC.
rSEC was derivatized with ADH as described previously (18). Six lots were prepared, and their derivatizations ranged from 1.23 to 5.54% (see Table 3).
TABLE 3.
Conjugation conditions and rSEC/CP ratio in conjugates and their IgG antibody responses in mice
| Immunogena | AH (%) of rSEC | pH | CDAP (mol) | Concn (mg/ml) of CP & Pr | rSEC/CP in the final (wt/wt) | IgG anti-CPb (EU, GM) |
|---|---|---|---|---|---|---|
| GAMP-rSEC1 | 1.23 | 7 | 0.01 | 7 | 2.96 | 26.6 |
| GAMP-rSEC2 | 1.54 | 7 | 0.02 | 10 | 1.95 | 31.7 |
| GAMP-rSEC3 | 2.81 | 7 | 0.02 | 10 | 1.01 | 80.1 |
| GAMP-rSEC4 | 2.81 | 7 | 0.04 | 20 | 0.85 | 56.6 |
| GAMP | NAc | NA | NA | NA | <0.2 | |
| W135P-rSEC1 | 2.81 | 7 | 0.02 | 10 | 0.61 | 0.69 |
| W135P-rSEC2 | 2.81 | 8 | 0.02 | 10 | 0.67 | 7.67 |
| W135P-rSEC3 | 5.54 | 8 | 0.02 | 10 | 0.90 | 8.73 |
| W135P-rSEC4 | 5.54 | 7 | 0.02 | 10 | 0.94 | 6.62 |
| W135P(H)-rSEC5 | 5.54 | 8 | 0.02 | 10 | 1.20 | 18.6 |
| W135P(H)-rSEC6 | 2.20 | 8 | 0.04 | 20 | 1.64 | 12.5 |
| W135P(S)-rSEC7 | 5.54 | 8 | 0.02 | 10 | NDd | 2.31 |
| W135P(S)-rSEC8 | 4.45 | 8 | 0.04 | 20 | 2.63 | 8.30 |
| W135P | NA | NA | NA | NA | <0.02 |
GAMP-rSEC1 to GAMP-rSEC4 and W135P-rSEC1 to W135P-rSEC4 were prepared with native CP; for W135P(H)-rSEC, hydrazine-treated CP was used in conjugation; for W135P(S)-rSEC, sonicated CP was used in conjugation.
GAMP-rSEC, 80.1 versus 26.6 and 31.74, P < 0.01; 56.6 versus 26.6, P < 0.05; W135-rSEC, 18.6, 12.5, and 8.30 versus 2.31, P < 0.01.
NA, not applicable.
ND, not done.
Conjugation.
The GAMP conjugates were prepared at pH 7 as described previously (18), and W135-rSEC conjugates were prepared at pH 7 or pH 8. Three W135P preparations were coupled to the rSEC: native (W135P-rSEC), hydrazine-treated [W135P(H)-rSEC], and sonicated [W135P(S)-rSEC]. W135P, W135P(H), and W135P(S) used in conjugation were from a single lot of W135P.
SEC-MALLS.
The use of high-performance size-exclusion chromatography with multi-angle laser light scattering detection (SEC-MALLS) for characterization of capsular polysaccharides has been reported (3, 11, 16). In the present study we expand upon this application for use with conjugate vaccines and their components. Mw and Pd (polydispersity, Mw/Mn; Mn, average molar mass; and Mw, weight average molar mass) were calculated for rSEC-AH, W135P, and W135P(H) and their corresponding conjugates: W135P-rSEC3 and W135P(H)-rSEC5 (see Table 2). The data were acquired and analyzed using a Wyatt Technology Corp. Dawn-DSP with Astra Version 4.73.04 software. Dextran standards from Polymer Standards Service-USA (Warwick, RI) were used to verify the instrument's calibration. All solvents were filtered by using 0.22-μm-pore-size Stericup (Millipore) filters. A 100 mM sodium chloride (pH 6.7) mobile phase was prepared in deionized H2O. All samples were dissolved in deionized H2O, stored overnight at 5°C, and then added to an equal volume of 200 mM NaCl. Samples were mixed gently at ambient temperature for at least 1 h, injected in 100-μl volumes, and fractionated through a Shodex OHPAK SB-806HQ column (300 by 8 mm). The samples were passed through a Dawn DSP Laser Photometer (633 nm) with 18 detectors for size, followed by an Optilab DSP refractometer for concentration. Astra software was then used to calculate the Mn and Mw of samples by summation of slices over the full volume of a user-defined peak region. All measurements were made at ambient temperature.
TABLE 2.
Comparison of the calculated Mw and Pd of rSEC-AH, W135P, W135P(H), W135P-rSEC, and W135P(H)-rSEC
| Sample | Mw (kg/mol kDa) | Mean Pd (Mw/Mn) ± SD |
|---|---|---|
| rSEC-AH | 31.5 | 1.008 ± 0.008 |
| W135P | 689.4 | 1.932 ± 0.067 |
| W135P(H)a | 63.4 | 1.265 ± 0.036 |
| W135P-rSECb | 9,267.0 | 1.408 ± 0.024 |
| W135(H)-rSECc | 5,026.0 | 1.107 ± 0.010 |
W135P(H), hydrazine-treated W135P.
