Abstract
Malaria and tuberculosis are endemic in many regions of the world, and coinfection with the two pathogens is common. In this study, we examined the effects of long- and short-term infection with Mycobacterium tuberculosis on the course of a lethal form of murine malaria in resistant (C57BL/6) and susceptible (BALB/c) mice. C57BL/6 mice coinfected with M. tuberculosis CDC1551 and Plasmodium yoelii 17XL had a lower peak parasitemia and increased survival compared to mice infected with P. yoelii 17XL alone. Splenic microarray analysis demonstrated potentiation of type 1 immune responses in coinfected C57BL/6 mice, which was especially prominent 5 days after infection with P. yoelii 17XL. Splenocytes from coinfected C57BL/6 mice produced higher levels of gamma interferon (IFN-γ) and tumor necrosis factor alpha than splenocytes from mice infected with either pathogen alone. Interestingly, mycobacterium-induced protection against lethal P. yoelii is mouse strain specific. BALB/c mice were significantly more susceptible than C57BL/6 mice to infection with P. yoelii 17XL and were not protected against lethal malaria by coinfection with M. tuberculosis. In addition, M. tuberculosis did not augment IFN-γ responses in BALB/c mice subsequently infected with P. yoelii 17XL. These data indicate that M. tuberculosis-induced potentiation of type 1 immune responses is associated with protection against lethal murine malaria.
Malaria and tuberculosis are major causes of morbidity and mortality worldwide, each accounting for over 2 million deaths a year (43, 61). There are approximately 500 million new cases of clinical malaria (53) and 8 million cases of active tuberculosis per year, and it is estimated that one-third of the world's population, or nearly 2 billion people, have latent tuberculosis infection (43). Given the significant geographic overlap between areas endemic for these two diseases, coinfection with tuberculosis and malaria is likely to be common.
The clinical manifestations of malaria may range from life-threatening anemia or neurologic involvement to asymptomatic infection. Although acquired immunity plays a significant role in determining the severity of disease, other host and environmental factors likely contribute to the variability in clinical outcomes (3). In coendemic areas, many children at risk for severe malaria have had prior mycobacterial infections, either from routine vaccination with live-attenuated Mycobacterium bovis BCG (bacillus Calmette-Guerin) or from exposure to Mycobacterium tuberculosis or environmental mycobacteria. The modulation of host immune responses to Plasmodium by concurrent mycobacterial infections remains poorly understood.
M. tuberculosis is a potent inducer of type 1 immune responses, characterized by high levels of gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) (24). The importance of cell-mediated immunity in the containment of M. tuberculosis has been demonstrated by the accelerated deaths of IFN-γ-knockout mice from uncontrolled tuberculosis (12). In clinical practice, dramatic increases in tuberculosis rates followed the onset of the human immunodeficiency virus epidemic, especially in patients with declining CD4 counts (24). Furthermore, individuals treated with the TNF-α antagonist, infliximab, have a markedly increased risk of developing active tuberculosis (26).
In contrast, the role of cell-mediated immunity in host responses to malaria is less well defined. Murine studies show that although an initial type 1 immune response is important in controlling malaria, unregulated type 1 responses can lead to immunopathology. Subsequently, a switch to a type 2 response is required for complete clearance of the parasite (31). Mice with defects in cell-mediated immunity, such as IFN-γ-knockout mice, succumb to infection with Plasmodium chabaudi (56), and recombinant IL-12 improves survival of mice infected with a lethal strain of P. chabaudi (55). Nonlethal strains of Plasmodium yoelii elicit stronger early type 1 responses than lethal P. yoelii (9, 51). However, proinflammatory cytokines can also exacerbate malaria-induced pathology. Neutralization of transforming growth factor β (TGF-β), a regulator of inflammation that decreases IFN-γ levels, increases the severity of malaria in mice infected with P. chabaudi or Plasmodium berghei (40). Furthermore, the appearance of neurologic signs of cerebral malaria after infection with P. berghei ANKA is associated with increased production of TNF-α and IFN-γ (16, 18). Depletion of IFN-γ prevents the development of cerebral malaria (17).
In areas of the world where multiple infections are endemic, coinfection with unrelated pathogens is common, and there are several examples where infection with one organism can modulate immune responses elicited by an unrelated pathogen (11, 25, 33, 34, 41). Previous work from our laboratory showed that malarial infection resulted in decreased containment of chronic tuberculosis infection in mice (48). Protection against Plasmodium knowlesi, Plasmodium cynomolgi, and Plasmodium inui in nonhuman primates infected with M. tuberculosis has been described (5, 52), and vaccination with M. bovis BCG protects mice against Plasmodium vinckei, P. yoelii, P. berghei, and P. chabaudi (6, 37, 54). In contrast, helminthic infections tend to worsen the outcome of P. chabaudi (15, 21) and P. yoelii (32, 38). However, the immunologic mechanisms underlying these phenomena have not been well elucidated.
We hypothesized that the type 1 immune response induced by mycobacteria can modulate the course of malaria. In this study, we evaluated the effects of short- and long-term exposure to M. tuberculosis on the course of infection and immune response after challenge with the lethal strain of P. yoelii (17XL) in C57BL/6 and BALB/c mice. Transcriptional and protein expression patterns revealed that protection against lethal malaria in C57BL/6 mice is associated with an enhanced type 1 immunity induced by M. tuberculosis infection. In striking contrast, neither short- nor long-term exposure of susceptible BALB/c mice to M. tuberculosis resulted in enhanced type 1 immunity and protection from challenge with P. yoelii 17XL.
MATERIALS AND METHODS
Mice.
Pathogen-free female C57BL/6 and BALB/c mice (6 to 8 weeks old) were obtained from Charles Rivers Laboratories (Raleigh, NC) and housed in a Biosafety Level 3 animal facility. Animals were kept in microisolator cages and provided food and water ad libitum. The Johns Hopkins Animal Care and Use Committee approved the experimental protocols used in this study.
M. tuberculosis CDC1551 was passaged once through mice and then grown in Middlebrook 7H9 medium (Difco) supplemented with 10% oleic acid-albumin-dextrose-catalase and 0.05% Tween 80. Once a concentration of 5 × 108 organisms/ml was reached, the mycobacteria were vortexed with large glass beads for 1 min and allowed to settle for 20 min. The supernatants were harvested, suspended in 10% glycerol, aliquoted, and stored at −70°C. For aerosol infections, the samples were thawed and diluted 100-fold in phosphate-buffered saline (PBS).
P. yoelii 17XL.
Parasitized erythrocytes from infected BALB/c or C57BL/6 mice were stored in glycerol, aliquoted, and frozen in liquid nitrogen. Prior to being used for experimental infections, P. yoelii 17XL was passaged three times in naïve C57Bl/6 or BALB/c mice.
M. tuberculosis infection.
Mice were infected in a Middlebrook Exposure Inhalation system (Glas-col Inc., Terre Haute, IN), using a low-dose aerosol exposure to M. tuberculosis CDC1551 (5 × 106 cells/ml). Uninfected control mice were kept in the same facility as M. tuberculosis-infected mice throughout the experiments. Five mice were sacrificed 1 day after aerosolization to quantify the number of mycobacteria deposited in the lung. Mice were also sacrificed 2 and 6 weeks postinfection to determine the organ burden of disease. Spleens and lungs were homogenized in 1 ml of PBS, using Ten-Broek homogenizers. Serial dilutions were plated on Middlebrook 7H10 agar supplemented with oleic acid, albumin, dextrose catalase enrichment media, and 5% glycerol and incubated at 37°C for 5 weeks for the enumeration of CFU.
P. yoelii infection.
Two weeks after being infected by aerosol with M. tuberculosis CDC1551, mice were infected by intraperitoneal injection with 105 P. yoelii 17XL-parasitized erythrocytes suspended in PBS. Parasitemia was monitored by daily examination of Giemsa-stained thin smears of whole blood (47).
