Abstract
The immunogenicity and protective efficacy of the recombinant 31-kDa outer membrane protein from Brucella melitensis (rOmp31), administered with incomplete Freund's adjuvant, were evaluated in mice. Immunization of BALB/c mice with rOmp31 conferred protection against B. ovis and B. melitensis infection. rOmp31 induced a vigorous immunoglobulin G (IgG) response, with higher IgG1 than IgG2 titers. In addition, spleen cells from rOmp31-immunized mice produced interleukin 2 (IL-2) and gamma interferon, but not IL-10 or IL-4, after in vitro stimulation with rOmp31, suggesting the induction of a T helper 1 (Th1) response. Splenocytes from rOmp31-vaccinated animals also induced a specific cytotoxic-T-lymphocyte activity, which led to the in vitro lysis of Brucella-infected macrophages. In vitro T-cell subset depletion indicated that rOmp31 immunization elicited specific CD4+ T cells that secrete IL-2 and gamma interferon, while CD8+ T cells induced cytotoxic-T-lymphocyte activity. In vivo depletion of T-cell subsets showed that the rOmp31-elicited protection against B. melitensis infection is mediated by CD4+ T cells while the contribution of CD8+ T cells may be limited. We then evaluated the immunogenicity and protective efficacy of a known exposed region from Omp31 on the Brucella membrane, a peptide that contains amino acids 48 to 74 of Omp31. Immunization with the synthetic peptide in adjuvant did not elicit a specific humoral response but elicited a Th1 response mediated by CD4+ T cells. The peptide in adjuvant induced levels of protection similar to those induced by rOmp31 against B. melitensis but less protection than was induced by rOmp31 against B. ovis. Our results indicate that rOmp31 could be a useful candidate for the development of subunit vaccines against B. melitensis and B. ovis.
Brucellae are gram-negative, facultative intracellular pathogens that may cause severe disease in both humans and animals. Brucellosis remains endemic in many developing countries, causing important economic losses (31). Brucella melitensis is the most pathogenic species for humans and may cause abortions in sheep, goats, and cows. Vaccination of sheep and goats against B. melitensis with live attenuated smooth B. melitensis Rev. 1, the strain most widely used for disease control, elicits a long-lasting serological response against the O polysaccharide (50). This makes differentiation between infected and vaccinated animals by standard serological tests a difficult task. Moreover, Rev 1 is resistant to streptomycin, one of the antibiotics of choice used to treat brucellosis (35), and is pathogenic for humans (6), and its use is prohibited in countries free of B. melitensis (28).
Killed vaccines are noninfectious, but they are considered less efficacious than live vaccines in inducing protective immunity against intracellular pathogens. In addition, with whole-microorganism vaccines, the regulatory requirements for the exact specifications of vaccine composition and the mechanisms to obtain immunity are difficult to meet. In this respect, recombinant subunit vaccines have numerous advantages: they are completely inert, their composition is predetermined, their production can be better controlled, and their homogeneity is much higher. However, the success of these vaccines depends on the selection of the right antigen(s) (Ags), adjuvant(s), and delivery systems. By optimizing these factors, the immune response can be tailored against a specific pathogen (33, 42).
Numerous cell surface and intracellular components have been assessed as protective antigens against Brucella infection. Until now, significant activity has been identified against B. abortus for only a few purified Ags: the L7/L12 ribosomal protein (38), Cu-Zn superoxide dismutase (43), a 22.9-kDa protein (12), the cytoplasmic protein p39 (1), and lumazine synthase (45). Conversely, against smooth B. melitensis, the most virulent strain of Brucella spp., the only protective Ag identified was the 31-kDa outer membrane protein (Omp) of Brucella delivered as a DNA vaccine (11). However, repeated doses and high concentrations of the plasmid containing the Omp31 gene are needed to generate an efficacious response (11), probably because of the low in vivo transfection efficiency for this type of plasmid vector (24, 25, 40). Thus, it remains possible that a different delivery system may lead to protection against B. melitensis using Omp31 as an Ag.
Protective immunity to Brucella spp. is incompletely understood but is similar to immunity to most of the intracellular bacterial infections; cell-mediated immunity plays a critical role in protection against virulent Brucella infection, although antibodies (Abs) specific for the O polysaccharide of the lipopolysaccharide (LPS) and certain membrane proteins can confer protection in some host species (8, 27). The outer membrane proteins (Omps) of Brucella spp. have been characterized as potential immunogenic and protective Ags (16). A monoclonal Ab (MAb) against Omp31 administered alone provided passive protection as strong as that obtained with an anti-B. ovis hot saline extract (rich in rough LPS and Omps) serum (28). The cognate epitope of this MAb is located in a hydrophilic loop situated between amino acids (aa) 43 and 83 (46) and conserved among strains of different geographic origins (47). Passive-protection experiments in mice have shown that mixtures of MAbs, previously shown to bind individually to several Omps, conferred no or poor protection against smooth Brucella strains in mice (14, 27), whereas they were protective against rough B. ovis (9). This could be attributed to the presence of the O polysaccharide-bearing LPS on smooth strains, which has been shown to hinder deeper Omp epitopes (8, 15). Indeed, vaccination with recombinant Omp31-enriched preparations, which induced a strong Ab response but a poor cellular response, provided protection against B. ovis (18) but not against B. melitensis (23) challenge. Moreover, it was demonstrated that an Omp31 extract immunization induces humoral and cellular immune mechanisms in sheep (19). Our previous results demonstrated that the Omp31 DNA vaccine conferred protection against B. ovis and B. melitensis in BALB/c mice. This vaccine induced a weak humoral response and no T helper 1 (Th1) but important Omp31 cytotoxic-T-lymphocyte (CTL) responses. The protective response could be related to the induction of Omp31-specific CD8+ T cells that eliminate Brucella-infected cells via the perforin pathway (11).
In this study, we evaluated the immunogenicity and protective efficacy of the purified recombinant Omp31 protein (rOmp31) inoculated with adjuvant. This vaccine conferred protection against B. ovis and B. melitensis infection. Our results demonstrated that rOmp31 could be efficacious against Brucella infection by eliciting a Th1 response mediated by CD4+ T cells. CD8+ T cells have a limited contribution. We further demonstrated that immunization with a 27-aa peptide derived from Omp31 induced protection against B. melitensis infection similar to that induced by the whole recombinant protein.
MATERIALS AND METHODS
Mice.
Six- to 8-week-old female BALB/c mice (obtained from the University of La Plata, Argentina) were acclimated and randomly distributed into experimental groups. The mice were kept in conventional animal facilities and received water and food ad libitum, in accordance with pertinent U.S. federal regulations and policies.
Bacterial strains.
Escherichia coli strain JM109 (Promega, Madison, WI) was used as the host for propagation of plasmids. Strain BL21(DE3) (Stratagene, La Jolla, CA) was used for expression of the recombinant protein. Bacterial strains were routinely grown at 37°C in LB broth or agar, supplemented when required with 100 μg/ml of ampicilin. B. melitensis H38S and B. ovis PA76250 (virulent strains) were cultured in tryptose-soy agar (Merck, Buenos Aires, Argentina) supplemented and incubated as described previously (18, 23).
Cell lines.
The J774.A1 cell line was purchased from the American Type Culture Collection (Manassas, VA). Cells were cultured in complete medium (RPMI 1640 supplemented with 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% heat-inactivated fetal bovine serum) (Gibco-BRL, Life technologies, Grand Island, NY). A20J cells expressing class I and II major histocompatibility complex molecules were kindly provided by Mauricio Rodriguez (Universidad Federal de Sao Paulo, Brazil) and were cultured in complete medium (Gibco).
Antigen production.
rOmp31 was cloned, expressed in E. coli, and purified as previously described (10). Purity was assessed by Coomassie blue staining, as reported elsewhere (10). An Omp31 synthetic peptide spanning aa 48 to 74 (Omp3148-74) was a gift of Fernando Goldbaum, who purchased it from the W. M. Keck Foundation Biotechnology Resource Laboratory (New Haven, CT). The peptide was further purified by high-performance liquid chromatography using a C18 reverse-phase column, and the molecular weight was confirmed by mass spectroscopy. The plasmid (pCIOmp31) was amplified in E. coli JM109 cells and isolated using Megaprep plasmid isolation columns (QIAGEN, Dorking, United Kingdom) as previously described (11). The purity and concentration of DNA were determined by spectrophotometry at 260/280 nm.