W135P-rSEC was prepared from native W135P.
W135P(H)-rSEC was from W135P(H).
Immunization.
Groups of 10 5- to 6-week-old National Institutes of Health general purpose mice were injected subcutaneously with 0.1 ml of a phosphate-buffered saline (PBS) solution containing 2.5 μg of CP alone or conjugated on days 0, 14, and 28. Groups of mice were exsanguinated 7 days after each injection or after the third injection.
ELISA and complement-mediated bactericidal assay.
Serum IgG GAMP, W135, and rSEC antibodies were measured by ELISA. Flat-bottom 96-well microtiter plates (Nunc-Immuno) were coated with GAMP, W135P, or rSEC (2μg/ml in PBS [pH 7.2]) followed by incubation at room temperature overnight. After washing (0.15 M NaCl, 0.1% Brij, 3 mM sodium azide), the plates were blocked with 1% BSA in PBS for 1 h at room temperature. Then the plates werewashed, and twofold serial dilutions of serum in 1% BSA-0.33% Brij- PBS were added. Again, the plates were incubated at room temperature overnight and washed, and then alkaline phosphatase-labeled goat anti-mouse IgG was added. After 4 h at room temperature, the plates were washed, and 4-nitrophenylphosphate substrate (1 mg/ml in 1 M Tris-3 mM MgCl2 [pH 9.8]) was added. The A405 was measured 20 min later by a MRX Dynatech reader. The results were computed with an ELISA data processing program provided by the Biostatistics and Information Management Branch, Centers for Disease Control and Prevention, based upon four parameters of logistic-log function using the Taylor Series linearization algorithm. The hyperimmune serum used as a reference was arbitrarily assigned a value of 100 ELISA units (EU). Serum IgG anti-CP and anti-rSEC levels were expressed as geometric means (GM). The complement-mediated bactericidal assay was done as described previously (18).
Toxin neutralization assay.
Five sera from mice injected three times with W135(S)-rSEC8 and nine sera from mice immunized with the bivalent conjugate [GAMP-rSEC3 + W135P(H)-rSEC6], containing high, medium, and low levels of IgG anti-SEC as measured by ELISA, were assessed for their ability to neutralize the T-cell proliferative activity of native SEC (5, 6). Enriched lymphocyte preparations were prepared from peripheral blood mononuclear cell suspensions obtained by density gradient centrifugation of human volunteer blood samples (obtained by venipuncture). Native SEC, purified by preparative isoelectric focusing, was used at 2 ng/ml of 10 mM PBS (pH 7.2), which generated ca. 50% maximal T-cell response. Toxin solution aliquots (50 μl) were preincubated at 37°C for 2 h with 5 μl of undiluted serum samples in duplicates in a 96-well microtiter plate. The T cells were adjusted to a concentration of 106cells/ml of RPMI 1640, supplemented with 2% fetal bovine serum, 2 mM glutamine, sodium penicillin at 200 U/ml, and streptomycin sulfate at 200 μg/ml. A total of 200 μl of the T-cell suspension was added to the wells containing the preincubated sera. Residual proliferative activity of the SEC was assessed after incubation at 37°C for 4 days. The cultures were pulsed on the fourth day with 1.0μCi of [3H]thymidine for 18 to 24 h. Cellular DNA was harvested with the Filtermate Harvester, and the incorporated radioactivity was quantified by using the TopCount NXT scintillation and luminescence counter.
Statistics.
The Student t test with Bonferroni correction was used to compare GMs between groups of mice. The Kendall procedure was used to evaluate the correlation between IgG anti-CP levels and bactericidal titers. Statistical significance was defined as a P value of <0.05.
RESULTS
rSEC.
rSEC was identified as 27-kDa band with a small amount of a dimer of 54 kDa by SDS-PAGE (Fig. 1).
FIG. 1.