P. yoelii antigen preparation.
P. yoelii 17XL-infected blood (∼50% parasitemia) was washed three times and diluted in PBS to a final concentration of 108 parasitized erythrocytes/ml. The infected cells were lysed by repeated freeze-thaw cycles, using liquid nitrogen, and the resulting lysate was stored at −80°C.
Cytokine production.
Spleens from the five mice in each experimental group were harvested and homogenized into single-cell suspensions. Spleen cells from each group were pooled and washed in RPMI 1640 medium supplemented with 2% heat-inactivated fetal calf serum (Sigma). The erythrocytes were lysed with NH4Cl-Tris solution, and the cells were washed twice and resuspended in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 1% glutamine, and 1% penicillin-streptomycin. Cells (5 × 106 cells/well) were aliquoted in triplicate into 24-well tissue culture plates and cultured at 37°C in 5% CO2 for 24 h in the presence or absence of phytohemoagglutinin (PHA; 10 μg/ml; Sigma), Old Tuberculin (10 μg/ml; Colorado Serum Co.), P. yoelii 17XL lysate (106 parasite equivalent), or erythrocyte lysate (control). Cell culture supernatants were harvested at 24°, centrifuged at 13,000 rpm for 5 min to remove cellular debris, aliquoted, and stored at −80°C.
Cytokine ELISAs.
Indirect sandwich enzyme-linked immunosorbent assays (ELISAs; Quantikine) for IFN-γ and TNF-α were conducted according to the manufacturer's instructions, using pairs of capture and detection antibodies (R&D Systems).
Splenic microarray analysis.
Three C57BL/6 mice from the four experimental groups (uninfected, infected with tuberculosis, infected with malaria, and infected with malaria and tuberculosis) were sacrificed at days 1, 3, and 5 after infection with P. yoelii 17XL. A portion of each spleen was immediately snap frozen in liquid nitrogen and stored at −80°C for RNA isolation. For RNA extraction, spleens were manually homogenized in 1 ml Trizol (Invitrogen) and subsequently processed according to the manufacturer's protocol with the following modifications: 5 micrograms of glycogen was used as a carrier for isopropanol precipitation, and all centrifugation times were extended to 15 min. RNA pellets were resuspended in 100 μl of nuclease-free water, and concentrations were determined using spectophotometry (DU640; Beckman). Quality assessment was done by RNA Nano LabChip analysis on a Bioanalyzer (model 1200; Agilent). A QIAGEN RNeasy total cleanup protocol was subsequently performed, followed by requantitation by spectrophotometry.
Processing of templates for analysis on the Murine Genome MOE430A GeneChip was done in accordance with methods described in the Affymetrix GeneChip Expression Analysis Technical Manual, Revision Three. Splenic RNA from two mice for each time point and condition were hybridized into the arrays. Gene expression data was preprocessed using the Affymetrix default settings and imported into GeneSpring 6.2.1 or 7.2 (Agilent Technologies) for further analysis. Gene expression patterns for each gene were normalized to the median array intensity for all chips, and expression data from infected animals were normalized to the expression levels from the uninfected control animals.
Real time PCR.
Custom primer sets were generated for each target gene using Primer Express software (Applied Biosystems): IFN-γ, 5′-GCTGCTGATGGGAGGAGATG-3′ and 5′-TGTCTGGCCTGCTGTTAAAGC-3′; IL-4, 5′-GGAGATGGATGTGCCAAACG-3′ and 5′-CGAGCTCACTCTCTGTGGTGTT-3′; TGF-β, 5′-CTGGGACCCTGCCCCTATAT-3′ and 5′-GGGCAAGGACCTTGCTGTAC-3′; IL-12, 5′-ACGCAGCACTTCAGAATCACA-3′ and 5′-CACCAGCATGCCCTTGTCTA-3′; IL-10, 5′-TCTATTCTAAGGCTGGCCACACT-3′ and 5′-CAATTGAAAGGACACCATAGCAAA-3′; and α-actin, 5′-AGCCCCATGTGCCTTGTC-3′ and 5′-TGCCCTCTGCTGGACTTCTT-3′.
Total splenic RNA from four mice per group was reverse transcribed using the SuperScript First-Strand Synthesis system for reverse transcription (RT)-PCR (Invitrogen). cDNA was amplified for real-time detection with iQ Supermix containing SYBR green, according to the manufacturer's protocol (Bio-Rad). Standard curves were generated, and quantities of each transcript were normalized to the levels of the α-actin. Expression levels for each gene relative to the values from the uninfected controls were reported.
Statistical analysis.
Kruskal-Wallis analysis of variance was used to compare gene expression patterns, and mean differences were considered statistically significant if P was <0.05. Differences in survival were analyzed using the log rank test. Unpaired t tests were performed to assess differences in cytokine production and mean parasitemia.
Additional data posted online.
A table listing the genes of the immune system differentially expressed in coinfected mice compared to those expressed in mice with malaria only can be found at https://jshare.johnshopkins.edu/kpage2/public_html. Gene expression levels from splenic microarrays of mice infected with P. yoelii 17XL, M. tuberculosis, or both at three different time points normalized to those of the uninfected controls are shown.
RESULTS
Enhanced survival of C57BL/6 mice coinfected with M. tuberculosis and P. yoelii 17XL compared to that of mice infected with P. yoelii 17XL alone.
To assess the impact of an ongoing infection with M. tuberculosis on the parasitic and immunologic outcome of P. yoelii 17XL malaria, a model system was established (Fig. 1). Mice were infected with M. tuberculosis CDC 1551 via a low-dose aerosol challenge that delivered 30 to 50 CFU to the lungs of each mouse. At days 14 or 52 postinfection with M. tuberculosis, mice were challenged with 105 P. yoelii 17XL-parasitized erythrocytes by intraperitoneal injection. Control groups included mice with M. tuberculosis-only infections or P. yoelii 17XL-only infections and noninfected animals.
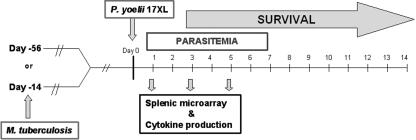
FIG. 1.
Experimental design. Two groups of mice were aerosol infected with M. tuberculosis on day −14 or day −56. On day 0, one group of mice with M. tuberculosis and another group of uninfected mice were given intraperitoneal injections with P. yoelii 17XL. Ten mice per group were followed for survival and parasitemia. Five mice per group were sacrificed at days 1, 3, and 5 after infection with P. yoelii for microarray analysis of the spleen and analysis of cytokine production.
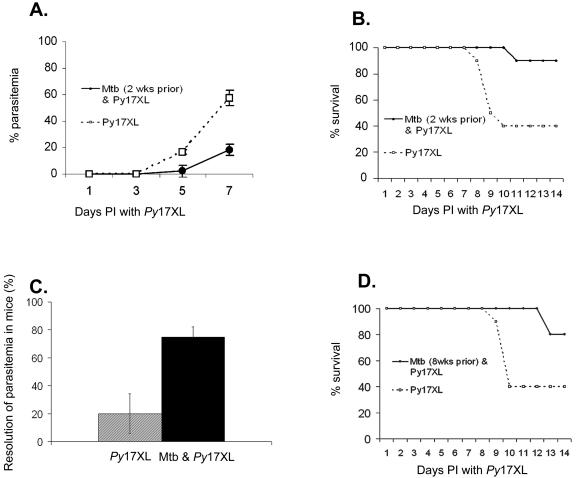
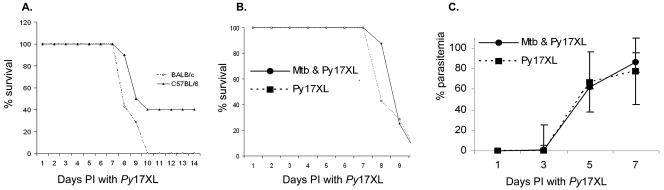
C57BL/6 mice infected with P. yoelii 17XL alone developed a rapidly progressing parasitemia, reaching a level of >50% parasitized erythrocytes at 7 days (Fig. 2A). In contrast, the parasitemia in coinfected animals rose at a slower rate and reached only 18% at day 7. The lower rate of parasitemia in coinfected mice was associated with increased survival. Two weeks after being infected with malaria, 90% of the coinfected animals were alive compared to 40% of the animals infected with P. yoelii 17XL alone (Fig. 2B). Furthermore, 28 days after infection, 80% of the coinfected animals resolved their parasitemia completely, compared to 20% of the mice that received only P. yoelii 17XL (Fig. 2C). After resolution of the malaria, the recovered animals from both groups were resistant to rechallenge with P. yoelii 17XL (data not shown).