Immunization.
Mice were anesthetized with methoxyfuorane (Mallinckrodt, Phillipsburg, NJ) and immunized by the intraperitoneal (i.p.) route with 30 μg of rOmp31, 30 μg of Omp3148-74, or phosphate-buffered saline (PBS) (as a control) in incomplete Freund's adjuvant (IFA) (Sigma, St. Louis, MO). Each mouse was injected on days 0 and 15. As a positive control (7), another group was immunized once subcutaneously on day 0 with 8 × 108 formalin-killed B. melitensis H38S (H38) bacteria in IFA. Sera were obtained at 15, 30, 45, 60, and 75 days after the first immunization (eight mice per group). Thirty days after the last protein injection, the mice were challenged intravenously with virulent Brucella organisms (eight mice per group) or were sacrificed to conduct the analysis of immune responses, including cytokine production and CTL induction (five mice per group).
As a positive control for CTL responses, other groups of mice (five mice per group) were immunized by the intramuscular route with 100 μg of pCIOmp31 or pCI as a control. Each mouse was injected on days 0, 15, 30, and 45.
Protection experiments.
rOmp31-, Omp3148-74-, PBS-, or H38-immunized mice were challenged, by intravenous injection, with 104 B. melitensis H38S or 104 B. ovis organisms. The mice were killed by cervical dislocation 30 days after being challenged, and their spleens and livers were removed aseptically. Each spleen or liver was homogenized in a stomacher bag, serially diluted, plated on supplemented tryptose-soy agar, and incubated as described previously (18, 23). The number of CFU per spleen or liver was determined, and the results were represented as the mean log CFU ± standard deviation (SD) per group. Log units of protection were obtained by subtracting the mean log CFU of the vaccinated group from the mean log CFU of the control immunized group.
Omp31 ELISA.
Serum reactivities against rOmp31 were determined by indirect enzyme-linked immunosorbent assay (ELISA) as described previously (10). Serum reactivities against Omp3148-74 were determined as for rOmp31, but in this case, Maxisorp polystyrene plates (Nunc, Roskilde, Denmark) were sensitized with 0.1 μg of Omp3148-74 per well. The cutoff value for the assays was calculated as the mean specific optical density plus 3 SD from 20 sera from nonimmunized mice assayed at 1:100 dilution. Serum titers were established as the reciprocal of the last serum dilution yielding an optical density higher than the cutoff.
Cytokine responses.
Spleen cell suspensions from immunized or control mice were prepared in complete medium and plated at 4 × 106/well in 24-well flat-bottom plates (Nunc). In some experiments, spleen cells were previously depleted of CD4+ or CD8+ T cells using mouse CD4 (L3T4) or mouse CD8 (Lyt2) Dynabeads according to the manufacturer's instructions (Dynal Biotech, Oslo, Norway). The efficacy of cell depletion was greater than 99% as determined by flow cytometric analysis of effector cells (not shown). After being depleted, effector cells were resuspended in the original volume. The cells were stimulated in vitro at 37°C in 5% CO2 with rOmp31 (1 μg/ml), concanavalin A (ConA) (2.5 μg/ml), or complete medium alone. Supernatants were taken after 48 h of culture and stored at −70°C until they were assayed for cytokine production. Interleukin 2 (IL-2), IL-4, IL-10 and gamma interferon (IFN-γ) in culture supernatants were measured by sandwich ELISA using paired cytokine-specific MAbs according to the manufacturer's instructions (PharMingen, San Diego, CA).
Generation of CTL targets.
For generation of CTL targets, pCIOmp31-transfected cells were incubated for 16 h with 10 μg/ml of rOmp31 (A20JOmp31) or pCI-transfected cells were cultured in medium alone (A20pCI) as previously described (11). Alternatively, J774.A1 macrophages at confluent growth were infected with opsonized live B. ovis at a ratio of 1:100 (cells to bacteria) for 6 h. Extracellular bacteria were rinsed away with RPMI containing 100 μg/ml of gentamicin and 50 μg/ml of streptomycin. Macrophages (J774 B. ovis) were scraped off with a sterile rubber policeman and centrifuged at 200 × g for 5 min. Target cells were harvested and labeled with 0.1 mCi 51Cr/2 × 106 cells (Amersham, Arlington Heights, IL) in 80 μl of complete medium for 1 h at 37°C. The cells were washed three times with RPMI, and their viability was determined by trypan blue exclusion.
Stimulator cells.
Stimulator cells were A20JOmp31 treated with 25 μg/ml of mitomycin C (Sigma) in a 37°C water bath for 45 min. The cells were washed three times by centrifugation and resuspended in complete RPMI.
Generation of effector cells.
For the generation of CTLs, 2.5 × 107 splenocytes were cocultured with 0.5 × 106 stimulator cells for 5 days in 15 ml of complete RPMI plus 2.5% (vol/vol) RAT-T-STIM without ConA (Becton Dickinson Labware, Bedford, MA).
51Cr release CTL assay.
Effector and target cells were incubated at different effector/target ratios in 96-well round-bottom plates (Costar Corporation, Cambridge, MA) for 6 h at 37°C in a final volume of 200 μl. After culture, the supernatants were harvested and counted in a gamma counter (Clinigamma, LKB, Turku, Finland). Spontaneous release was determined by culturing target cells in medium alone, and maximum release was determined by lysis of target cells in 5% (vol/vol) Triton X-100-containing medium. The percentage of specific lysis was calculated by the following formula: percent specific lysis = [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100. The data given are the means of triplicate determinations. Spontaneous-release values were always <10% of maximum-release values. In some experiments, effector cells were previously depleted of CD4+ or CD8+ T cells using mouse CD4 (L3T4) or mouse CD8 (Lyt2) Dynabeads according to the manufacturer's instructions (Dynal). The efficacy of cell depletion was greater than 99% as determined by flow cytometric analysis of effector cells (not shown). After being depleted, effector cells were resuspended in the original volume and used in the cytotoxic assay.
Flow cytometry for intracellular IFN-γ and cell surface marker staining.
Intracellular cytokine staining was used to determine the IFN-γ production at the single-cell level. Stimulator cells were A20JOmp31 or A20J cells that were incubated for 16 h with 10 μg/ml of Omp3148-74 (A20JOmp3148-74). Briefly, after 18 h of primary stimulation with mitomycin C-treated A20JOmp31 cells or mitomycin C-treated A20JOmp3148-74 cells, splenocytes from rOmp31- or PBS-immunized mice were extensively washed and restimulated for 6 h with mitomycin C-treated A20JOmp31 cells and rOmp31 (1 μg/ml) or mitomycin C-treated A20JOmp3148-74 cells and Omp3148-74 (2 μg/ml). Phorbol myristate acetate (20 ng/ml; Sigma) plus ionomycin (750 ng/ml; Sigma) were added in parallel as a positive control for the assay (not shown). Brefeldin A (10 μg/ml; Sigma) was added to the cells during the last 4 h of culture. The cells were then harvested, stained for surface expression with fluorescein isothiocyanate-conjugated anti-CD4+ (clone GK1.5) and Cy-chrome-conjugated anti-CD8+ (clone 53-6.7; PharMingen) and washed with PBS-2% fetal bovine serum. Intracellular IFN-γ staining was performed with phycoerythrin-conjugated anti-IFN-γ (clone XMG1.2) (PharMingen), using the Fix & Perm kit (PharMingen) according to the manufacturer's instructions. Negative control samples were incubated with irrelevant, isotype-matched Abs in parallel with all experimental samples. Samples were analyzed for a total of 300,000 events on a FACScan flow cytometer (BD Biosciences, Mountain View, CA).