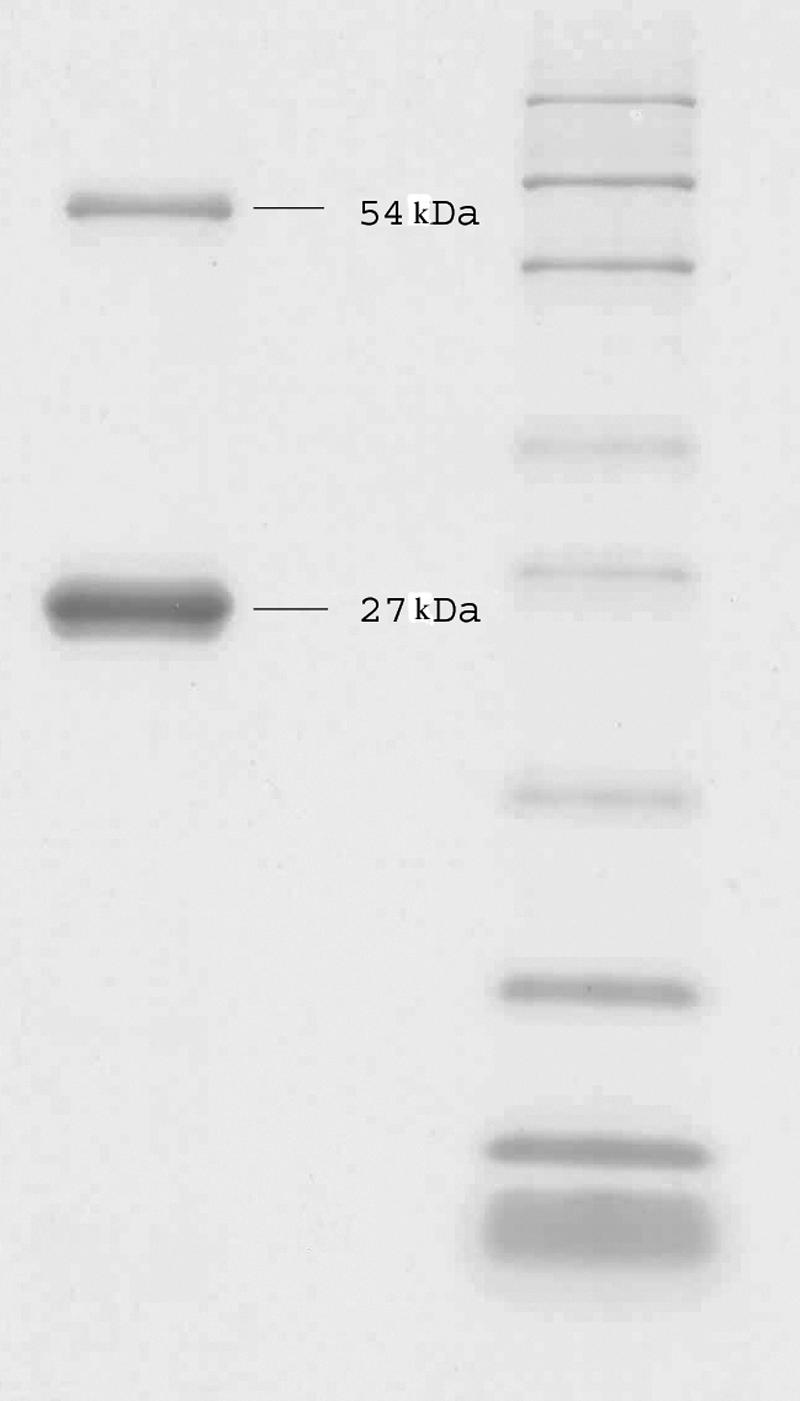
SDS-PAGE of rSEC. Left lane, rSEC, 2 μl, 70 μg/ml; right lane, Mark12 unstained standard, 5 μl.
Characterization of GAMP and W135P.
GAMP and W135P contained less than 1% (wt/wt) of nucleic acids and protein, except the W135P purified without phenol extraction that contained 3.25% protein (Table 1). However, this value still conformed to the WHO requirements (protein less than 5% for W135P); the purification procedure without phenol extraction increased the CP yield from 30 to 60% (data not shown). GAMP contained O-acetyl at 2.1 mmol/g and W135P at 0.020 mmol/g. Treatment of W135P with hydrazine reduced its O-acetyl content to 0.007 mmol/g; a lesser reduction to 0.015mmol/g, followed ultrasonication. GAMP had a Kd value of 0.35 on Sepharose CL-4B. W135P had two peaks on Sepharose Cl-4B: a major peak at Kd of 0.06 and a minor peak Kd of 0.46. Treatment of W135P with hydrazine or ultrasonication reduced it to a single peak of 0.46 kDa.
TABLE 1.
Characterizations of GAMP and native, hydrazine-treated, or sonicated W135P
| GAMP or W135 | Nucleic acids (%) | Protein (%) | O-acetyl (mmol/g) | O-acetyl after treatmenta (%) | Kd on Sepharose CL-4B |
|---|---|---|---|---|---|
| GAMP | 0.08 | <0.05 | 2.130 | NA | 0.35 |
| W135Pb | 0.59 | <0.05 | 0.020 | NA | 0.06, 0.46 |
| W135Pc | 0.38 | 3.25 | 0.021 | NA | 0.06, 0.46 |
| W135P(H)d | 0.36 | 0.16 | 0.007 | 65 | 0.46 |
| W135P(S)e | 0.62 | 0.26 | 0.015 | 25 | 0.46 |
Reduced O-acetyl percentage compared with native W135P. NA, not applicable.
W135P was purified by both phenol extraction and Sepharose CL-6B gel filtration.
W135P was purified by Sepharose CL-6B gel filtration only.
W135P(H), hydrazine-treated W135P.
W135P(S), sonicated W135P.
Antigenicity of GAMP, W135P, W135P(H), and rSEC and their conjugates.
GAMP, W135P, W135P(H), and rSEC each had a precipitation line in immunodiffusion with their corresponding serum (H49, H54, or rabbit anti-SEC). GAMP conjugates showed a line of identity with both H49 and anti-SEC serum, W135P conjugates showed a line of identity with both H54 and anti-SEC sera (data not shown).