FIG. 2.
M. tuberculosis infection protects C57BL/6 mice against lethal infection with P. yoelii 17XL. Two weeks after being infected with M. tuberculosis CDC1551 by low-dose aerosol, mice were infected intraperitoneally with 105 P. yoelii 17XL-parasitized erythrocytes. Control mice were infected with P. yoelii 17XL alone. (A) Results for coinfected C57BL/6 mice (Mtb and Py17XL, circles) and C57BL/6 mice infected with P. yoelii 17XL (Py17XL, squares) alone (P value = 0.007) are shown. (B) Improved survival rate in C57BL/6 mice coinfected with Mtb and Py17XL (P value = 0.026) is indicated. (C) Coinfected C57BL/6 mice had complete resolution of parasitemia compared to those with malaria alone. (D) Chronic infection with M. tuberculosis also protects C57BL/6 mice against infection with P. yoelii 17XL. Eight weeks after being infected with M. tuberculosis CDC1551 by low-dose aerosol, mice were infected intraperitoneally with 105 P. yoelii 17XL-parasitized erythrocytes. Control mice were infected with P. yoelii 17XL alone. The data shown is representative of 3 experiments with 10 mice included in each group. Bars represent the means ± the standard errors of the means.
In the murine model, the first 3 weeks of infection with M. tuberculosis represent a period of exponential mycobacterial growth, which is thought to mimic acute tuberculosis infection in humans. In our mice, the average burden of disease 2 weeks after low-dose aerosol infection was 106 CFU per lung and 104 CFU per spleen. Since most M. tuberculosis infections in humans are chronic in nature, it was of interest to determine the impact that a longer-term exposure to M. tuberculosis would have on the ability of C57Bl/6 mice to respond to subsequent infection with malaria. Mice infected with M. tuberculosis 8 weeks prior to infection with P. yoelii 17XL exhibited a higher survival rate (80%) after infection with P. yoelii 17XL than mice infected with P. yoelii 17XL alone (40% survival rate) (Fig. 2D). Thus, the M. tuberculosis-induced changes that result in protection against challenge with malaria are established during the first 2 weeks after the bacteria are instilled in the lungs, and they persist for at least 8 weeks.
M. tuberculosis significantly enhances expression of genes encoding proteins associated with type 1 immune responses.
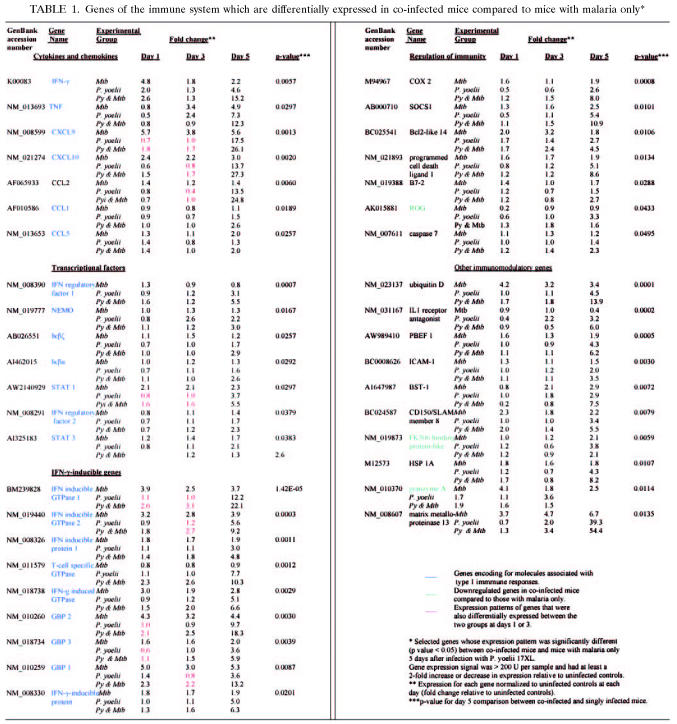
Microarray-based expression analyses of cells from the spleens of coinfected and singly infected animals were used to determine whether the observed alterations in parasitological outcome could be correlated with differences in the expression of genes associated with immunity. Gene expression in the spleens of mice infected with M. tuberculosis only showed upregulation of several proinflammatory genes associated with type 1 responses, including IFN-γ, TNF-α, STAT 1, and other IFN-γ-inducible genes (Table 1).
TABLE 1.
Genes of the immune system which are differentially expressed in co-infected mice compared to mice with malaria only*
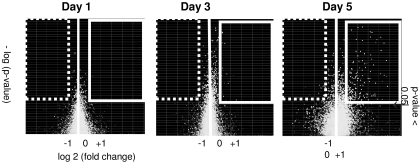
To specifically evaluate immune responses that could account for the differences in malaria outcomes observed, our analysis focused on the comparison between coinfected animals and those infected with P. yoelii alone. Of the ∼34,000 genes represented on the array, 14,480 (∼42%) had detectable transcripts in at least one of the samples. Of these, 231 had significant (P < 0.05) differences in expression levels between coinfected mice and those infected with malaria only. The raw gene expression signal was at least 200 U in 202 of 231 genes, and 144 of 202 genes had at least a twofold difference in expression from that of the uninfected controls (Fig. 3). Of these 144 genes, 75 genes encoded proteins with ascribed immune function. Subsequent analysis was based on the examination of the genes shown in Table 1 (40 out of 75 genes) and in the table found at https://jshare.johnshopkins.edu/kpage2/public_html (complete list of the 75 genes).
FIG. 3.
Volcano plots of levels of differentially expressed genes in coinfected mice compared to those in mice with malaria only at days 1, 3, and 5 after infection with P. yoelii 17XL. The horizontal axis represents fold regulation, and the vertical axis represents the P value. The genes enclosed in the white box are greater than twofold upregulated (P < 0.05), and the genes enclosed in the dashed box are greater than twofold downregulated compared to genes expressed in mice with malaria only. The number of significantly upregulated genes is greatest 5 days after infection with P. yoelii 17XL. Relatively few genes are downregulated in coinfected mice compared to those in mice with malaria only.
Overall, coinfected mice had an enhanced inflammatory response compared to mice with malaria alone. A majority of the 75 genes were upregulated in the spleens of both coinfected and malaria-only mice compared to those of the uninfected controls; however, the degree of upregulation was significantly higher in spleens from coinfected animals, particularly at day 5 (Table 1). We observed significant upregulation of genes associated with proinflammatory responses in coinfected animals 5 days after infection with P. yoelii 17XL, including chemokines implicated in neutrophil recruitment (CCL2 and CX3CL1), heat shock protein 1A, pre-B cell colony-enhancing factor 1 (PBEF1), and matrix metalloproteinases. Furthermore, there was a dramatic upregulation of cytokines, chemokines, and transcriptional factors associated with type 1 immune responses, such as IFN-γ, TNF-α, CCL5, CXCL9, CXCL10, STAT 1 and STAT 3, IFN regulatory factors 1 and 2, NF-κB essential modulator, Iκβζ, Iκβα, and other IFN-γ-inducible genes (Table 1).
