Abstract
Cryptococcus neoformans capsular glucuronoxylomannan (GXM) is shed during cryptococcosis and taken up by macrophages. The roles of the putative GXM receptors CD14, CD18, Toll-like receptor 2 (TLR2), and TLR4 in GXM clearance from serum and deposition in the liver and spleen in receptor-deficient mice were studied. While alterations in the kinetics of GXM redistribution were seen in the mutant mice, none of the receptors was absolutely required for serum clearance or hepatosplenic accumulation.
The encapsulated yeast Cryptococcus neoformans causes infections primarily in persons with defects in cell-mediated immunity. The major virulence factor of the organism is the capsule, which is composed mostly of the high-molecular- weight polysaccharide glucuronoxylomannan (GXM). GXM has numerous immunomodulatory properties, including inhibition of leukocyte migration, alteration of cytokine production, and inhibition of neutrophil anticryptococcal activity (2, 10).
During cryptococcosis, GXM is shed into the blood and cerebrospinal fluid (CSF) at up to μg/ml concentrations and often can be detected in the blood and CSF for months after successful antifungal therapy (14). GXM in the brains of patients with cryptococcal meningitis is associated with macrophages/microglial cells, which may serve as a reservoir for GXM after the organism is cleared (7).
Due to the postulated contribution of shed GXM to the pathogenesis of cryptococcosis, the fate of circulating GXM has received much investigation. Following intravenous (i.v.) injection in mice, the serum half-life of GXM was shown to be 14 to 48 h (5, 8). The GXM subsequently accumulated in the liver and spleen, where it could be detected for weeks after injection (5, 8, 12). Injected GXM localizes primarily to marginal zone macrophages in the spleen and Kupffer cells in the liver (4, 5). Depletion of tissue macrophages in vivo results in decreased accumulation of GXM in the liver and spleen and fivefold lower total body GXM levels, suggesting that the sequestering of GXM by macrophages prevents clearance from the body (5).
In vitro studies have shown that CD14, CD11/CD18 heterodimers, TLR2, and TLR4 are receptors for GXM (1, 15). Uptake of GXM by human monocytes and neutrophils was demonstrated to involve CD14 and CD11/CD18, respectively (10). Similarly, GXM uptake by human monocyte-derived macrophages (MDM) was inhibited by antibodies against CD14, TLR4, and CD18 (9). Supporting a role for CD18 as a GXM receptor, CD11b/CD18, also known as complement receptor 3 (CR3), and CD11c/CD18 (CR4) can mediate antibody-dependent, complement-independent phagocytosis of C. neoformans (16).
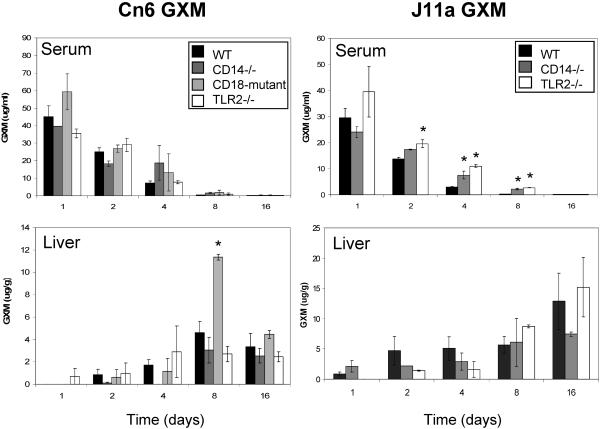
To elucidate the role of these receptors in GXM clearance in vivo, C57BL/6 wild-type (WT), CD14−/−, TLR2−/−, TLR4−/−, and CD18 mutant mice were injected i.v. with GXM, and the serum, liver, and spleen were harvested on various days after injection. GXM from serotype A C. neoformans strains 6 (ATCC 62066) and J11a (a gift of Arturo Casadevall, Albert Einstein College of Medicine, New York, NY) was prepared as described previously (15). The GXM preparations had undetectable levels (<0.03 endotoxin U/ml) of endotoxin as measured by Limulus amoebocyte lysate assay (Associates of Cape Cod, East Falmouth, MA). C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The CD14−/−, TLR2−/−, and TLR4−/− mice were engineered as described and backcrossed at least 5 generations to a C57BL/6 background (6, 11, 17). The TLR2−/− and TLR4−/− mice were provided by Shizuo Akira (Osaka University, Osaka, Japan) via Douglas Golenbock (University of Massachusetts Medical School, Worcester, MA), and the CD14−/− mice were provided by Mason Freeman (Massachusetts General Hospital, Harvard Medical School, Boston, MA). The CD18 mutant mice (The Jackson Laboratory) exhibit 2 and 16% of normal CD18 expression on resting and activated granulocytes, respectively (18). Blood was obtained by cardiac puncture and centrifuged, and the serum was collected and stored at −80°C. The spleens and livers were homogenized in 1 ml phosphate-buffered saline and stored at −80°C. The levels of GXM were quantified by enzyme-linked immunosorbent assay using the anti-GXM monoclonal antibody 3C2 (gift of Thomas Kozel, University of Nevada School of Medicine, Reno, NV) as described previously (5). Data are expressed as micrograms of GXM per ml of serum or gram of organ ± standard error of the mean (SEM). The organ GXM levels were corrected for plasma GXM by subtracting the plasma GXM from total organ GXM, using published mouse organ plasma volumes (3) as described previously (5). Statistical comparisons utilized Student's t test. Bonferroni's correction was applied to account for the multiple comparisons made at each time point. P values of <0.05 after Bonferroni's correction were considered significant. In the first experiment, WT, CD14−/−, CD18 mutant, and TLR2−/− mice were injected i.v. with GXM from strain 6 and GXM levels were measured in the serum, liver, and spleen on days 1, 2, 4, 8, and 16 postinjection. Consistent with the data of Grinsell et al. (5), over the course of the experiment GXM was cleared from the serum and accumulated in the liver (Fig. 1) and spleen (data not shown). Thus, by day 16, the levels of GXM in the serum were less than 1 μg/ml. There was a gradual accumulation of GXM in the liver that peaked at day 8, while the GXM concentrations in the spleen increased from day 1 to day 2 but then remained steady through day 16. The clearances were similar between both CD14−/− and TLR2−/− mice and WT mice. However, in an experiment using strain J11a GXM, CD14−/− and TLR2−/− mice demonstrated significant differences in clearance (Fig. 1). The TLR2−/− mice had significantly higher GXM levels in the serum on days 2, 4, and 8, indicating delayed serum clearance. The CD14−/− mice also had significantly higher GXM levels in the serum on day 4 and day 8. Not shown in Fig. 1, there were no significant differences in splenic concentrations of GXM except that the TLR2 and CD14 knockout mice displayed significantly increased GXM levels at day 16 compared with WT mice (20 ± 1 μg/g for TLR2−/− and 18 ± 1 μg/g for CD14−/− versus 13 μg/g for WT; P < 0.005 for both comparisons).
FIG. 1.
GXM clearance from serum and accumulation in the liver and spleen in WT, CD14−/−, TLR2−/−, and CD18 mutant mice. Mice were injected i.v. with 75 μg strain 6 or J11a GXM. GXM concentrations then were measured in the serum, liver, and spleen on days 1, 2, 4, 8, and 16 after injection. The data are expressed as the mean ± SEM of four WT and two mutant mice per day. *, P ≤ 0.01.
When compared with WT mice, the CD18 mutant mice displayed significantly increased accumulation of strain 6 GXM in the liver on day 8 (Fig. 1). Significant differences were also seen when a higher dose of strain 6 GXM (250 μg) was injected into WT and CD18 mutant mice. The CD18 mutant mice demonstrated increased accumulation of GXM in the liver on days 4 (95 ± 1 versus 43 ± 4 μg/g for WT; P < 0.01) and 8 (143 ± 21 versus 67 ± 1 μg/g for WT; not significant), as well as in the spleen on day 4 (97 ± 1 versus 52 μg/g for WT; P < 0.005) (n = 2 WT and 2 mutant mice per day).