SEC-MALLS analysis of rSEC-AH, W135P, and W135P(H) and their conjugates.
rSEC-AH was homogeneous (Pd = 1; molecular size, 31.5 kDa), and the size of W135P was reduced from 689.4 to 63.4 kDa after treatment with hydrazine (Table 2). SEC-MALLS also showed that W135P(H) was more homogeneous than W135P, with polydispersities of 1.265 and 1.932, respectively. Their conjugates, W135-rSEC and W135(H)-rSEC, followed suit. Figure 2 shows the cumulative weight fraction as a function of molar mass. As the value of a sample's Pd approaches 1, indicating a monodispersed sample, the slope of the line increases to infinity; the size and the degree of heterogeneity were lower for W135(H)-rSEC than for W135-rSEC.
FIG. 2.
Cumulative molar mass distribution plot of rSEC-AH, native and hydrazine-treated W135P and their conjugates. (A) Native W135; (B) W135-rSEC; (C) W135(H)-rSEC; (D) rSEC-AH; (E) hydrazine-treated W135P.
Conjugation conditions and rSEC/CP ratio in conjugates.
Four GAMP conjugates and eight W135 conjugates (Table 3) varied in the degree of AH derivatization of rSEC, the molar concentration of CDAP, and the initial polysaccharide and protein concentrations in reactions. At pH 7, GAMP conjugates showed different rSEC/CP ratios (0.85 to 2.96) related to varying AH derivatization of rSEC (1.23 to 2.81), molar concentration of CDAP (0.01 to 0.04), and initial concentration ofpolysaccharide and protein in the reaction mixture (7 to 20 mg/ml). Precipitation occurred using rSEC 5% with AH (data not shown), a CDAP concentration of 0.02 M, and 10 mg/ml of CP and rSEC. Between W135P-rSEC1 and W135P-rSEC2 or between W135P-rSEC3 and W135P-rSEC4 the rSEC/CP ratios were similar (0.61 to 0.67 or 0.90 to 0.94) at both pH 7 and 8 under the same coupling conditions. Using an AH derivative of 5.54% and a CDAP concentration of 0.02 M, the rSEC/CP ratio of W135P(H)-rSEC1 (1.20) was higher than it was in W135P-rSEC (0.90) at pH 8.
Immunogenicity.
All four GAMP-rSEC and eight W135P-rSEC conjugates elicited IgG anti-CP responses, whereas GAMP and W135P alone did not induce detectable CP antibodies after the third injection (Table 3). GM IgG levels for the GAMP conjugates ranged from 26.6 to 80.1 EU. GAMP-rSEC3 had the highest IgG response among GAMP conjugates (80.1 EU), a response significantly different from those of GAMP-rSEC1 and GAMP-rSEC2 (80.1 versus 26.6 and 31.7 EU, P < 0.05). The W135P-rSEC2 conjugate, prepared at pH 8, elicited a statistically higher level of IgG antibodies than W135P-rSEC1 synthesized at pH 7 (W135P-rSEC1) (7.67 versus 0.69 EU, P < 0.05). Between W135P-rSEC3 (8.73 EU) and W135P-rSEC4 (6.62 EU), the difference was not significant. Conjugates of W135P(H)-rSEC5 and W135P(H)-rSEC6 elicited the highest GM IgG levels (18.6 and 12.5 EU) among W135P conjugates, there were significant differences only between W135P(H)-rSEC5 and W135(H)-rSEC6 versus W135P-rSEC1 or W135P(S)-rSEC7 (18.6 and 12.5 versus 0.69 or 2.31 EU, P < 0.05).
Based on the GM IgG levels induced by GAMP and W135P conjugates (Table 3), we formulated the bivalent vaccine composed of GAMP-rSEC3 and W135P(H)-rSEC6, which elicited the highest IgG response among GAMP and W135P conjugates. No detectable IgG responses to GAMP, W135P, and rSEC were observed after the first injection (<0.2 EU) (Table 4). After the second immunization, GM IgG levels to these three components increased significantly (33.2, 2.5, and 5.5 EU for GAMP, W135P, and rSEC, respectively; P < 0.01). The IgG levels of anti-W135P and anti-rSEC showed booster responses after the third injections (2.5 versus 8.5 and 5.5 versus 45.7 EU; P < 0.05). Although the GM IgG levels of anti-GAMP and anti-W135P (39.7 and 8.5 EU) of the bivalent conjugate group were lower than the levels induced by each conjugate alone (80.1 and 12.5 EU), there was no statistical difference between these levels.
TABLE 4.
GM IgG anti-CP and anti-rSEC levels elicited in mice by the bivalent conjugatea
| Antibody | GM (EU)
|
||
|---|---|---|---|
| First injection | Second injection | Third injection | |
| Anti-GAMP | <0.2 | 33.2 | 39.7 |
| Anti-W135P | <0.2 | 2.5 | 8.5 |
| Anti-rSEC | <0.2 | 5.5 | 45.7 |
The bivalent conjugate was composed with GAMP-rSEC3 and W135P(H)-rSEC6 to a final concentration of 25 μg/ml of each CP; each mouse was immunized 0.1 ml. IgG responses in the second and third injections had statistic difference with the corresponding first injections (P < 0.01). Also, for 8.5 versus 2.5 and 45.7 versus 5.5, P < 0.05.
Bactericidal titers.