In vivo T-cell depletion.
rOmp31-vaccinated mice were depleted of CD4+ or CD8+ T cells by i.p. injection of 200 μg of purified GK1.5 or 2.43 (American Type Culture Collection) MAbs, respectively, on days −2, 1, 4, 7, and 10 after bacterial challenge. The efficacy of cell depletion was greater than 99% as determined by flow cytometric analysis of splenocytes (not shown). Nonspecific rat immunoglobulin G (IgG) purified MAb was used as an isotype control.
Statistical analysis.
The CFU data were normalized by log transformation and evaluated by analysis of variance, followed by Dunnett's post hoc test. The cellular responses were compared using the nonparametric Mann-Whitney U test (InStat; GraphPad version 4).
RESULTS
rOmp31 protects BALB/c mice against B. melitensis and B. ovis infection.
Protection experiments were carried out by challenging rOmp31-vaccinated and control mice with B. melitensis or B. ovis, and the level of infection was evaluated by determining the numbers of CFU in spleens and livers. In the spleen, mice given rOmp31 exhibited a significant degree of protection against B. melitensis (P < 0.05) and B. ovis (P < 0.01) compared with controls receiving PBS (1.16- and 2-log-unit protection, respectively) (Table 1). B. melitensis H38S (control vaccine) induced 2.65-log-unit protection against B. melitensis and 2.61-log-unit protection against B. ovis. rOmp31 was also protective against B. melitensis and B. ovis infection in the liver (Table 1). These results indicate that rOmp31 could be a useful candidate for the development of subunit vaccines against brucellosis, since it elicits protection against smooth and rough species of Brucella.
TABLE 1.
Protection against B. melitensis or B. ovis in mice immunized with Omp31
| Treatment group (n = 8) | Log10aB. melitensis organisms in:
|
Log units of protection in:
|
Log10aB. ovis organisms in:
|
Log units of protection in:
|
||||
|---|---|---|---|---|---|---|---|---|
| Spleen | Liver | Spleen | Liver | Spleen | Liver | Spleen | Liver | |
| PBS | 5.75 ± 0.13 | 2.53 ± 0.15 | 4.83 ± 0.17 | 3.12 ± 0.08 | ||||
| rOmp31 | 4.59 ± 0.67b | 1.86 ± 0.53c | 1.16 | 0.67 | 2.83 ± 0.3c | 2.32 ± 0.06c | 2.00 | 0.80 |
| H38 | 3.10 ± 0.25c | 1.41 ± 0.07c | 2.65 | 1.12 | 2.22 ± 0.57c | 2.29 ± 0.01c | 2.61 | 0.83 |
The content of bacteria in spleens and livers is represented as the mean log CFU ± SD per group.
Significantly different from PBS-immunized mice (P < 0.05) as estimated by Dunnett's test.
Significantly different from PBS-immunized mice (P < 0.01) as estimated by Dunnett's test.
rOmp31 induces humoral, T helper 1, and cytotoxic responses.
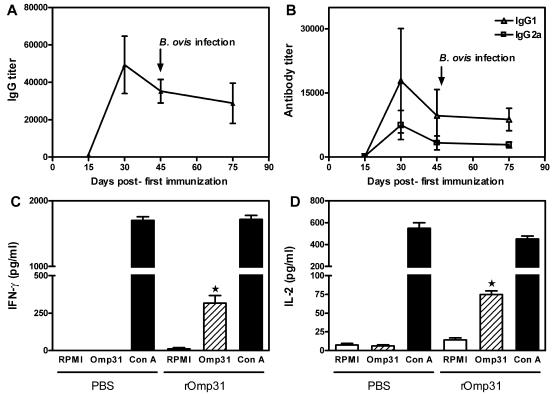
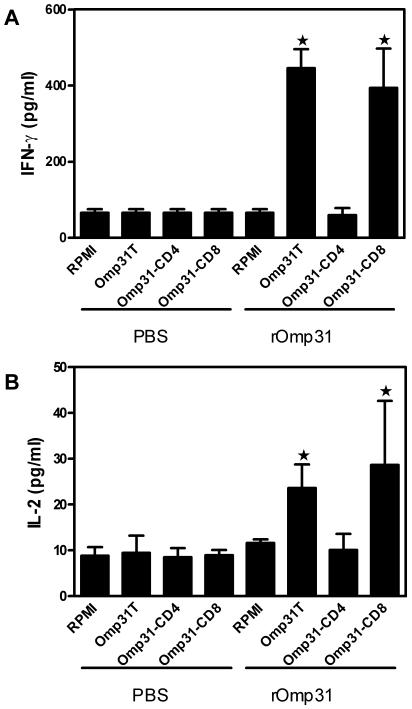
Omp31 is an excellent model to evaluate the role of the humoral response in protection, since it is well exposed in B. ovis but hindered in B. melitensis. Anti-Omp31 Ab titers were measured by ELISA in sera from immunized mice. Immunization with rOmp31 elicited a vigorous IgG response that was detectable after the first immunization, increased steadily, and reached a maximum after the second Ag injection (IgG mean titer, 50,000) and, at the time of bacterial challenge, reached an IgG mean titer of 35,000 (Fig. 1A). Omp31 IgG1 titers predominated over IgG2a titers during the whole immunization schedule, and at the time of challenge, the IgG2a/IgG1 ratio was 0.30 ± 0.15 (Fig. 1B and data not shown). Neither the animals injected with PBS nor the H38-immunized animals showed specific anti-Omp31 Abs (data not shown). To get further information on the type of immune response induced by rOmp31 at the time of bacterial challenge, cytokine secretion in culture supernatants of spleen cells from immunized mice was evaluated by ELISA. rOmp31 significantly stimulated the production of IFN-γ and IL-2 in spleen cells from rOmp31- but not from PBS-immunized animals (Fig. 1C and D). Spleen cells from all immunized mice were unable to produce IL-4 or IL-10 (data not shown). ConA induced the production of the corresponding proteins in all groups (Fig. 1C and D and data not shown). To further determine the contribution of CD8+ and CD4+ T cells in the Omp31-specific Th1 responses, spleen cells were depleted of CD4+ T cells or CD8+ T cells using immunobeads. When splenocytes from rOmp31-immunized animals were depleted of CD4+ T cells, IFN-γ or IL-2 production in response to rOmp31 was completely abrogated; in contrast, depletion of CD8+ T cells did not affect cytokine production (Fig. 2A and B). Thus, rOmp31 immunization induces Omp31-specific CD4+ T cells that secrete IFN-γ and IL-2.
FIG. 1.
Kinetics of the (A) IgG response and (B) IgG1 or IgG2a response elicited after immunization with the rOmp31 vaccine. Mice were immunized with rOmp31 in incomplete Freund's adjuvant and bled retroorbitally on the indicated days. Specific Abs against rOmp31 were evaluated by ELISA. Each symbol represents the mean ± SD of eight animals. The arrow indicates the time of B. ovis infection. Determination of IFN-γ (C) or IL-2 (D) production in cells from PBS- or rOmp31-immunized mice. Spleen cells (4 × 106/ml) from mice were stimulated with complete medium (RPMI) or rOmp31 (1 μg/ml) or ConA (2.5 μg/ml) for 48 h. IFN-γ or IL-2 in cell supernatants was quantified (pg/ml) by MAb capture ELISA. Each value represents the mean plus SD of the responses of spleen cells from five individual mice. The data are representative of three separate experiments. ★, significantly different from the same stimulus in PBS-immunized mice (P < 0.01).
FIG. 2.
CD4+ or CD8+ T-cell cytokine responses from mice immunized with rOmp31. Determination of IFN-γ (A) or IL-2 (B) production in cells from PBS- or rOmp31-immunized mice. Splenocytes were depleted of CD4+ T cells (-CD4+) or CD8+ T cells (-CD8+) using mouse CD4 (L3T4) Dynabeads or mouse CD8 (Lyt2) Dynabeads or were not depleted (T). Cells (4 × 106/ml) from mice were stimulated with complete medium (RPMI) or rOmp31 (1 μg/ml) for 48 h. IFN-γ or IL-2 in cell supernatants was quantified (pg/ml) by MAb capture ELISA. Each value represents the mean plus SD of the responses of spleen cells from five individual mice. The data are representative of two separate experiments. ★, significantly different from the same stimulus in PBS-immunized mice (P < 0.01).