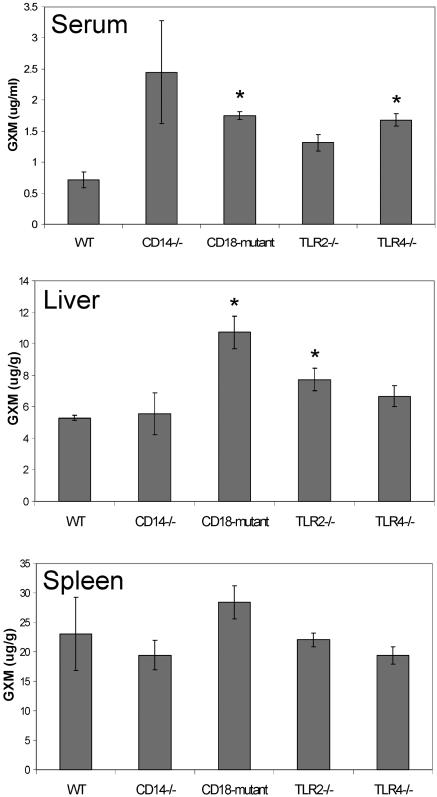
In the final experiment, WT, CD14−/−, CD18 mutant, TLR2−/−, and TLR4−/− mice were injected with 75 μg GXM from strain 6 and serum, liver, and spleen GXM levels determined (Fig. 2). The mice were studied on day 8 following injection because previous experiments demonstrated maximal differences at this time point. The CD18 mutant and TLR4−/− mice had significantly higher serum GXM levels than WT mice. As in previous experiments, the CD18 mutant mice demonstrated significantly higher GXM concentrations in the liver. While the TLR2−/− mice also had significantly higher liver GXM levels, the difference was modest and, taken together with the data in Fig. 1 showing no difference, unlikely to be of biological significance. There were no significant differences found in splenic GXM levels between WT and receptor-deficient mice.
FIG. 2.
GXM levels in the serum, liver, and spleen in WT, CD14−/−, CD18 mutant, TLR2−/−, and TLR4−/− mice 8 days postinjection. Mice were injected i.v. with 75 μg strain 6 GXM. GXM concentrations were measured in the serum, liver, and spleen on day 8 after injection. The data are expressed as the mean ± SEM of five WT and four mutant mice. ★, P < 0.01.
Thus, our results demonstrate differences in the kinetics of GXM clearance and organ accumulation between WT and receptor-deficient mice. In particular, the CD18 mutant mice had consistent increases in hepatic accumulation of GXM. While this was not due to decreased splenic uptake, it is possible there was reduced deposition in other organs, such as the kidneys or bone marrow. It is also conceivable that the higher liver GXM levels in the CD18 mutant mice do not reflect increased uptake, but rather decreased degradation and/or excretion. In fact, neutrophils (which internalize GXM in part via CD18) have been shown to expel or degrade GXM after 1 h of incubation (10). In addition, as the CD18 mutant mice express some CD18, it is possible the phenotype would be more dramatic in mice totally lacking CD18. Overall, though, the differences seen in the CD14-, TLR2-, TLR4-, and CD18-deficient mice were relatively modest, suggesting that, individually, none of the receptors studied has a dominant role in GXM clearance.
Although some GXM may be taken up by fluid-phase endocytosis in vivo, Lendvai et al. demonstrated that nonlabeled cryptococcal capsular polysaccharide competed with labeled capsular polysaccharide for uptake in the liver and spleen, suggesting a receptor-mediated process (8). While blocking CD14, CD18, or TLR4 inhibited the uptake of GXM by MDM in vitro, the inhibition was not additive, or complete, when all three receptors were blocked, suggesting the existence of another GXM receptor(s) (9). One such candidate is FcγRII, as blocking this receptor partially inhibited antibody-independent uptake by MDM (9).
In vivo, the administration of anti-GXM monoclonal antibodies reduces serum GXM levels and enhances GXM sequestration in the liver and spleen (4, 5, 8, 13). While clearance of GXM from the serum could prevent some of the polysaccharide's immunoinhibitory effects, including the inhibition of leukocyte migration, it is possible enhanced GXM accumulation in the tissues could have deleterious effects. In particular, monoclonal antibody-mediated increased GXM uptake by macrophages in the spleen could impair the ability of these cells to activate T cells, as demonstrated for human MDM in vitro (9). The fate of intracellular GXM differs between human monocytes and neutrophils in vitro (10), and it is likely that the receptor(s) used for internalization affects the outcome. If so, it would be beneficial to target GXM in patients to a receptor(s) that mediates internalization followed by degradation, rather than persistence.
Acknowledgments
We thank Douglas Golenbock, Mason Freeman, and Shizuo Akira for providing the mice, Thomas Kozel for the 3C2 antibodies, and Al Ozonoff for help with the statistical analysis.
This work was supported in part by National Institutes of Health grants RO1 AI25780, RO1 AI37532, and T32 AI07309. All mouse experiments were approved by the Boston University Institutional Animal Care and Use Committee.