Only six genes with ascribed immune function were downregulated in coinfected mice compared to levels in mice infected with malaria only, three of which encode for regulatory molecules that inhibit T-cell activation. ROG (repressor of GATA) expression peaked 5 days after infection with P. yoelii 17XL in mice with malaria only but was not overexpressed in coinfected mice at any time point. Although the gene encoding the FK506 binding protein was upregulated in both groups at day 5 after P. yoelii 17XL infection, gene expression was significantly higher in mice with malaria alone. Likewise, the gene encoding granzyme A, a cytolytic enzyme associated with caspase-independent apoptosis, was more highly expressed at day 5 in singly-infected animals.
However, a feedback loop leading to downregulation of inflammation was also apparent in coinfected mice. Five days after infection with P. yoelii 17XL, suppressor of cytokine signaling-1 (SOCS-1), caspase 7, and members of the B7 family (B7-2 and PD-L1), which play a role in regulating T-cell activation and tolerance (19), were upregulated in coinfected mice compared to levels in mice infected with malaria only. Increased expression of COX-2 in coinfected animals suggests activation of prostaglandin-mediated anti-inflammatory pathways, which are associated with reduced pathology in both murine and human malaria (4, 42).
Real-time PCR for microarray validation.
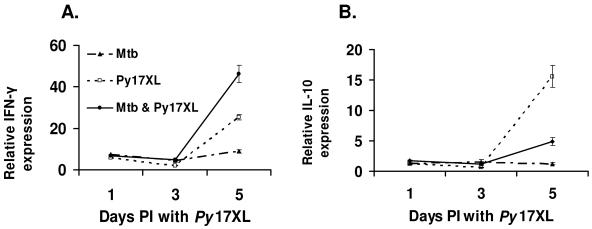
We performed real time RT-PCR using RNA samples from four mice per group, two of which had been also tested by the arrays. In concordance with our microarray data, 5 days after mice were infected with P. yoelii 17XL, IFN-γ expression was higher in the coinfected animals than in those with malaria alone (P = 0.003) (Fig. 4A). As indicated by the gene array results, transcription of interleukin-4 (IL-4), IL-12, and TGF-β was not differentially changed in any of the treatment groups (data not shown). Differences in IL-10 expression were not captured by our microarray analysis, due to the low raw gene expression signal (<200) of IL-10. However, real-time PCR showed a significant upregulation of IL-10 with progression of malaria in mice infected with P. yoelii 17XL only compared to coinfected or M. tuberculosis-only animals (P = 0.009) (Fig. 4B).
FIG. 4.
Gene expression patterns (determined by real-time PCR) of IFN-γ (A) and IL-10 (B) in spleens of C57BL/6 mice infected with M. tuberculosis (Mtb), P. yoelii 17XL (Py17XL), or both (Mtb & Py17XL). Gene expression data from infected animals were normalized to the expression levels of uninfected controls sacrificed at each time point.
Enhanced in vitro INF-γ and TNF-α production by splenocytes from coinfected mice.
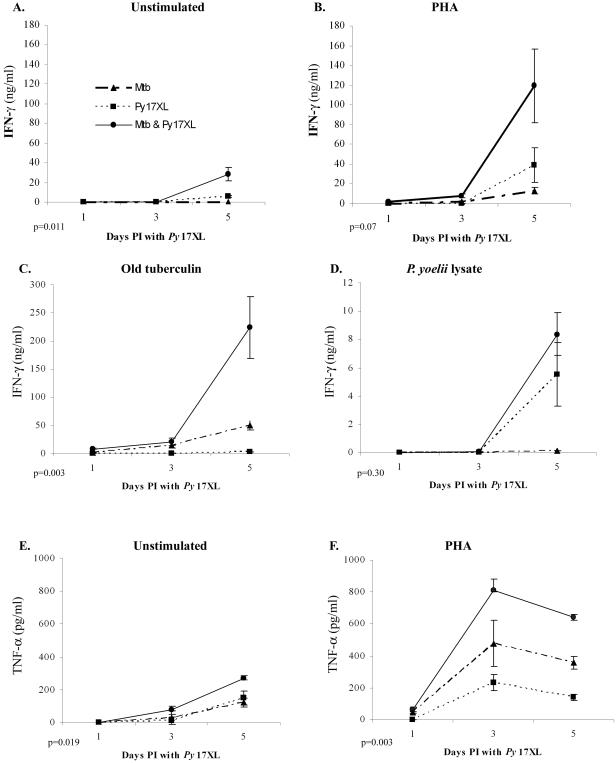
To determine whether the enhanced transcription identified by microarray and real-time RT-PCR analyses resulted in an increase in translation of the protein products, IFN-γ and TNF-α secretion was measured from the splenocytes of coinfected, M. tuberculosis-only and P. yoelii 17XL-only C56BL/6 mice at days 1, 3, and 5 post-malaria challenge (Fig. 5). On days 1 and 3 after infection with P. yoelii 17XL, IFN-γ secretion in coinfected mice was below the level of detection in unstimulated and PHA-stimulated splenocytes and was detected at low levels in splenocytes stimulated with tuberculin antigen (9 ng/ml on day 1 and 21 ng/ml on day 3). In parallel with the transcription results (Table 1, Fig. 4), there was higher IFN-γ production in coinfected animals at day 5, when the cells were cultured without stimulation (P = 0.01) or stimulated with PHA (P = 0.07) (Fig. 5A and B). The lower responses from the cells when exposed to crude malaria antigen presumably reflect a combination of a low number of antigen-specific cells and the short incubation time of the assay (supernatants were collected at 24 h). Interestingly, stimulation with tuberculin antigen resulted in significantly more IFN-γ production from spleen cells from coinfected mice than from those isolated from M. tuberculosis-only animals (P = 0.003) (Fig. 5C). As expected, there was no IFN-γ production by spleen cells from mice infected with malaria only after stimulation with tuberculin antigen.
FIG. 5.
Enhanced IFN-γ and TNF-α production in splenocytes from mice coinfected with M. tuberculosis and P. yoelii 17XL compared to that in splenocytes from mice infected with P. yoelii 17XL alone, as measured by ELISA. Five mice per group were sacrificed at each time point. IFN-γ secretion by unstimulated splenocytes (A) and splenocytes stimulated with PHA (B), old tuberculin (C), P. yoelii lysate (D), and TNF-α secretion by unstimulated (E) and PHA-stimulated (F) splenocytes was measured in the supernatants collected after 24 h of incubation. Mtb, mice infected with M. tuberculosis; Py17XL, mice infected with P. yoelii 17XL; Mtb & Py17XL, mice infected with M. tuberculosis and P. yoelii 17XL. PI, postinfection.
The patterns of TNF-α protein secretion at days 3 and 5 post-P. yoelii 17XL infection (Fig. 5E) were not totally consistent with the transcriptional profiles detected on gene arrays (Table 1). While there are inconsistencies between the protein and transcription data, the trend for the coinfected animals to have the most vigorous response holds for TNF-α as well (P = 0.019, unstimulated; P = 0.003, PHA-stimulated).
BALB/c mice are more susceptible to infection with P. yoelii 17XL than C57BL/6 mice and are not protected by coinfection with M. tuberculosis.
Next, we sought to determine the degree to which the enhanced type 1 immune response observed in coinfected animals might play a role in the outcome of malaria infection. The high susceptibility of IFN-γ- or inducible nitric oxide gene-deletion mice to M. tuberculosis (12) precluded their use in our experiments. Instead, we used BALB/c mice, which are known to be more susceptible to M. tuberculosis than C57BL/6 mice but do not succumb to the disease. BALB/c mice mount a weaker type 1 response than C57BL/6 mice to tuberculosis, with higher expression of type 2-associated cytokines in their spleens (23, 27).