Sera from groups immunized with GAMP and W135P conjugates showed complement-dependent bactericidal activity against their respective organisms (Table 5). The GM reciprocal titer for GAMP-rSEC3 was 520, and the titers for W135P conjugates were 56, 320, and 394 for W135P-rSEC1, W135P-rSEC3, and W135P(H)-rSEC5, respectively. GAMP-rSEC3, W135P-rSEC1, and W135P(H)-rSEC5 had bactericidal levels that correlated roughly with their IgG levels (r = 0.56 to 0.68). The bactericidal activity of W135P-rSEC3 conjugate, although high, did not correlate (r = 0.26) with its corresponding ELISA IgG level.
TABLE 5.
Correlation between serum IgG anti-CP levels and bactericidal titersa
| Conjugate | IgG (GM, EU) | Reciprocal titer (GM, EU) | r (correlation coefficient) |
|---|---|---|---|
| GAMP-rSEC3 | 80.1 | 520 | 0.68 |
| W135P-rSEC1 | 0.69 | 56 | 0.56 |
| W135P-rSEC3 | 8.73 | 320 | 0.26 |
| W135P(H)-rSEC5 | 18.62 | 394 | 0.58 |
Bactericidal titers were expressed as the reciprocal titer of serum dilution yielding 50% killing compared to the number of bacteria in controls.
SEC neutralization test.
All tested sera had SEC neutralizing activity, ranging from 9.1 to 95.9% (Table 6). Sera 6 to 11 from the bivalent conjugate (after the second injection) with lower IgG EUs had generally lower neutralization values compared to the sera 1 to 5 from the group of W135(S)-rSEC8 with higher GM IgG levels.
TABLE 6.
SEC neutralization assay for the sera from conjugates
| Serum no.a | Avg cpm ± SD | Neutralization (%)b | IgG (EU) |
|---|---|---|---|
| 1 | 8,847 ± 1246 | 91.7 | 20.26 |
| 2 | 18,021 ± 1,831 | 83.2 | 21.92 |
| 3 | 10,068 ± 3,740 | 90.6 | 27.02 |
| 4 | 4,385 ± 98 | 95.9 | 15.16 |
| 5 | 52,684 ± 4,889 | 50.8 | 18.37 |
| 6 | 96,793 ± 82 | 9.6 | 5.71 |
| 7 | 75,315 ± 936 | 29.6 | 10.19 |
| 8 | 48,505 ± 2,358 | 54.7 | 13.45 |
| 9 | 82,623 ± 36 | 22.8 | 10.40 |
| 10 | 97,296 ± 4,173 | 9.1 | 7.39 |
| 11 | 64,437 ± 7,724 | 39.8 | 55.60 |
| 12 | 5,577 ± 2,778 | 94.8 | 100.96 |
| 13 | 71,770 ± 1,840 | 32.9 | 60.12 |
| 14 | 46,801 ± 4,074 | 56.3 | 113.57 |
| Control (−) | 107,023 ± 2,778 | 0.0 | <0.01 |
| Control (+) | 2,448 ± 224 | 97.7 | NDc |
Sera 1 to 5 were obtained from the third injection of W135P(S)-rSEC8 (rSEC was coupled with the sonicated W135P). Sera 6 to 11 were obtained after the second injection of the bivalent conjugate (GAMP-rSEC3 + W135P(H)-rSEC6). Sera 12 to 14 were from the third injection of the bivalent conjugate. Serum in the control (−) was from a normal mouse with no detectable antibodies specific for SEC. Control (+) was a hyperimmune serum from a rabbit immunized with SEC.
The neutralization percent was calculated by comparing the average cpm's in sera of immunized mice with the cpm in control (−).
ND, not determined.
DISCUSSION
As demonstrated by the Haemophilus influenzae type b conjugate vaccine, polysaccharide-protein conjugates are more immunogenic at all ages than the corresponding polysaccharide alone, elicit protective antibody levels in infants, and can be incorporated into the schedule of the Extended Program on Immunization. However, despite the availability of a licensed ACYW135 conjugate vaccine for older children and adults, there are no licensed conjugate vaccines against diseases caused by N. meningitidis group A, B, W135, and Y for children younger than 5 years of age. This is because group A conjugates have not been shown to have an advantage over the polysaccharide vaccine for that age group (8, 14, 19, 21, 22, 27, 33). GAMP vaccine was shown to be immunogenic and protective, including infants and children less than 2 years of age. This was also the case for W135P vaccine for those over the age of 2 years (28). Conjugates of these two CPs could be included in the routine infant vaccinations. Based on the experience with GAMP conjugate (18), we prepared both GAMP and W135P conjugates with CDAP-activated CP coupled to a potentially medically useful carrier, the recombinant SEC from S. aureus. In contrast to the CPs alone, the conjugates were immunogenic and induced both anti-CP and rSEC antibodies. Sera from mice immunized with GAMP and W135P conjugates showed complement-dependent bactericidal activity against their respective organisms and neutralized SEC-induced T-cell proliferative activity. This provides the rationale for considering a clinical evaluation of these conjugates.