Editor: A. Casadevall
REFERENCES
- 1.Dong, Z. M., and J. W. Murphy. 1997. Cryptococcal polysaccharides bind to CD18 on human neutrophils. Infect. Immun. 65:557-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellerbroek, P. M., A. M. Walenkamp, A. I. Hoepelman, and F. E. Coenjaerts. 2004. Effects of the capsular polysaccharides of Cryptococcus neoformans on phagocyte migration and inflammatory mediators. Curr. Med. Chem. 11:253-266. [DOI] [PubMed] [Google Scholar]
- 3.Friedman, J. J. 1955. Organ plasma volume of normal unanesthetized mice. Proc. Soc. Exp. Biol. Med. 88:323-325. [DOI] [PubMed] [Google Scholar]
- 4.Goldman, D. L., S. C. Lee, and A. Casadevall. 1995. Tissue localization of Cryptococcus neoformans glucuronoxylomannan in the presence and absence of specific antibody. Infect. Immun. 63:3448-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grinsell, M., L. C. Weinhold, J. E. Cutler, Y. Han, and T. R. Kozel. 2001. In vivo clearance of glucuronoxylomannan, the major capsular polysaccharide of Cryptococcus neoformans: a critical role for tissue macrophages. J. Infect. Dis. 184:479-487. [DOI] [PubMed] [Google Scholar]
- 6.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 7.Lee, S. C., A. Casadevall, and D. W. Dickson. 1996. Immunohistochemical localization of capsular polysaccharide antigen in the central nervous system cells in cryptococcal meningoencephalitis. Am. J. Pathol. 148:1267-1274. [PMC free article] [PubMed] [Google Scholar]
- 8.Lendvai, N., A. Casadevall, Z. Liang, D. L. Goldman, J. Mukherjee, and L. Zuckier. 1998. Effect of immune mechanisms on the pharmacokinetics and organ distribution of cryptococcal polysaccharide. J. Infect. Dis. 177:1647-1659. [DOI] [PubMed] [Google Scholar]
- 9.Monari, C., F. Bistoni, A. Casadevall, E. Pericolini, D. Pietrella, T. R. Kozel, and A. Vecchiarelli. 2005. Glucuronoxylomannan, a microbial compound, regulates expression of costimulatory molecules and production of cytokines in macrophages. J. Infect. Dis. 191:127-137. [DOI] [PubMed] [Google Scholar]
- 10.Monari, C., C. Retini, A. Casadevall, D. Netski, F. Bistoni, T. R. Kozel, and A. Vecchiarelli. 2003. Differences in outcome of the interaction between Cryptococcus neoformans glucuronoxylomannan and human monocytes and neutrophils. Eur. J. Immunol. 33:1041-1051. [DOI] [PubMed] [Google Scholar]
- 11.Moore, K. J., L. P. Andersson, R. R. Ingalls, B. G. Monks, R. Li, M. A. Arnaout, D. T. Golenbock, and M. W. Freeman. 2000. Divergent response to LPS and bacteria in CD14-deficient murine macrophages. J. Immunol. 165:4272-4280. [DOI] [PubMed] [Google Scholar]
- 12.Muchmore, H. G., E. N. Scott, F. G. Felton, and R. A. Fromtling. 1982. Cryptococcal capsular polysaccharide clearance in nonimmune mice. Mycopathologia 78:41-45. [DOI] [PubMed] [Google Scholar]
- 13.Mukherjee, J., L. S. Zuckier, M. D. Scharff, and A. Casadevall. 1994. Therapeutic efficacy of monoclonal antibodies to Cryptococcus neoformans glucuronoxylomannan alone and in combination with amphotericin B. Antimicrob. Agents Chemother. 38:580-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powderly, W. G., G. A. Cloud, W. E. Dismukes, and M. S. Saag. 1994. Measurement of cryptococcal antigen in serum and cerebrospinal fluid: value in the management of AIDS-associated cryptococcal meningitis. Clin. Infect. Dis. 18:789-792. [DOI] [PubMed] [Google Scholar]
- 15.Shoham, S., C. Huang, J. M. Chen, D. T. Golenbock, and S. M. Levitz. 2001. Toll-like receptor 4 mediates intracellular signaling without TNF-alpha release in response to Cryptococcus neoformans polysaccharide capsule.J. Immunol. 166:4620-4626. [DOI] [PubMed] [Google Scholar]
- 16.Taborda, C. P., and A. Casadevall. 2002. CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are involved in complement-independent antibody-mediated phagocytosis of Cryptococcus neoformans. Immunity 16:791-802. [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 18.Wilson, R. W., C. M. Ballantyne, C. W. Smith, C. Montgomery, A. Bradley, W. E. O'Brien, and A. L. Beaudet. 1993. Gene targeting yields a CD18 mutant mouse for study of inflammation. J. Immunol. 151:1571-1578. [PubMed] [Google Scholar]