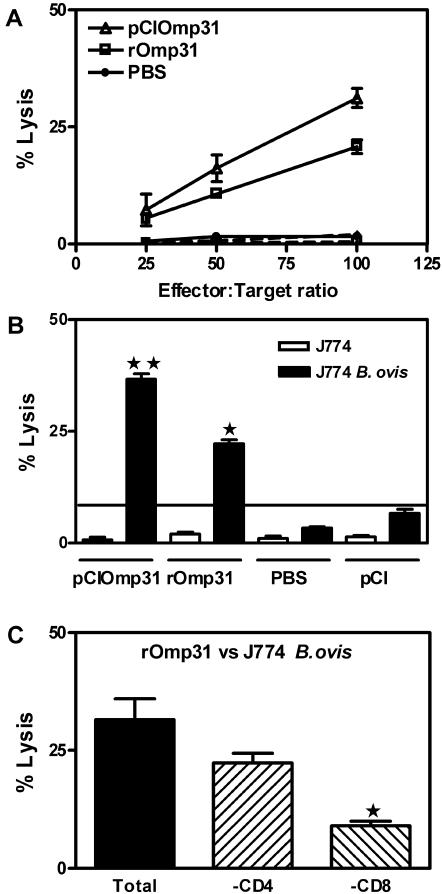
Omp31 DNA immunization has been shown to be effective in inducing CTLs (11). Therefore, the induction of Omp31-specific CTLs was examined in rOmp31-immunized mice. Splenocytes from PBS- or rOmp31-immunized mice were harvested at the time of bacterial challenge. As a positive control of CTL activity, splenocytes from pCIOmp31-immunized mice were included. CTL responses were assessed by the 51Cr release assay after in vitro stimulation with mitomycin C-treated A20JOmp31 stimulator cells. Target cells were A20JOmp31 or A20JpCI (control). Specific lysis of A20JOmp31 cells was observed at effector/target ratios of 50:1 or higher using CTLs from mice that were immunized with rOmp31. Splenocytes from PBS-immunized animals failed to lyse A20JpCI or A20JOmp31 cells. As expected (11), spleen cells from pCIOmp31-vaccinated mice lysed A20JOmp31 cells. However, the cytotoxicity elicited by Omp31 DNA vaccination at a 100:1 effector/target ratio was significantly higher (P = 0.0079) than that achieved by rOmp31 immunization (Fig. 3A). We next investigated whether rOmp31 vaccination could induce a cytotoxic response against Brucella-infected macrophages. Splenocytes from immunized mice were cultured for 5 days with mitomycin C-treated A20JOmp31 stimulator cells, and then their ability to recognize and kill a macrophage cell line (J774) infected with B. ovis was analyzed. Uninfected J774 cells served as a control. Splenocytes from rOmp31-immunized mice were able to lyse J774-infected cells but did not lyse uninfected J774 cells. Again, the cytotoxic response against Brucella was greater for DNA vaccination than for recombinant Omp31 immunization (Fig. 3B). To further determine the contribution of CD8+ and CD4+ T cells in the anti-Brucella cytotoxic responses, effector cells were depleted of CD4+ T cells or CD8+ T cells using immunobeads. When CTLs from rOmp31-immunized animals were depleted of CD4+ T cells, lysis of J774-infected cells was reduced 25%. In contrast, depletion of CD8+ T cells significantly reduced (by 75%) the specific lysis (P < 0.05) (Fig. 3C). Thus, rOmp31 immunization induced mainly CD8+ T cells, but also CD4+ T cells that elicited lysis of Brucella-infected cells. The same results were obtained when Brucella-infected A20J cells were used (data not shown). These results indicate that CTLs induced by rOmp31 immunization have the capacity to recognize and destroy Brucella-infected cells, but to a lesser extent than CTLs elicited by DNA vaccination with the same Ag.
FIG. 3.
Induction of Omp31-specific CTLs in spleen cells from rOmp31-immunized mice. Cytotoxicity was detected in a standard 6-h 51Cr-release assay. (A) Target cells were A20JpCI (dashed lines) or A20JOmp31 (solid lines) cells labeled with 51Cr. Effector cells were the splenocytes from rOmp31-, PBS-, or pCIOmp31-immunized mice previously cultured for 5 days with mitomycin C-treated A20JOmp31. The cytotoxicity was measured at the indicated effector/target ratios. Each value represents the mean ± SD of the responses of spleen cells from five individual mice. The data are representative of two separate experiments. (B) Vaccination with rOmp31 elicited CTLs that lyse Brucella-infected macrophages in vitro. Cytotoxicity was detected in a standard 6-h 51Cr release assay. The effector/target ratio was 100:1. Target cells were J774 or J774 B. ovis. Effector cells were the splenocytes from rOmp31-, PBS-, pCIOmp31-, or pCI-immunized mice previously cultured for 5 days with mitomycin C-treated A20JOmp31. Each value represents the mean plus SD of the responses of spleen cells from five individual mice. The data are representative of two separate experiments. ★★, significantly different from pCI-immunized mice (P < 0.01); ★, significantly different from PBS-immunized mice (P < 0.05). (C) Target cells were J774 B. ovis. The effector/target ratio was 100:1. rOmp31-specific effector cells were depleted of CD4+ T cells (-CD4+) or CD8+ T cells (-CD8+) using mouse CD4 (L3T4) Dynabeads or mouse CD8 (Lyt2) Dynabeads or were not depleted (Total). Each value represents the mean of triplicates plus SD of the responses of a pool of spleen cells from five mice. ★, significantly different from undepleted (Total) cells (P < 0.05). The data are representative of three separate experiments.
Taken together, these results indicate that rOmp31 immunization induces a specific humoral response, a Th1 cytokine response mediated exclusively by CD4+ T cells, and a cytotoxic-T-cell response mediated mainly by CD8+ T cells.
Protection induced by rOmp31 immunization against Brucella infection in vivo is mediated by CD4+ T cells.
We next focused on the in vivo role of CD4+ or CD8+ T cells in the protective immunity induced by the rOmp31 vaccine. rOmp31-vaccinated mice were injected i.p. with MAb 2.43 to deplete CD8+ T cells or MAb GK1.5 to deplete CD4+ T cells. In the absence of immune depletion, mice given rOmp31 exhibited a significant (P < 0.01) degree of protection against B. melitensis (1.23-log-unit protection) compared with controls receiving PBS. rOmp31 vaccination induced 1.13-log-unit protection in mice depleted of CD8+ T cells (P < 0.05) and 0.37-log-unit protection in CD4+-depleted mice (P > 0.05). Isotype control IgG injections had no effect on the degree of protection induced by rOmp31 immunization (Table 2).
TABLE 2.
Protection against B. melitensis infection induced by rOmp31 immunization is mediated by CD4+ T cells
| Vaccine (n = 8) | Treatment | Log10aB. melitensis organisms in spleen | Log units of protection |
|---|---|---|---|
| PBS | None | 5.43 ± 0.50 | |
| rOmp31 | None | 4.20 ± 0.40c | 1.23 |
| rOmp31 | Anti-CD4− | 5.06 ± 0.51d | 0.37 |
| rOmp31 | Anti-CD8− | 4.30 ± 0.21b | 1.13 |
| rOmp31 | IgG | 4.22 ± 0.60c | 1.21 |
| H38 | None | 3.11 ± 0.40c | 2.32 |
The content of bacteria in spleens is represented as the mean log CFU ± SD per group.
Significantly different from PBS-immunized mice (P < 0.05) as estimated by Dunnett's test.
Significantly different from PBS-immunized mice (P < 0.01) as estimated by Dunnett's test.
Significantly different from rOmp31-immunized mice (P < 0.05) as estimated by Dunnett's test.