One of the problems of producing of GAMP vaccines is the instability of the CP, as exemplified by a GAMP vaccine that afforded no protection in a field trial in Nigeria in 1969 (29): vaccine samples returned from the field showed a considerable decrease in molecular weight of the GAMP, probably due to storage at ambient temperatures. The addition of saccharide, such as lactose, inhibited hydrolysis of GAMP (32). Unlike CP activated at alkaline pH by CNBr, using CDAP-activated CP bound to AH or an AH derivatized protein has advantages in maintaining stability of GAMP and preparation of GAMP conjugates. At neutral pH, the reaction is effective and the phosphate esters of GAMP can be mostly protected during covalent reactions. While a pH 7 is optimal for GAMP conjugate preparation, pH 8 was shown to be favorable for W135P conjugation, probably owing to the stability of W135P at this pH optimal for the CDAP activation. W135P purified by Sepharose Cl-6B gel-filtration satisfied the WHO requirements for both protein and nucleic acid content. This procedure simplifies the process of W135P purification and improves the CP yield. It may be useful in polysaccharide or lipopolysaccharide purification of other bacteria.
SEC-MALLS and refractive index detection are relatively new techniques for measuring the weight average molar mass and polydispersity of capsular polysaccharides. Previous techniques have been limited to low-pressure chromatography, which defines molar mass estimates relative to an external standard, such as dextran. It has been shown that these values can be misleading for polysaccharides due to their polydisperse nature. The molecular weights and distribution profiles measured by SEC-MALLS revealed that the molecular size of hydrazine-treated W135P was more homogeneous and smaller than that of the native W135P. The conjugate prepared with hydrazine-treated W135P had improved immunogenicity and will be considered for clinical evaluation.
O-acetyl moieties influence the immunological properties of the two major pathogenic meningococcal serogroups. The presence of at least 2 mmol/g of O-acetyl is essential for the immunogenicity of GAMP (4, 41). The presence of O-acetyl groups on group B meningococcal polysaccharide, as occurs on OAc+ K1 Escherichia coli CP, renders it less reactive with antisera to the native CP (25). However, O-acetyl negative variants of group C CP have comparable or slightly higher immunogenicity than O-acetyl positive (26, 35). A survey of W135 isolates from Europe indicated that their CP had similar O-acetyl levels (24). Treatment of the W135P with anhydrous hydrazine reduced its O-acetyl content but did not reduce its immunogenicity, thus O-acetyl content may not be considered in the requirements of W135P conjugate vaccine. We also measured the O-acetyl content of native and hydrazine-treated W135P by nuclear magnetic resonance (not shown). The structures and O-acetyl contents of the native and hydrazine-treated W135P were similar. These disparate results may have occurred because of the low content of 7-OAc and 9-OAc that neared the limit of detection by nuclear magnetic resonance.
The bivalent GAMP and W135P conjugates induced significant immune responses against GAMP, W135P and rSEC. These antibodies had bactericidal activity against both GAM and W135 organisms. Multivalent N. meningitidis conjugate vaccine including groups A and W135 along with groups C and Y could be used in all age groups and replace the present tetravalent polysaccharide vaccine.
Acknowledgments
We are grateful to Arthur Karpas for assistance with statistical analysis of data and manuscript review.
This work was supported by grants by the U.S. Public Health Service (grants U54AI57141 and P20-RR15587) and the M. J. Murdock Charitable Trust Foundation (to G.A.B.).
Editor: J. N. Weiser
REFERENCES
- 1.Aguilera, J. F., A. Perrocheau, C. Meffre, S. Hahne, et al. 2002. Outbreak of serogroup W135 meningococcal disease after the Hajj pilgrimage, Europe, 2000. Emerg. Infect. Dis. 8:761-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander, H. E., G. Leidy, and C. Macpherson. 1946. Production of types A, B, C, D, E, and F, Haemophilus influenzae antibody for diagnostic and therapeutic purposes. J. Immunol. 54:207-214. [PubMed] [Google Scholar]
- 3.Bednar, B., and J. P. Hennessey, Jr. 1993. Molecular size analysis of capsular polysaccharide preparations from Streptococcus pneumoniae. Carbohydr. Res. 243:115-130. [DOI] [PubMed] [Google Scholar]