BALB/c mice were infected with P. yoelii 17XL at 2 or 8 weeks, following a low-dose aerosol exposure to M. tuberculosis. At the time P. yoelii 17XL was injected, groups of M. tuberculosis-uninfected BALB/c and C57BL/6 mice were also infected with malaria. BALB/c mice were significantly more susceptible to P. yoelii 17XL alone than C57BL/6 mice, with a higher parasitemia at day 7 (mean, 87% and 58%, respectively) and 100% mortality by day 10 (Fig. 6A). In contrast to C57BL/6 mice, BALB/c mice received no protection against malaria through coinfection with M. tuberculosis. Singly and dually infected BALB/c mice had equally high parasitemia (Fig. 6B) and mortality (Fig. 6C). Similar results were obtained when BALB/c mice were chronically infected with M. tuberculosis for 8 weeks prior to infection with P. yoelii 17XL (data not shown).
FIG. 6.
M. tuberculosis infection does not protect BALB/c mice against infection with P. yoelii 17XL. Two weeks after being infected via aerosol with low-dose M. tuberculosis CDC1551, mice were infected intraperitoneally with 105 P. yoelii 17XL (Py17XL)-parasitized erythrocytes. Control BALB/c and C57BL/6 mice were infected with P. yoelii 17XL only. (A) Mortality 14 days after mice were infected with P. yoelii 17XL was significantly higher in BALB/c (diamonds) mice than in C57BL/6 (triangles) mice (P = 0.001). (B) Parasitemia in coinfected BALB/c mice (Mtb & Py17XL, circles) and mice infected with P. yoelii 17XL (squares) was greater than 80% by day 7 in both groups. (C) By day 10, 100% mortality was observed in both groups. The data shown is representative of two experiments with 10 mice included in each group. Bars represent the means ± the standard errors of the means.
Gene expression pattern in spleens from BALB/c mice.
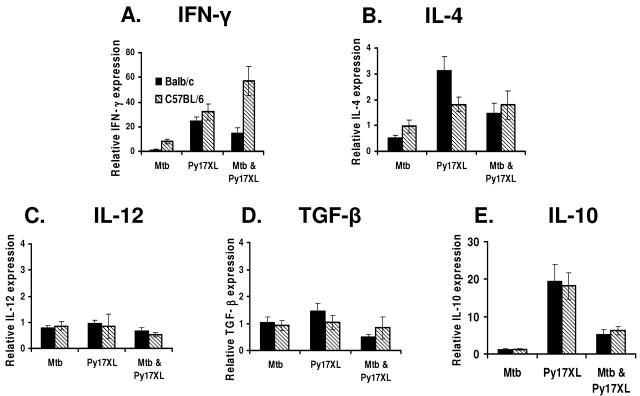
To evaluate differences in the immune responses of BALB/c and C57BL/6 mice under the challenge conditions used here, the transcription of a select group of cytokines, IFN-γ, IL-4, IL-12, IL-10, and TGF-β, from the spleen cells isolated 5 days post-malaria challenge was evaluated (Fig. 7). The relative expression levels of IL-12, TGF-β, and IL-10 were similar between BALB/c and C57BL/6 mice harboring infection with a single pathogen or a coinfection. In mice infected with P. yoelii 17XL only, IL-4 expression was significantly higher (P = 0.015) in BALB/c mice than in C57BL/6 mice (Fig. 7 B), consistent with published results (22). However, IL-4 expression levels were comparable in coinfected BALB/c and C57BL/6 mice, a result consistent with the known ability of M. tuberculosis to induce Th1 responses and the capacity of Th1 responses to counterregulate Th2 responses.
FIG. 7.
Cytokine splenic expression pattern (real-time-PCR) in BALB/c and C57BL/6 mice infected with M. tuberculosis (Mtb), P. yoelii 17XL (Py17XL), or both (Mtb & Py17XL). Mice were infected with M. tuberculosis CDC1551 2 weeks prior to being infected with P. yoelii 17XL. Spleens were harvested on day 5 after the mice were infected with P. yoelii 17XL, and the gene expression data from the infected animals were normalized to the expression levels from the same-strain uninfected controls.
In animals infected with M. tuberculosis alone, IFN-γ expression was slightly elevated in spleen cells from C57BL/6 mice but nearly undetectable in the cells from BALB/c mice (Fig. 7A). P. yoelii 17XL-only infection resulted in similar levels of INF-γ transcription in the spleen cells from both mouse strains, but coinfection effected a dramatic increase in IFN-γ expression in C57BL/6 mice compared to that in BALB/c mice (P ≤ 0.01). These results are consistent with the notion that type 1 immune responses are attenuated in BALB/c mice compared to those in C57BL/6 mice and suggest that the different malarial outcomes observed in coinfected BALB/c and C57BL/6 mice depend on the ability of M. tuberculosis to potentiate type 1 responses to P. yoelii 17XL in susceptible and resistant strains of mice.
DISCUSSION
In a murine model of malaria and tuberculosis coinfection, we demonstrate significant protection against infection with P. yoelii 17XL in C57BL/6 mice previously infected with M. tuberculosis. Coinfected animals had a lower parasitemia and increased survival and displayed enhanced type 1 immune responses compared to mice infected with P. yoelii 17XL alone. Our findings for C57BL/6 mice are consistent with older studies showing protection against malaria in animals infected with mycobacteria (5, 6, 35, 52, 54). A previous study evaluating coinfection in different strains of mice found that treatment with BCG protected both genetically resistant and susceptible mice against P. chabaudi (54). In contrast, we show that susceptible BALB/c mice are not protected against challenge with P. yoelii 17XL by prior infection with M. tuberculosis. This is the first study to demonstrate host-related differences in the immunopathologic outcome of Plasmodium and Mycobacterium coinfection. Our findings indicate that potentiation of type 1 immune responses plays a protective role in coinfected resistant mice.
Analysis of the gene expression profiles in spleens of infected C57BL/6 mice showed that many genes encoding proinflammatory molecules were upregulated as the course of infection with P. yoelii 17XL progressed. Enhanced expression of type 1 immune response genes in C57BL/6 mice infected with P. berghei ANKA has been described previously (49). In fact, a number of genes upregulated during the course of infection with P. yoelii 17XL were also found by other investigators to be upregulated after infection with P. berghei ANKA (49). For example, genes encoding transcriptional factors that regulate interferon, such as STAT 1 and IFN-regulatory factor, were upregulated during the course of both infections. Members of the 47-kDa family (IFN-inducible GTPase, T-cell-specific GTPase, and IFN-γ-inducible protein), which are important in the host defense against intracellular pathogens (57), and type 1 chemokines and chemokine receptors (CXCL10 and CCL5) were expressed after infection with either P. berghei ANKA or P. yoelii 17XL.
In our coinfected C57BL/6 mice, we saw early upregulation of some genes associated with type 1 immune responses (CXCL9, CXCL10, STAT 1, IFN-inducible GTPase 1 and GTPase 2, and guanylate nucleotide binding protein 1), but the most dramatic difference in the transcriptional immune profile between both groups was observed after 5 days of infection with P. yoelii 17XL, immediately prior to the rapid increase in parasitemia seen in both groups. Fewer genes encoding representative type 2 cytokines were expressed than genes encoding molecules typifying characteristic type 1 responses, which accounted for 39 out of 75 differentially expressed immunoregulatory genes. In addition to increased expression of IFN-γ and TNF-α, which are classically associated with the host response to mycobacteria, coinfected animals had upregulation of genes encoding chemokines found in tuberculosis lymphadenitis (CX3CL1) (13) and in activated M. tuberculosis-infected macrophages (CCL5, CXCL9, and CXCL10) (1).