These results suggest a main role of CD4+ T cells in rOmp31-mediated immunity against B. melitensis. As CD4+ T cells were the cells that secreted IFN-γ and IL-2 while CD8+ T cells were the cytotoxic cells, we speculate that the secretion of IFN-γ is the principal mechanism that allows rOmp31 vaccine to confer protection.
A 27-amino-acid peptide derived from Omp31 is a Th1 epitope.
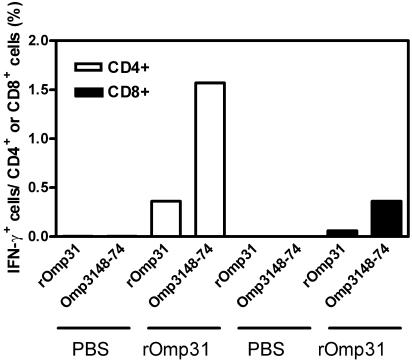
We then investigated the cellular response against an exposed region on Omp31, a protective B epitope that is located in a hydrophilic loop on Omp31 (46). We designed a synthetic peptide spanning aa 48 to 74 from the hydrophilic loop of Omp31. Spleen cells from rOmp31- or PBS-immunized animals were stimulated with rOmp31, the 27-aa synthetic peptide (Omp3148-74), or complete medium alone. Intracellular IFN-γ production by CD4+ or CD8+ T cells was determined by three-color flow cytometric analysis. As reported above (Fig. 2A), CD4+ T cells, but not CD8+ T cells, were capable of synthesizing intracellular IFN-γ in response to rOmp31 (Fig. 4). rOmp31 was unable to induce intracellular IFN-γ production in cells from PBS-immunized mice. Omp3148-74 also induced IFN-γ in CD4+ T cells from rOmp31-immunized mice (Fig. 4). This result suggests that Omp3148-74 also contains a CD4+ Th1 epitope. Since CD4+ T-cell epitopes (major histocompatibility complex class II ligands) consist of 12 to 25 aa (39) and their lengths are more heterogeneous than the lengths of CD8+ T-cell epitopes (29), the minimal epitope length was not determined. Notably, the same peptide also induced IFN-γ in CD8+ T cells from rOmp31-immunized mice, although this response was lower than that induced by CD4+ T cells (Fig. 4).
FIG. 4.
Three-color flow cytometric analysis of intracellular IFN-γ expression versus cell surface markers in spleen cells from PBS- or rOmp31-immunized mice. The graph shows the percentages of CD4+ or CD8+ cells producing intracellular IFN-γ after stimulation with rOmp31 or Omp3148-74 as described in Materials and Methods. Spontaneous (unstimulated) percentages have been subtracted. Each value represents the responses of a pool of spleen cells from five mice. The data are representative of two separate experiments.
Immunization with Omp3148-74 induced weak humoral but good Th1 responses and protection against B. melitensis similar to that induced by rOmp31.
On the basis of the above results, and knowing that rOmp31-specific CD4+ T-cell responses are protective against Brucella, we decided to immunize animals with Omp3148-74 in adjuvant and evaluate the humoral and cellular responses and the protection conferred against Brucella.
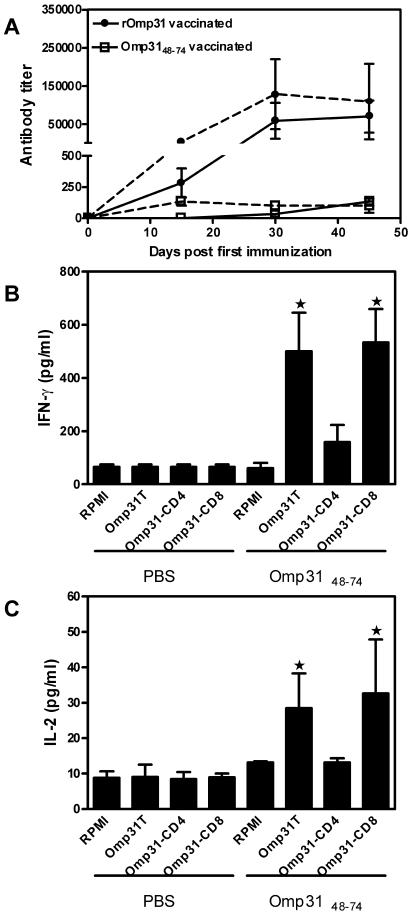
Immunization with Omp3148-74 elicited a weak IgG response against Omp3148-74, as well as rOmp31; the response was detectable only 45 days after the first immunization (IgG titer range, 100 to 200) and declined thereafter. On the other hand, rOmp31 immunization induced a vigorous IgG response against both rOmp31 and Omp3148-74 (Fig. 5A).
FIG. 5.
(A) Kinetics of the humoral response elicited after immunization with Omp3148-74. Mice were immunized with Omp3148-74 or rOmp31 in incomplete Freund's adjuvant and bled retroorbitally on the indicated days. IgG-specific Abs against rOmp31 (dashed lines) or Omp3148-74 (solid lines) were evaluated by ELISA. Each symbol represents the mean ± SD of eight animals. Determination of IFN-γ (B) or IL-2 (C) production in cells from PBS- or Omp3148-74-immunized mice. Splenocytes were depleted of CD4+ T cells (-CD4+) or CD8+ T cells (-CD8+) using mouse CD4 (L3T4) Dynabeads or mouse CD8 (Lyt2) Dynabeads or were not depleted (T). Cells (4 × 106/ml) from mice were stimulated with complete medium (RPMI) or rOmp31 (1 μg/ml) for 48 h. IFN-γ or IL-2 in cell supernatants was quantified (pg/ml) by MAb capture ELISA. Each value represents the mean plus SD of the responses of spleen cells from five individual mice. The data are representative of two separate experiments. ★, significantly different from the same stimulus in PBS-immunized mice (P < 0.01).
To evaluate the cellular response, spleen cells from Omp3148-74- or PBS-immunized animals were stimulated with rOmp31 or complete medium. rOmp31 induced the secretion of IFN-γ or IL-2 in spleen cells from Omp3148-74-vaccinated, but not from PBS-immunized, mice (Fig. 5B and C). Neither IL-4 nor IL-10 was detected in any of the culture supernatants from PBS- or Omp3148-74-immunized mice (data not shown). When splenocytes from Omp3148-74-immunized animals were depleted of CD4+ T cells, IFN-γ or IL-2 production in response to rOmp31 was completely abrogated; in contrast, depletion of CD8+ T cells did not affect cytokine production (Fig. 5B and C). Thus, Omp3148-74 immunization induced Omp31-specific CD4+ T cells that secrete IFN-γ and IL-2.
Finally, Omp3148-74-, rOmp31-, or PBS-immunized mice were challenged with B. ovis or B. melitensis, and the level of protection in the spleen was determined as described above. Omp3148-74 conferred significant protection (1.15 log units) against B. melitensis (a strain in which Omp31 is not exposed in the membrane) (P < 0.01) (Table 3). The levels of protection conferred by Omp3148-74 were statistically similar to those obtained with Omp31 (1.25 log units) (P > 0.05). On the other hand, Omp3148-74 also conferred protection against B. ovis infection (0.85 log units), but it conferred statistically significantly less protection (P < 0.05) than rOmp31 (1.81 log units).
TABLE 3.
Protection against B. melitensis or B. ovis in mice immunized with the 27-aa peptide
| Treatment group (n = 8) | Log10aB. melitensis organisms in spleen | Log units of protection in spleen | Log10aB. ovis organisms in spleen | Log units of protection in spleen |
|---|---|---|---|---|
| PBS | 5.10 ± 0.38 | 4.77 ± 0.49 | ||
| Omp3148-74 | 3.95 ± 0.51c | 1.15 | 3.92 ± 0.58b,d | 0.85 |
| rOmp31 | 3.85 ± 0.50c | 1.25 | 2.96 ± 0.36c | 1.81 |
| H38 | 2.97 ± 0.45c | 2.13 | 2.35 ± 0.49c | 2.42 |
The content of bacteria in spleens is represented as the mean log CFU ± SD per group.
Significantly different from PBS-immunized mice (P < 0.05) as estimated by Dunnett's test.