- 4.Berry, D. S., F. Lynn, C. H. Lee, C. E. Frasch, and M. C. Bash. 2002. Effect of acetylation of Neisseria meningitidis serogroup A capsular polysaccharide on development of functional immune response. Infect. Immun. 70:3707-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohach, G. A., and P. M. Schlievert. 1987. Expression of staphylococcal enterotoxin C1 in Escherichia coli. Infect. Immun. 55:428-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohach, G. A., C. J. Hovde, J. P. Handley, and P. M. Schlievert. 1988. Cross-neutralization of staphylococcal and streptococcal pyrogenic toxins by monoclonal and polyclonal antibodies. Infect. Immun. 56:400-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brawley, R. E. 1982. Neisseria meningitidis W135 disease. JAMA 247:2779. [PubMed] [Google Scholar]
- 8.Campagne, G., A. Gaba, P. Fabre, A. Schuchat, R. Ryall, D. Boulanger, M. Bybel, G. Carlone, P. Briantis, B. Ivanoff, B. Xerri, and J.-P. Chippaux. 2000. Safety and immunogenicity of three doses of a Neisseria meningitidis A+C diphtheria conjugate vaccine in infants from Niger. Pediatr. Infect. J. 19:144-150. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention/MMER. 2000. Serogroup W-135 meningococcal disease among travelers returning from Saudi Arabia-United States, 2000. JAMA 283:2647. [PubMed] [Google Scholar]
- 10.Chi, Y. I., I. Sadler, L. M. Jablonski, S. D. Callantine, C. F. Deobald, C. V. Stauffacher, and G. A. Bohach. 2002. Zinc-mediated dimerization and its effect on activity and conformation of staphylococcal enterotoxin type C. J. Biol. Chem. 277:22839-22846. [DOI] [PubMed] [Google Scholar]
- 11.D'ambra, A. J., J. E. Baugher, P. E. Concannon, R. A. Pon, and F. Michon. 1997. Direct and indirect methods for molar-mass analysis of fragments of the capsular polysaccharide of Haemophilus influenzae type b. Anal. Biochem. 250:228-236. [DOI] [PubMed] [Google Scholar]
- 12.Decosas, J., and J. B. Koama. 2002. Chronicle of an outbreak foretold: meningococcal meningitis W135 in Burkina Faso. Lancet Infect. Dis. 2:763-765. [DOI] [PubMed] [Google Scholar]
- 13.Denis, F., J. L. Rey, A. Amadou, P. Saliou, M. Prince-David, S. M'Boup, M. Cadox, I. D. Mar, and J. Etienne. 1982. Emergence of meningitis caused by W 135 subgroup in Africa. Lancet ii:1135-1136. [DOI] [PubMed] [Google Scholar]
- 14.Fairley, C. K., N. Begg, N. Borrow, A. J. Fox, D. M. Jones, and K. Cartwright. 1996. Conjugate meningococcal serogroup A and C vaccine: reactogenicity and immunogenicity in United Kingdom infants. J. Infect. Dis. 174:1360-1363. [DOI] [PubMed] [Google Scholar]
- 15.Gupta, R. K., W. Egan, D. A. Bryla, J. B. Robbins, and S. C. Szu. 1995. Comparative immunogenicity of conjugates of Escherichia coli O111 O-specific polysaccharide, prepared by treatment with acetic acid or hydrazine, bound to tetanus toxoid by two synthetic schemes. Infect. Immun. 63:2805-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hennessey, J. P., Jr., B. Bednar, and V. Manam. 1993. Molecular size analysis of Haemophilus influenzae type b capsular polysaccharide. J. Liquid Chromatogr. 16:1715-1729. [Google Scholar]
- 17.Hestrin, S. 1949. The reaction of acetylcholine and other carboxylic acid derivatives with hydroxylamine, and its analytical application. J. Biol. Chem. 180:249-261. [PubMed] [Google Scholar]
- 18.Jin, Z., C. Chu, J. B. Robbins, and R. Schneerson. 2003. Preparation and characterization of group A meningococcal capsular polysaccharide conjugates and evaluation of their immunogenicity in mice. Infect. Immun. 71:5115-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joseph, H., R. Ryall, M. Bybel, T. Papa, J. MacLennan, J. Buttery, and R. Borrow. 2003. Immunogenicity and immunological priming of the serogroup A portion of a bivalent meningococcal A/C conjugate vaccine in 2-year-old children. J. Infect. Dis. 187:1142-1146. [DOI] [PubMed] [Google Scholar]
- 20.Lapeysonnie, L. 1963. Cerebrospinal meningitis in Africa. Bull. W. H. O. 28(Suppl.):53-114. [Google Scholar]
- 21.Leech, A., P. A. Twumasi, S. Kumah, W. S. Banya, D. E. Libutti, G. N. Carlone, and B. M. Greenwood. 1997. Induction of immunologic memory in Gambian children by vaccination in infancy with a group A plus group C meningococcal polysaccharide-protein conjugate vaccine. J. Infect. Dis. 175:200-204. [DOI] [PubMed] [Google Scholar]
- 22.Lieberman, J. M., S. S. Chiu, V. K. Wong, S. Patridge, S. J. Chang, C. Y. Chiu, L. L. Gheesling, G. M. Carlone, and J. I. Ward. 1996. Safety and immunogenicity of serogroup A/C Neisseria meningitidis oligosaccharide-protein conjugate vaccine in young children. JAMA 275:1499-1503. [PubMed] [Google Scholar]