Overall, the gene expression data showed that prior infection with M. tuberculosis does not qualitatively alter the type of immune responses elicited by P. yoelii 17XL but, instead, measurably enhances the magnitude of type 1 responses. The association between a dominant type 1 immune response and protection from malaria in coinfected animals is consistent with the established role of cell-mediated immunity in controlling the erythrocytic stage of the disease. Previous studies comparing cytokine production in lethal and nonlethal strains of P. yoelii have revealed that an early IFN-γ response is important in controlling intraerythrocytic parasite replication (9). However, modulation of the inflammatory response may be important as malaria progresses (3, 31). For example, a recent study of malaria-filariae coinfection showed an association between high IFN-γ responsiveness late in the course of infection with P. chabaudi (18 to 20 days after infection) and severe malaria, suggesting that prolonged type 1 responses are not advantageous (15). Furthermore, the timing of TGF-β and IL-10 secretion, which can modulate the immune response, may determine the outcome of infection with lethal and nonlethal strains of P. yoelii (39). Although we did not detect differences in TGF-β expression between coinfected mice and mice infected with P. yoelii 17XL only by microarray analysis or RT-PCR, we detected upregulation of IL-10 by RT-PCR in mice infected with P. yoelii 17XL alone.
The lack of protection observed in BALB/c mice coinfected with M. tuberculosis and P. yoelii supports the hypothesis that the protective effect in coinfected C57BL/6 mice is type 1 immune mediated. Classic studies in Leishmania have demonstrated that BALB/c mice have type 2-biased immune responses compared to those of C57BL/6 mice (20, 46). Interestingly, increased susceptibility of BALB/c mice to murine cytomegalovirus has been attributed to the lack of Ly49H+ natural killer (NK) cells, which account for >80% of IFN-γ-producing cells during murine cytomegalovirus infection in C57BL/6 mice (8). Although the polarity of the immune response to M. tuberculosis is not as clearly demarcated as in Leishmania, BALB/c mice are more susceptible to M. tuberculosis infection than C57BL/6 mice and have a diminished generalized type 1 response with increased type 2 markers after mycobacterial infections (27, 44, 58, 59). Cytokine profiles confirmed that BALB/c mice have lower IFN-γ expression than C57BL/6 mice when infected with M. tuberculosis and higher IL-4 expression when infected with P. yoelii 17XL alone. Prior infection with M. tuberculosis did not augment IFN-γ expression in BALB/c mice with malaria as it did in coinfected C57BL mice, indicating that the inability to modulate the course of malaria in BALB/c mice could result from the lack of a robust type 1 response following infection with M. tuberculosis. The discrepancy of our data with previous findings showing that BCG infection can modulate innate susceptibility to P. chabaudi (54) may reflect differences in mouse strains and species of coinfecting pathogens and suggests that different immune mechanisms are playing a role in these two models.
Heterologous immunity has been best characterized in murine models of viral infections where cross-reactive memory T-cells appear to play a critical role (60). Although we did not directly measure the expansion of specific T-cell epitopes in this model, our findings suggest that protection in coinfected mice is not primarily mediated by cross-reactive memory T-cells. For example, tuberculin or malarial antigens did not stimulate IFN-γ production by splenocytes from animals singly infected with P. yoelii 17XL or M. tuberculosis, respectively. Furthermore, protection against malaria in C57BL/6 mice occurred prior to the onset of adaptive immunity to M. tuberculosis, as the outcome of malaria infection was the same regardless of whether mice were chronically or acutely infected with M. tuberculosis. In a previously reported model of BCG and Babesia microti coinfection, Clark and colleagues showed that protection against B. microti was not affected by the length of BCG infection, which ranged from 5 to 180 days, also suggesting that protection is not primarily mediated by the adaptive immune response elicited by mycobacteria (7). We postulate that the enhanced type 1 response elicited by M. tuberculosis in resistant coinfected C57BL/6 mice but not in susceptible coinfected BALB/c mice promotes a systemic immunologic milieu that may prime the immune response against a second infection by enhancing both innate and pathogen-specific cellular responses.
Our findings cannot be directly extrapolated to predict the effect of coinfection on human malaria because there are significant differences between the murine model of malaria and human disease. Nonetheless, lethal P. yoelii infection may model important aspects of severe human erythrocytic disease. Early TNF-α, IFN-γ, and nitric oxide responses are important in parasite clearance in human disease (10, 28). In addition, clinical vaccine trials have shown that adjuvants which enhance cell-mediated immunity may improve the protective effect against malaria (2, 30).
Despite the limitations of our model, we show that modulation of infection can occur through the systemic activation of immune responses elicited by an unrelated pathogen. Our study corroborates the pioneering work by Clark and colleagues (6, 7) and provides new insight into protective immune mechanisms which may play a role in the modulation of immune responses by coinfecting organisms. In particular, as with BCG infection (36), protection against lethal P. yoelii appears to be mediated by the potentiation of type 1 immune responses induced by M. tuberculosis. However, as evidenced by the lack of protection in BALB/c mice, the immunomodulatory properties of M. tuberculosis are influenced by the host's genetic profile. These findings may have implications for regions of the world where tuberculosis is coendemic with other infections.
Epidemiologic studies have found that vaccines such as M. bovis BCG and measles, which predominantly elicit type 1 immune responses, may reduce childhood mortality beyond the direct effect on their targeted diseases. In contrast, vaccines such as DTP, which primarily elicit type 2 immune responses, have the opposite effect (14, 29, 50). A recent retrospective study found a reduced risk of death from malaria in children with a BCG scar (45). However, this finding must be confirmed in large prospective trials before any firm conclusions can be drawn. Clark and colleagues showed that, in contrast to intraperitoneal or intravenous inoculation with BCG, subcutaneous BCG vaccination did not protect mice against malaria (6), suggesting that BCG vaccination in children may not be protective against malaria either. Our data indicates that the ability to mount polarized systemic responses may vary between individuals, depending on genetic factors and prior infections. Future studies may be warranted to specifically evaluate whether infection with M. tuberculosis, exposure to environmental mycobacteria, or M. bovis BCG vaccination may modulate systemic responses in humans and affect the outcome of clinical malaria.
Acknowledgments
The expertise, facilities, and instrumentation for Affymetrix GeneChip experimentation and analyses were provided and supported by the Johns Hopkins University Malaria Research Institute. We thank Sabra Klein and Meg Mintz for their assistance with the microarray analyses. We greatly appreciate the helpful comments of Fidel Zavala, Christopher Karp, and Joe Vinetz.
This work was supported by a grant from the Johns Hopkins University Malaria Research Institute.