Significantly different from PBS-immunized mice (P < 0.01) as estimated by Dunnett's test.
Significantly different from rOmp31-immunized mice (P < 0.05) as estimated by Dunnett's test.
DISCUSSION
To design a new generation of vaccines, more information on the antigenic makeup of Brucella spp. must be obtained in order to identify immunodominant proteins and epitopes. Also, it is important to understand the immune effector mechanisms elicited by these immunogens. It is thought that a coordinated response of the cellular immune system is fundamental. Consequently, both CD4+ and CD8+ T lymphocytes are believed to play important roles in immunity to Brucella (2), in part because they secrete IFN-γ for the activation of bactericidal functions in macrophages (31). In addition, lysis of infected cells and subsequent killing of Brucella by CTLs, including CD4+, CD8+, and γδ T cells, may be important in maintaining continuous immune surveillance (37). Th1-type Ab isotypes, such as IgG2a and IgG3, may also opsonize the pathogen to facilitate phagocytosis (31).
In this report, we investigated the immune response and protection elicited by the purified recombinant Omp31 from B. melitensis in adjuvant. Mice immunized with rOmp31 were significantly protected against B. ovis and B. melitensis infection (Tables 1, 2, and 3). The presence of immune memory T cells in nonlymphoid organs has been reported in the cases of viral and bacterial infections (32, 34); in that respect, protection was observed in the spleen and also in the liver, an important site for the control of Brucella infection (13, 26) (Table 1). Remarkably, levels of protection afforded after B. ovis challenge were comparable to those achieved by the H38 control vaccine in the spleen and the liver. Although the level of immune protection against B. melitensis infection never reached the level of the H38 vaccine, the rOmp31 vaccine could be used in combination with other protective proteins as a successful alternative in the immunoprophylaxis of B. melitensis infection.
In a recent report, we demonstrated that a DNA vaccine coding for Omp31 confers protection against B. melitensis and B. ovis, along with a weak humoral response, without inducing Th1 responses but inducing strong CD8+ CTL responses. In vivo depletion showed that CD8+ T cells were the cells responsible for the protection afforded against B. melitensis (11). In the present study, rOmp31 in adjuvant induced high Ab IgG titers with a predominance of IgG1 over IgG2a. It also induced a specific Th1 response characterized by the induction of IFN-γ- and IL-2-secreting CD4+ T cells. These results are in agreement with previous work in the field indicating that protective Ags against B. abortus infection induce T-cell proliferation and IFN-γ secretion (1, 38, 43, 44).
rOmp31 also induced a CTL response, but to a lesser extent than the Omp31 DNA vaccine (Fig. 3A and B). Omp31-specific CTLs were able to lyse Brucella-infected macrophages. Mainly CD8+, but also CD4+ T cells were involved in the CTL activity induced by rOmp31. As with the Omp31 DNA vaccine (11), CD8+ T cells elicit lysis of Brucella-infected macrophages via the perforin pathway, while CD4+ T cells use the Fas-FasL pathway (data not shown). Thus, cells involved in, and mechanisms of CTL response induced by, the DNA vaccine are similar to the ones induced by rOmp31 in adjuvant. The main difference observed is that the DNA vaccine induced stronger CTL responses. Recent data indicate that CD8+ and CD4+ T cells are fundamentally different in their requirements for activation and clonal expansion (21). It has been suggested that CD8+ T-cell proliferation requires less Ag for activation and is not influenced by the duration of Ag presentation. Upon activation, these cells enter a developmental program that instructs them to continue division and differentiation into effectors and memory cells in the absence of further Ag stimulation (4, 21, 30). This may partially explain why DNA vaccines (which express low levels of Ag) are particularly good at inducing CTLs. The production of Omp31 detected in transiently transfected COS-7 cells and the immune response induced in vaccinated mice argue for an in vivo expression of this Ag (11). Nevertheless, the amount of Ag produced in vivo after DNA immunization is usually in the picogram or the nanogram range (40). In contrast, CD4+ Th1 cells appear to require repeated Ag exposure and increased amounts of Ag for the survival of proliferating cells and for differentiation into cytokine-producing effector cells, although not for initial proliferation (4). This would explain why higher doses of rOmp31 preferentially induce CD4+ Th1 cells.
In vivo T-cell subset depletion experiments indicated a major role of CD4+ T cells in rOmp31-mediated protective immunity against B. melitensis. These results strongly suggest that Omp31 harbors at least a protective CD4+ T-cell epitope. Even though this vaccine was able to induce a CTL response mediated by CD8+ T cells in vitro, these cells were not protective in vivo. Central memory CTLs have recently been shown to have a greater capacity for in vivo expansion following exposure to Ag and are thus presumably more efficient in mediating protective immunity than are effector memory CTLs (5, 48). Therefore, the subset of memory CTLs generated by vaccination may determine the ultimate effectiveness of vaccine-elicited immune protection against infection (41). We speculate that differences in the levels or the types of CTLs induced in vivo by either rOmp31 or the DNA vaccine could reflect the different roles of CD8+ T cells in mediating protection in each case.
The importance of IFN-γ in resolution of Brucella infection is supported by many studies (20, 49). Indeed, BALB/c or C57BL/6 IFN-γ knockout mice died approximately 6 weeks after infection with B. abortus strain 2308 (36). Immunization with rOmp31 induced IFN-γ-secreting CD4+ T cells and cytotoxic CD8+ T cells. As in vivo only CD4+ T cells were important in immune protection, we speculate that the secretion of IFN-γ is the principal mechanism that allows the rOmp31 vaccine to confer protection against B. melitensis infection. Together, these and previous results (11) suggest that different delivery methods induce different Omp31 responses—Omp31-specific CD4+ Th1 cells (rOmp31 immunization) or CD8+ CTL cells (pCIOmp31 immunization)—yet these responses are able to induce protection against B. melitensis infection. The results also suggest that Omp31 harbors CD4+ and CD8+ T-cell epitopes that, depending on the way they are presented to the immune system, are able to confer protection against Brucella spp.
The ability to produce a peptide Ag that is molecularly defined and pure is highly beneficial in terms of safety, efficacy, and large-scale production. Moreover, the development of peptide-based vaccines does not require reliance on the cold chain for storage. This is an important fact when considering mass population vaccine administration in rural areas of developing countries (17). In the Brucella research field, Tabatabai and Pugh have tested the immunogenicities and protective efficacies against B. abortus strain 2308 of three synthetic peptides derived from Cu-Zn superoxide dismutase, administered singly or in combination, with or without adjuvant, in BALB/c mice. Their results indicated that only one peptide induced significant protection (43). In an attempt to identify one of the epitopes that is responsible for the protective capacity of Omp31, we chose a known exposed region of Omp31 situated between aa 48 and 74 (47). Notably, this peptide stimulated IFN-γ production in CD4+ T cells from rOmp31-immunized mice and also induced intracellular IFN-γ production in CD8+ T cells. These results suggest that within aa 48 to 74, there is a Th1 epitope. On the basis of these results, we also evaluated the humoral and T helper responses elicited after immunization with Omp3148-74 in adjuvant. The peptide induced an extremely weak humoral response. This suggests that either the peptide is not folded as in the native protein and there are important B-cell epitopes that are not efficiently presented to B lymphocytes or that it needs some carrier properties of Omp31 (22). Immunization with Omp3148-74 also induced IFN-γ-secreting CD4+ T cells.
To put forward our hypothesis that this would be a protective Th1 epitope, we evaluated the protection conferred by immunizing mice with this peptide in adjuvant. As we expected, Omp3148-74 conferred significant levels of protection against B. melitensis (a strain in which Omp31 is not exposed in the membrane), and the protection was similar to that obtained with rOmp31, further indicating that it contains at least an immunodominant protective T-cell epitope. On the other hand, Omp3148-74 conferred less protection than rOmp31 against B. ovis (a strain in which Omp31 is exposed in the membrane) (Table 3). A predominant role of hot saline extract-specific Abs relative to T cells as effectors of immunity to B. ovis was described (28). Indeed, a MAb against the epitope of Omp3148-74 administered alone provided passive protection as strong as that obtained with an anti-B. ovis hot saline extract serum (28). Thus, the fact that Omp3148-74 immunization induces a very weak humoral response could explain why protection afforded by Omp3148-74 is lower against B. ovis.