- 23.Lingappa, J. R., A. M. Al-Rabeah, R. Hajjeh, T. Mustafa, A. Fatani, T. Al-Bassam, A. Badukhan, A. Turkistani, S. Makki, N. Al-Hamdan, M. Al-Jeffri, Y. Al Mazrou, B. A. Perkins, T. Popovic, L. W. Mayer, and N. E. Rosenstein. 2003. Serogroup W-135 meningococcal disease during the Hajj, 2000. Emerg. Infect. Dis. 9:665-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Longword, E., P. Fernstein, T. L. Mininni, U. Vogel, H. Claus, E. Kaczmarski, and R. Borrow. 2002. O-acetylation status of the capsular polysaccharides of systemic infections of groups Y and 135 meningococci from the United Kingdom. FEMS. Immunol. Med. Microbiol. 32:119-123. [DOI] [PubMed] [Google Scholar]
- 25.(swsl)Orskov, F., I. (swsl)Orskov, A. Sutton, R. Schneerson, W. Lin, W. Egan, G. E. Hoff, and J. B. Robbins. 1979. Form variation in Escherichia coli K1: determined by O-acetylation of the capsular polysaccharide. J. Exp. Med. 149:669-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peltola, H., A. Safary, H. Kayhty, V. Karanko, and F. E. Andre. 1985. Evaluation of two tetravalent (ACYW135) meningococcal vaccines in infants and small children: a clinical study comparing immunogenicity of O-acetyl-negative and O-acetyl-positive group C polysaccharides. Pediatr. 76:91-96. [PubMed] [Google Scholar]
- 27.Rennels, M., J. King, R. Ryall, T. Papa, and J. Froeschle. 2004. Dose escalation, safety and immunogenicity study of four dosages of a tetravalent meningococcal polysaccharide diphtheria toxoid conjugate vaccine in infants. Pediatr. Infect. Dis. J. 23:429-435. [DOI] [PubMed] [Google Scholar]
- 28.Robbins, J. B., R. Schneerson, E. C. Gotschlich, I. Mohammed, A. Nasidi, J. P. Chippaux, L. Bernardino, and M. A. Maiga. 2003. Meningococcal meningitis in sub-Saharan Africa: the case for mass and routine vaccination with available polysaccharide vaccines. Bull. W. H. O. 81:745-755. [PMC free article] [PubMed] [Google Scholar]
- 29.Sanborn, W. R., Z. Benčić, B. Cvjetanović, E. C. Gotschlich, and T. M. Pollock. 1972. Trial of a serogroup A meningococcus polysaccharide vaccine in Nigeria. Prog. Immunobiol. Standard 5:497-505. [PubMed] [Google Scholar]
- 30.Szu, S. C., G. Zon, R. Schneerson, and J. B. Robbins. 1986. Ultrasonic irradiation of bacterial polysaccharide: characterization of the depolymerized products and some applications of the process. Carbohydr. Res. 152:7-20. [DOI] [PubMed] [Google Scholar]
- 31.Taha, M. K., I. Parent Du Chatelet, M. Schlumberger, I. Sanou, S. Djibo, F. de Chabalier, and J. M. Alonso. 2002. Neisseria meningitidis serogroups W135 and A were equally prevalent among meningitis cases occurring at the end of the 2001 epidemics in Burkina Faso and Niger. J. Clin. Microbiol. 40:1083-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiesjema, R., E. C. Beuvery, and B. J. te Pas. 1977. Enhanced stability of meningococcal polysaccharide vaccine by using lactose as a menstrum for lyophilization. Bull. W. H. O. 55:43-48. [PMC free article] [PubMed] [Google Scholar]
- 33.Twumasi, P. A., S. Kumah, A. Leach, T. J. D. O'Dempsey, S. J. Ceesay, J. Todd, C. V. Broome, G. M. Carlone, L. B. Pais, P. K. Holder, B. D. Plikaytis, and B. M. Greenwood. 1995. A trial of group A plus group C meningococcal polysaccharide-protein conjugate vaccine in African infants. J. Infect. Dis. 171:632-638. [DOI] [PubMed] [Google Scholar]
- 34.Vann, W. F., T.-Y. Liu, and J. B. Robbins. 1976. Bacillus pumilus polysaccharide cross-reactive with meningococcal group A polysaccharide. Infect. Immun. 13:1654-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vodopija, I., Z. Baklaic, P. Hauser, P. Roelants, F. E. André, and A. Safary. 1983. Reactvity and immunogenicity of bivalent (AC) and tetravalent(ACW135Y) menngococcal vaccines containing O-acetyl-negative or O-acetyl-positive group C polysaccharide. Infect. Immun. 42:599-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilder-Smith, A., T. M. Barkham, S. K. Chew, and N. I. Paton. 2003. Absence of Neisseria meningitidis W-135 electrophoretic type 37 during the Hajj, 2002. Emerging Infect. Dis. 9:734-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization. 1999. Group A and C meningococcal vaccines. Wkly. Epidemiol. Rec. 74:297-303. [PubMed] [Google Scholar]
- 38.World Health Organization. 2001. Meningococcal disease, serogroup W135. Wkly. Epidemiol. Rec. 76:141-142. [PubMed] [Google Scholar]
- 39.World Health Organization. 2002. Meningococcal disease, serogroup W135, Burkina Faso: preliminary report. Wkly. Epidemiol. Rec. 77:152-155. [PubMed] [Google Scholar]
- 40.World Health Organization. 2002. Urgent call for action on meningitis in Africa-vaccine price and shortage are major obstacles. Wkly. Epidemiol. Rec. 77:330-331. [PubMed] [Google Scholar]
- 41.World Health Organization. 1976. Requirements for meningococcal polysaccharide vaccine. Tech. Rep. Ser. 594:50-75. [Google Scholar]
- 42.World Health Organization. 1980. Requirements for meningococcal polysaccharide vaccine. Tech. Rep. Ser. 658:174-175. [Google Scholar]