Editor: J. L. Flynn
REFERENCES
- 1.Algood, H. M., P. L. Lin, D. Yankura, A. Jones, J. Chan, and J. L. Flynn. 2004. TNF influences chemokine expression of macrophages in vitro and that of CD11b+ cells in vivo during Mycobacterium tuberculosis infection. J. Immunol. 172:6846-6857. [DOI] [PubMed] [Google Scholar]
- 2.Alonso, P. L., J. Sacarlal, J. J. Aponte, A. Leach, E. Macete, J. Milman, I. Mandomando, B. Spiessens, C. Guinovart, M. Espasa, Q. Bassat, P. Aide, O. Ofori-Anyinam, M. M. Navia, S. Corachan, M. Ceuppens, M. C. Dubois, M. A. Demoitie, F. Dubovsky, C. Menendez, N. Tornieporth, W. R. Ballou, R. Thompson, and J. Cohen. 2004. Efficacy of the RTS, S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet 364:1411-1420. [DOI] [PubMed] [Google Scholar]
- 3.Artavanis-Tsakonas, K., J. E. Tongren, and E. M. Riley. 2003. The war between the malaria parasite and the immune system: immunity, immunoregulation and immunopathology. Clin. Exp. Immunol. 133:145-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ball, H. J., H. G. MacDougall, I. S. McGregor, and N. H. Hunt. 2004. Cyclooxygenase-2 in the pathogenesis of murine cerebral malaria. J. Infect. Dis. 189:751-758. [DOI] [PubMed] [Google Scholar]
- 5.Bazaz-Malik, G. 1973. Increased resistance to malaria after Mycobacterium tuberculosis infection. Indian J. Med. Res. 61:1014-1024. [PubMed] [Google Scholar]
- 6.Clark, I. A., A. C. Allison, and F. E. Cox. 1976. Protection of mice against Babesia and Plasmodium with BCG. Nature 259:309-311. [DOI] [PubMed] [Google Scholar]
- 7.Clark, I. A., E. J. Wills, J. E. Richmond, and A. C. Allison. 1977. Suppression of babesiosis in BCG-infected mice and its correlation with tumor inhibition. Infect. Immun. 17:430-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniels, K. A., G. Devora, W. C. Lai, C. L. O'Donnell, M. Bennett, and R. M. Welsh. 2001. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J. Exp. Med. 194:29-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Souza, J. B., K. H. Williamson, T. Otani, and J. H. Playfair. 1997. Early gamma interferon responses in lethal and nonlethal murine blood-stage malaria. Infect. Immun. 65:1593-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodoo, D., F. M. Omer, J. Todd, B. D. Akanmori, K. A. Koram, and E. M. Riley. 2002. Absolute levels and ratios of proinflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to Plasmodium falciparum malaria. J. Infect. Dis. 185:971-979. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira, A. P., A. S. Aguiar, M. W. Fava, J. O. Correa, F. M. Teixeira, and H. C. Teixeira. 2002. Can the efficacy of bacille calmette-guerin tuberculosis vaccine be affected by intestinal parasitic infections? J. Infect. Dis. 186:441-443. [DOI] [PubMed] [Google Scholar]
- 12.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraticelli, P., M. Sironi, G. Bianchi, D. D'Ambrosio, C. Albanesi, A. Stoppacciaro, M. Chieppa, P. Allavena, L. Ruco, G. Girolomoni, F. Sinigaglia, A. Vecchi, and A. Mantovani. 2001. Fractalkine (CX3CL1) as an amplification circuit of polarized Th1 responses. J. Clin. Invest. 107:1173-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garly, M. L., C. L. Martins, C. Bale, M. A. Balde, K. L. Hedegaard, P. Gustafson, I. M. Lisse, H. C. Whittle, and P. Aaby. 2003. BCG scar and positive tuberculin reaction associated with reduced child mortality in West Africa. A non-specific beneficial effect of BCG? Vaccine 21:2782-2790. [DOI] [PubMed] [Google Scholar]
- 15.Graham, A. L., T. J. Lamb, A. F. Read, and J. E. Allen. 2005. Malaria-filaria coinfection in mice makes malarial disease more severe unless filarial infection achieves patency. J. Infect. Dis. 191:410-421. [DOI] [PubMed] [Google Scholar]
- 16.Grau, G. E., L. F. Fajardo, P. F. Piguet, B. Allet, P. H. Lambert, and P. Vassalli. 1987. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science 237:1210-1212. [DOI] [PubMed] [Google Scholar]
- 17.Grau, G. E., H. Heremans, P. F. Piguet, P. Pointaire, P. H. Lambert, A. Billiau, and P. Vassalli. 1989. Monoclonal antibody against interferon gamma can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc. Natl. Acad. Sci. USA 86:5572-5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grau, G. E., P. F. Piguet, H. D. Engers, J. A. Louis, P. Vassalli, and P. H. Lambert. 1986. L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J. Immunol. 137:2348-2354. [PubMed] [Google Scholar]
- 19.Greenwald, R. J., G. J. Freeman, and A. H. Sharpe. 2005. The B7 family revisited. Annu. Rev. Immunol. 23:515-548. [DOI] [PubMed] [Google Scholar]
- 20.Gumy, A., J. A. Louis, and P. Launois. 2004. The murine model of infection with Leishmania major and its importance for the deciphering of mechanisms underlying differences in Th cell differentiation in mice from different genetic backgrounds. Int. J. Parasitol. 34:433-444. [DOI] [PubMed] [Google Scholar]
- 21.Helmby, H., M. Kullberg, and M. Troye-Blomberg. 1998. Altered immune responses in mice with concomitant Schistosoma mansoni and Plasmodium chabaudi infections. Infect. Immun. 66:5167-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helmby, H., and M. Troye-Blomberg. 2000. Differential immunoglobulin E and cytokine responses in BALB/c and C57Bl/6 mice during repeated infections with blood-stage Plasmodium chabaudi malaria. Parasite Immunol. 22:185-190. [DOI] [PubMed] [Google Scholar]
- 23.Howard, A. D., and B. S. Zwilling. 1998. Cytokine production by CD4 and CD8 T cells during the growth of Mycobacterium tuberculosis in mice. Clin. Exp. Immunol. 113:443-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iseman, M. 2000. A clinician's guide to tuberculosis. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 25.Kamal, S. M., J. W. Rasenack, L. Bianchi, A. Al Tawil, K. El Sayed Khalifa, T. Peter, H. Mansour, W. Ezzat, and M. Koziel. 2001. Acute hepatitis C without and with schistosomiasis: correlation with hepatitis C-specific CD4+ T-cell and cytokine response. Gastroenterology 121:646-656. [DOI] [PubMed] [Google Scholar]
- 26.Keane, J., S. Gershon, R. P. Wise, E. Mirabile-Levens, J. Kasznica, W. D. Schwieterman, J. N. Siegel, and M. M. Braun. 2001. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 345:1098-1104. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi, K., N. Nakata, M. Kai, T. Kasama, Y. Hanyuda, and Y. Hatano. 1997. Decreased expression of cytokines that induce type 1 helper T cell/interferon-gamma responses in genetically susceptible mice infected with Mycobacterium avium. Clin. Immunol. Immunopathol. 85:112-116. [DOI] [PubMed] [Google Scholar]
- 28.Kremsner, P. G., S. Winkler, E. Wildling, J. Prada, U. Bienzle, W. Graninger, and A. K. Nussler. 1996. High plasma levels of nitrogen oxides are associated with severe disease and correlate with rapid parasitological and clinical cure in Plasmodium falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 90:44-47. [DOI] [PubMed] [Google Scholar]
- 29.Kristensen, I., P. Aaby, and H. Jensen. 2000. Routine vaccinations and child survival: follow up study in Guinea-Bissau, West Africa. Br. Med. J. 321:1435-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lalvani, A., P. Moris, G. Voss, A. A. Pathan, K. E. Kester, R. Brookes, E. Lee, M. Koutsoukos, M. Plebanski, M. Delchambre, K. L. Flanagan, C. Carton, M. Slaoui, C. Van Hoecke, W. R. Ballou, A. V. Hill, and J. Cohen. 1999. Potent induction of focused Th1-type cellular and humoral immune responses by RTS, S/SBAS2, a recombinant Plasmodium falciparum malaria vaccine. J. Infect. Dis. 180:1656-1664. [DOI] [PubMed] [Google Scholar]