For standard prophylactic immunization in healthy individuals, only adjuvants that induce minimal adverse effects will prove acceptable. However, despite extensive evaluation of a large number of candidates over many years, the only adjuvants currently approved by the U.S. Food and Drug Administration are aluminum-based mineral salts (generically called alum) (3). In relation to that concern, we also tested the protective capacity of rOmp31 in alum hydroxide. rOmp31 induced in BALB/c mice statistically similar (1.2- versus 1.3 log-unit) levels of protection in alum hydroxide and in IFA against B. melitensis (data not shown), further reinforcing the use of this protein as a vaccine candidate in larger animals and humans.
In conclusion, our results indicate that rOmp31 would be a useful candidate for the development of subunit vaccines against brucellosis, since it elicits high levels of protection against smooth and rough species of Brucella. Protection against B. melitensis takes place in virtue of the induction of CD4+ T cells. CD8+ T cells seem to have a minor contribution. Apart from T cells, the strong humoral response induced by rOmp31 would be important in developing protection against B. ovis, as was described previously (28). Finally, the finding that Omp3148-74 is a Th1 protective peptide would be of value in the development of a multivalent subunit vaccine carrying only protective epitopes.
Acknowledgments
We thank Fernando Goldbaum from Leloir Institute, Argentina, for kindly providing the synthetic peptide and Mauricio Rodriguez from Universidad Federal de Sao Paulo, Brazil, for A20J cells.
This work was supported by grant 05-06324 from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT-Argentina) and grants 14116-160 and 14156-33 from Fundación Antorchas. K.A.P. is a recipient of a doctoral fellowship from CONICET (Argentina). J.C, S.D.L.B, C.A.F, and G.H.G. are members of the Research Career of CONICET. S.M.E is a member of CIC (Argentina). C.A.F. is also a member of the Facultad de Ciencias Exactas, Universidad Nacional de La Plata.
Editor: J. D. Clements
REFERENCES
- 1.Al-Mariri, A., A. Tibor, P. Mertens, X. De Bolle, P. Michel, J. Godefroid, K. Walravens, and J. J. Letesson. 2001. Protection of BALB/c mice against Brucella abortus 544 challenge by vaccination with bacterioferritin or P39 recombinant proteins with CpG oligodeoxynucleotides as adjuvant. Infect. Immun. 69:4816-4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araya, L. N., P. H. Elzer, G. E. Rowe, F. M. Enright, and A. J. Winter. 1989. Temporal development of protective cell-mediated and humoral immunity in BALB/c mice infected with Brucella abortus. J. Immunol. 143:3330-3337. [PubMed] [Google Scholar]
- 3.Aucouturier, J., L. Dupuis, and V. Ganne. 2001. Adjuvants designed for veterinary and human vaccines. Vaccine 19:2666-2672. [DOI] [PubMed] [Google Scholar]
- 4.Bajenoff, M., O. Wurtz, and S. Guerder. 2002. Repeated antigen exposure is necessary for the differentiation, but not the initial proliferation, of naive CD4+ T cells. J. Immunol. 168:1723-1729. [DOI] [PubMed] [Google Scholar]
- 5.Barber, D. L., E. J. Wherry, and R. Ahmed. 2003. Cutting edge: rapid in vivo killing by memory CD8 T cells. J. Immunol. 171:27-31. [DOI] [PubMed] [Google Scholar]
- 6.Blasco, J. M., and R. Diaz. 1993. Brucella melitensis Rev-1 vaccine as a cause of human brucellosis. Lancet 342:805. [DOI] [PubMed] [Google Scholar]
- 7.Bosseray, N., and M. Plommet. 1983. A laboratory reference vaccine to titrate immunogenic activity of antibrucella vaccines in mice. Ann. Rech. Vet. 14:163-168. [PubMed] [Google Scholar]
- 8.Bowden, R. A., A. Cloeckaert, M. S. Zygmunt, and G. Dubray. 1995. Outer-membrane protein- and rough lipopolysaccharide-specific monoclonal antibodies protect mice against Brucella ovis. J. Med. Microbiol. 43:344-347. [DOI] [PubMed] [Google Scholar]
- 9.Bowden, R. A., S. M. Estein, M. S. Zygmunt, G. Dubray, and A. Cloeckaert. 2000. Identification of protective outer membrane antigens of Brucella ovis by passive immunization of mice with monoclonal antibodies. Microbes Infect. 2:481-488. [DOI] [PubMed] [Google Scholar]
- 10.Cassataro, J., K. Pasquevich, L. Bruno, J. C. Wallach, C. A. Fossati, and P. C. Baldi. 2004. Antibody reactivity to Omp31 from Brucella melitensis in human and animal infections by smooth and rough Brucellae. Clin. Diagn. Lab. Immunol. 11:111-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassataro, J., C. Velikovsky, S. de la Barrera, S. Estein, L. Bruno, R. Bowden, K. Pasquevich, C. Fossati, and G. Giambartolomei. 2005. A DNA vaccine coding for the Brucella outer membrane protein 31 confers protection against B. melitensis and B. ovis infection by eliciting a specific cytotoxic response. Infect. Immun. 73:6537-6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cespedes, S., E. Andrews, H. Folch, and A. Onate. 2000. Identification and partial characterisation of a new protective antigen of Brucella abortus. J. Med. Microbiol. 49:165-170. [DOI] [PubMed] [Google Scholar]
- 13.Cheville, N. F., R. A. Kunkle, A. E. Jensen, and M. V. Palmer. 1995. Persistence of Brucella abortus in the livers of T cell-deficient nude mice. Lab. Investig. 73:96-102. [PubMed] [Google Scholar]
- 14.Cloeckaert, A., I. Jacques, N. Bosseray, J. N. Limet, R. Bowden, G. Dubray, and M. Plommet. 1991. Protection conferred on mice by monoclonal antibodies directed against outer-membrane-protein antigens of Brucella. J. Med. Microbiol. 34:175-180. [DOI] [PubMed] [Google Scholar]
- 15.Cloeckaert, A., P. Kerkhofs, and J. N. Limet. 1992. Antibody response to Brucella outer membrane proteins in bovine brucellosis: immunoblot analysis and competitive enzyme-linked immunosorbent assay using monoclonal antibodies. J. Clin. Microbiol. 30:3168-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cloeckaert, A., N. Vizcaino, J. Y. Paquet, R. A. Bowden, and P. H. Elzer. 2002. Major outer membrane proteins of Brucella spp.: past, present and future. Vet. Microbiol. 90:229-247. [DOI] [PubMed] [Google Scholar]
- 17.Demotz, S., C. Moulon, M. A. Roggero, N. Fasel, and S. Masina. 2001. Native-like, long synthetic peptides as components of sub-unit vaccines: practical and theoretical considerations for their use in humans. Mol. Immunol. 38:415-422. [DOI] [PubMed] [Google Scholar]
- 18.Estein, S. M., J. Cassataro, N. Vizcaino, M. S. Zygmunt, A. Cloeckaert, and R. A. Bowden. 2003. The recombinant Omp31 from Brucella melitensis alone or associated with rough lipopolysaccharide induces protection against Brucella ovis infection in BALB/c mice. Microbes Infect. 5:85-93. [DOI] [PubMed] [Google Scholar]
- 19.Estein, S. M., P. C. Cheves, M. A. Fiorentino, J. Cassataro, F. A. Paolicchi, and R. A. Bowden. 2004. Immunogenicity of recombinant Omp31 from Brucella melitensis in rams and serum bactericidal activity against B. ovis. Vet. Microbiol. 102:203-213. [DOI] [PubMed] [Google Scholar]
- 20.Eze, M. O., L. Yuan, R. M. Crawford, C. M. Paranavitana, T. L. Hadfield, A. K. Bhattacharjee, R. L. Warren, and D. L. Hoover. 2000. Effects of opsonization and gamma interferon on growth of Brucella melitensis 16M in mouse peritoneal macrophages in vitro. Infect. Immun. 68:257-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foulds, K. E., L. A. Zenewicz, D. J. Shedlock, J. Jiang, A. E. Troy, and H. Shen. 2002. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J. Immunol. 168:1528-1532. [DOI] [PubMed] [Google Scholar]
- 22.Golding, B., and D. E. Scott. 1995. Vaccine strategies: targeting helper T cell responses. Ann. N. Y. Acad. Sci. 754:126-137. [DOI] [PubMed] [Google Scholar]
- 23.Guilloteau, L. A., K. Laroucau, N. Vizcaino, I. Jacques, and G. Dubray. 1999. Immunogenicity of recombinant Escherichia coli expressing the omp31 gene of Brucella melitensis in BALB/c mice. Vaccine 17:353-361. [DOI] [PubMed] [Google Scholar]
- 24.Gurunathan, S., D. M. Klinman, and R. A. Seder. 2000. DNA vaccines: immunology, application, and optimization. Annu. Rev. Immunol. 18:927-974. [DOI] [PubMed] [Google Scholar]
- 25.Gurunathan, S., C. Y. Wu, B. L. Freidag, and R. A. Seder. 2000. DNA vaccines: a key for inducing long-term cellular immunity. Curr. Opin. Immunol. 12:442-447. [DOI] [PubMed] [Google Scholar]
- 26.Izadjoo, M. J., Y. Polotsky, M. G. Mense, A. K. Bhattacharjee, C. M. Paranavitana, T. L. Hadfield, and D. L. Hoover. 2000. Impaired control of Brucella melitensis infection in Rag1-deficient mice. Infect. Immun. 68:5314-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacques, I., A. Cloeckaert, J. N. Limet, and G. Dubray. 1992. Protection conferred on mice by combinations of monoclonal antibodies directed against outer-membrane proteins or smooth lipopolysaccharide of Brucella. J. Med. Microbiol. 37:100-103. [DOI] [PubMed] [Google Scholar]
- 28.Jimenez de Bagues, M. P., P. H. Elzer, J. M. Blasco, C. M. Marin, C. Gamazo, and A. J. Winter. 1994. Protective immunity to Brucella ovis in BALB/c mice following recovery from primary infection or immunization with subcellular vaccines. Infect. Immun. 62:632-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung, G., B. Fleckenstein, F. von der Mulbe, J. Wessels, D. Niethammer, and K. H. Wiesmuller. 2001. From combinatorial libraries to MHC ligand motifs, T-cell superagonists and antagonists. Biologicals 29:179-181. [DOI] [PubMed] [Google Scholar]
- 30.Kaech, S. M., and R. Ahmed. 2001. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ko, J., and G. A. Splitter. 2003. Molecular host-pathogen interaction in brucellosis: current understanding and future approaches to vaccine development for mice and humans. Clin. Microbiol. Rev. 16:65-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lefrancois, L., and D. Masopust. 2002. T cell immunity in lymphoid and non-lymphoid tissues. Curr. Opin. Immunol. 14:503-508. [DOI] [PubMed] [Google Scholar]
- 33.Liljeqvist, S., and S. Stahl. 1999. Production of recombinant subunit vaccines: protein immunogens, live delivery systems and nucleic acid vaccines. J. Biotechnol. 73:1-33. [DOI] [PubMed] [Google Scholar]
- 34.Masopust, D., V. Vezys, A. L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413-2417. [DOI] [PubMed] [Google Scholar]
- 35.Moriyon, I., M. J. Grillo, D. Monreal, D. Gonzalez, C. Marin, I. Lopez-Goni, R. C. Mainar-Jaime, E. Moreno, and J. M. Blasco. 2004. Rough vaccines in animal brucellosis: structural and genetic basis and present status. Vet. Res. 35:1-38. [DOI] [PubMed] [Google Scholar]
- 36.Murphy, E. A., J. Sathiyaseelan, M. A. Parent, B. Zou, and C. L. Baldwin. 2001. Interferon-gamma is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunology 103:511-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliveira, S. C., and G. A. Splitter. 1995. CD8+ type 1 CD44hi CD45 RBlo T lymphocytes control intracellular Brucella abortus infection as demonstrated in major histocompatibility complex class I- and class II-deficient mice. Eur. J. Immunol. 25:2551-2557. [DOI] [PubMed] [Google Scholar]
- 38.Oliveira, S. C., and G. A. Splitter. 1996. Immunization of mice with recombinant L7/L12 ribosomal protein confers protection against Brucella abortus infection. Vaccine 14:959-962. [DOI] [PubMed] [Google Scholar]
- 39.Rammensee, H., J. Bachmann, N. P. Emmerich, O. A. Bachor, and S. Stevanovic. 1999. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 50:213-219. [DOI] [PubMed] [Google Scholar]
- 40.Rush, C., T. Mitchell, and P. Garside. 2002. Efficient priming of CD4+ and CD8+ T cells by DNA vaccination depends on appropriate targeting of sufficient levels of immunologically relevant antigen to appropriate processing pathways. J. Immunol. 169:4951-4960. [DOI] [PubMed] [Google Scholar]
- 41.Seaman, M. S., F. W. Peyerl, S. S. Jackson, M. A. Lifton, D. A. Gorgone, J. E. Schmitz, and N. L. Letvin. 2004. Subsets of memory cytotoxic T lymphocytes elicited by vaccination influence the efficiency of secondary expansion in vivo. J. Virol. 78:206-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh, M., and D. O'Hagan. 1999. Advances in vaccine adjuvants. Nat. Biotechnol. 17:1075-1081. [DOI] [PubMed] [Google Scholar]
- 43.Tabatabai, L. B., and G. W. Pugh, Jr. 1994. Modulation of immune responses in Balb/c mice vaccinated with Brucella abortus Cu-Zn superoxide dismutase synthetic peptide vaccine. Vaccine 12:919-924. [DOI] [PubMed] [Google Scholar]
- 44.Velikovsky, C. A., J. Cassataro, G. H. Giambartolomei, F. A. Goldbaum, S. Estein, R. A. Bowden, L. Bruno, C. A. Fossati, and M. Spitz. 2002. A DNA vaccine encoding lumazine synthase from Brucella abortus induces protective immunity in BALB/c mice. Infect. Immun. 70:2507-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Velikovsky, C. A., F. A. Goldbaum, J. Cassataro, S. Estein, R. A. Bowden, L. Bruno, C. A. Fossati, and G. H. Giambartolomei. 2003. Brucella lumazine synthase elicits a mixed Th1-Th2 immune response and reduces infection in mice challenged with Brucella abortus 544 independently of the adjuvant formulation used. Infect. Immun. 71:5750-5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vizcaino, N., A. Cloeckaert, M. S. Zygmunt, and G. Dubray. 1996. Cloning, nucleotide sequence, and expression of the Brucella melitensis omp31 gene coding for an immunogenic major outer membrane protein. Infect. Immun. 64:3744-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vizcaino, N., R. Kittelberger, A. Cloeckaert, C. M. Marin, and L. Fernandez-Lago. 2001. Minor nucleotide substitutions in the omp31 gene of Brucella ovis result in antigenic differences in the major outer membrane protein that it encodes compared to those of the other Brucella species. Infect. Immun. 69:7020-7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wherry, E. J., V. Teichgraber, T. C. Becker, D. Masopust, S. M. Kaech, R. Antia, U. H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225-234. [DOI] [PubMed] [Google Scholar]
- 49.Zhan, Y., and C. Cheers. 1993. Endogenous gamma interferon mediates resistance to Brucella abortus infection. Infect. Immun. 61:4899-4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zygmunt, M. S., H. S. Debbarh, A. Cloeckaert, and G. Dubray. 1994. Antibody response to Brucella melitensis outer membrane antigens in naturally infected and Rev1 vaccinated sheep. Vet. Microbiol. 39:33-46. [DOI] [PubMed] [Google Scholar]