- 31.Li, C., E. Seixas, and J. Langhorne. 2001. Rodent malarias: the mouse as a model for understanding immune responses and pathology induced by the erythrocytic stages of the parasite. Med. Microbiol. Immunol. 189:115-126. [DOI] [PubMed] [Google Scholar]
- 32.Lwin, M., C. Last, G. A. Targett, and M. J. Doenhoff. 1982. Infection of mice concurrently with Schistosoma mansoni and rodent malarias: contrasting effects of patent S. mansoni infections on Plasmodium chabaudi, P. yoelii and P. berghei. Ann. Trop. Med. Parasitol. 76:265-273. [DOI] [PubMed] [Google Scholar]
- 33.Malhotra, I., P. Mungai, A. Wamachi, J. Kioko, J. H. Ouma, J. W. Kazura, and C. L. King. 1999. Helminth- and Bacillus Calmette-Guerin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J. Immunol. 162:6843-6848. [PubMed] [Google Scholar]
- 34.Marshall, A. J., L. R. Brunet, Y. van Gessel, A. Alcaraz, S. K. Bliss, E. J. Pearce, and E. Y. Denkers. 1999. Toxoplasma gondii and Schistosoma mansoni synergize to promote hepatocyte dysfunction associated with high levels of plasma TNF-alpha and early death in C57BL/6 mice. J. Immunol. 163:2089-2097. [PubMed] [Google Scholar]
- 35.Matsumoto, S., H. Yukitake, H. Kanbara, H. Yamada, A. Kitamura, and T. Yamada. 2000. Mycobacterium bovis bacillus calmette-guerin induces protective immunity against infection by Plasmodium yoelii at blood-stage depending on shifting immunity toward Th1 type and inducing protective IgG2a after the parasite infection. Vaccine 19:779-787. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto, S., H. Yukitake, H. Kanbara, and T. Yamada. 1998. Recombinant Mycobacterium bovis bacillus Calmette-Guerin secreting merozoite surface protein 1 (MSP1) induces protection against rodent malaria parasite infection depending on MSP1-stimulated interferon gamma and parasite-specific antibodies. J. Exp. Med. 188:845-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy, J. R. 1981. Host defenses in murine malaria: nonspecific resistance to Plasmodium berghei generated in response to Mycobacterium bovis infection or Corynebacterium parvum stimulation. Infect. Immun. 33:199-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noland, G. S, T. K. Graczyk, B. Fried, E. J. Fitzgerald, and N. Kumar. 2005. Exacerbation of Plasmodium yoelii malaria in Echinostoma caproni-infected mice and abatement through anthelmintic treatment. J. Parasitol. 91:944-948. [DOI] [PubMed] [Google Scholar]
- 39.Omer, F. M., J. B. de Souza, and E. M. Riley. 2003. Differential induction of TGF-beta regulates proinflammatory cytokine production and determines the outcome of lethal and nonlethal Plasmodium yoelii infections. J. Immunol. 171:5430-5436. [DOI] [PubMed] [Google Scholar]
- 40.Omer, F. M., and E. M. Riley. 1998. Transforming growth factor beta production is inversely correlated with severity of murine malaria infection. J. Exp. Med. 188:39-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pasquetto, V., L. G. Guidotti, K. Kakimi, M. Tsuji, and F. V. Chisari. 2000. Host-virus interactions during malaria infection in hepatitis B virus transgenic mice. J. Exp. Med. 192:529-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perkins, D. J., P. G. Kremsner, and J. B. Weinberg. 2001. Inverse relationship of plasma prostaglandin E2 and blood mononuclear cell cyclooxygenase-2 with disease severity in children with Plasmodium falciparum malaria. J. Infect. Dis. 183:113-118. [DOI] [PubMed] [Google Scholar]
- 43.Raviglione, M. C., D. E. Snider, Jr., and A. Kochi. 1995. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA 273:220-226. [PubMed] [Google Scholar]
- 44.Roch, F., and M. A. Bach. 1991. Strain differences in mouse cellular responses to Mycobacterium lepraemurium and BCG subcutaneous infections. II. Production of interleukins 2, 4, and 6 and of interferon-gamma by draining lymph node cells. Clin. Immunol. Immunopathol. 60:443-454. [DOI] [PubMed] [Google Scholar]
- 45.Roth, A., P. Gustafson, A. Nhaga, Q. Djana, A. Poulsen, M. L. Garly, H. Jensen, M. Sodemann, A. Rodriques, and P. Aaby. 2005. BCG vaccination scar associated with better childhood survival in Guinea-Bissau. Int. J. Epidemiol. 34:540-547. [DOI] [PubMed] [Google Scholar]
- 46.Sacks, D., and N. Noben-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2:845-858. [DOI] [PubMed] [Google Scholar]
- 47.Sanni, L. A., L. F. Fonseca, and J. Langhorne. 2002. Mouse models for erythrocytic-stage malaria. Methods Mol. Med. 72:57-76. [DOI] [PubMed] [Google Scholar]
- 48.Scott, C. P., N. Kumar, W. R. Bishai, and Y. C. Manabe. 2004. Short report: modulation of Mycobacterium tuberculosis infection by Plasmodium in the murine model. Am. J. Trop. Med. Hyg. 70:144-148. [PubMed] [Google Scholar]
- 49.Sexton, A. C., R. T. Good, D. S. Hansen, M. C. D'Ombrain, L. Buckingham, K. Simpson, and L. Schofield. 2004. Transcriptional profiling reveals suppressed erythropoiesis, up-regulated glycolysis, and interferon-associated responses in murine malaria. J. Infect. Dis. 189:1245-1256. [DOI] [PubMed] [Google Scholar]
- 50.Shann, F. 2004. Heterologous immunity and the nonspecific effects of vaccines: a major medical advance? Pediatr. Infect. Dis. J. 23:555-558. [DOI] [PubMed] [Google Scholar]
- 51.Shear, H. L., R. Srinivasan, T. Nolan, and C. Ng. 1989. Role of IFN-gamma in lethal and nonlethal malaria in susceptible and resistant murine hosts. J. Immunol. 143:2038-2044. [PubMed] [Google Scholar]
- 52.Singh, J. P., A. P. Ray, and C. P. Nair. 1956. Relationship of tuberculosis on the course and intensity of plasmodial infections in M. mulatta. Indian J. Malariol. 10:3-10. [PubMed] [Google Scholar]
- 53.Snow, R. W., C. A. Guerra, A. M. Noor, H. Y. Myint, and S. I. Hay. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434:214-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stevenson, M., S. Lemieux, and E. Skamene. 1984. Genetic control of resistance to murine malaria. J. Cell. Biochem. 24:91-102. [DOI] [PubMed] [Google Scholar]
- 55.Stevenson, M. M., M. F. Tam, S. F. Wolf, and A. Sher. 1995. IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-gamma and TNF-alpha and occurs via a nitric oxide-dependent mechanism. J. Immunol. 155:2545-2556. [PubMed] [Google Scholar]
- 56.Su, Z., and M. M. Stevenson. 2000. Central role of endogenous gamma interferon in protective immunity against blood-stage Plasmodium chabaudi AS infection. Infect. Immun. 68:4399-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor, G. A., C. G. Feng, and A. Sher. 2004. p47 GTPases: regulators of immunity to intracellular pathogens. Nat. Rev. Immunol. 4:100-109. [DOI] [PubMed] [Google Scholar]
- 58.Teixeira, H. C., M. E. Munk, and S. H. Kaufmann. 1995. Frequencies of IFN gamma- and IL-4-producing cells during Mycobacterium bovis BCG infection in two genetically susceptible mouse strains: role of alpha/beta T cells and NK1.1 cells. Immunol. Lett. 46:15-19. [DOI] [PubMed] [Google Scholar]
- 59.Wakeham, J., J. Wang, and Z. Xing. 2000. Genetically determined disparate innate and adaptive cell-mediated immune responses to pulmonary Mycobacterium bovis BCG infection in C57BL/6 and BALB/c mice. Infect. Immun. 68:6946-6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Welsh, R. M., and L. K. Selin. 2002. No one is naive: the significance of heterologous T-cell immunity. Nat. Rev. Immunol. 2:417-426. [DOI] [PubMed] [Google Scholar]
- 61.World Health Organization. 2002. What is malaria? Roll back malaria info sheet:1. Roll Back Malaria Department, World Health Organization, Geneva, Switzerland.