Abstract
In vitro, Clostridium perfringens enterotoxin (CPE) binds to human ileal epithelium and induces morphological damage concurrently with reduced short-circuit current, transepithelial resistance, and net water absorption. CPE also binds to the human colon in vitro but causes only slight morphological and transport changes that are not statistically significant.
Clostridium perfringens enterotoxin (CPE) is produced by ≤5% of C. perfringens isolates. Epidemiological evidence suggests that CPE plays an important role in the pathogenesis of both food-borne and non-food-borne human gastrointestinal (GI) illnesses caused by cpe-positive C. perfringens type A isolates (9). Specifically, feces from patients suffering from GI illnesses caused by cpe-positive isolates contain CPE at concentrations that cause GI effects in animal models (1, 2). Moreover, ingestion of highly purified CPE by human volunteers induces diarrhea (24), a predominant symptom of GI illnesses involving cpe-positive C. perfringens type A isolates. Finally, inactivation of the cpe gene abrogates the ability of cpe-positive C. perfringens human GI disease isolates to cause GI disease in experimental animals (20).
Although CPE intestinal activity in animal models has been demonstrated (12, 14-21) and the cytotoxicity mechanism is relatively well characterized in cell lines (4, 6-11, 22, 23, 26), how those results correspond to CPE effects on the human intestine remains uncertain. Therefore, we examined whether CPE induces physiological and morphological changes to the human small and large intestine in vitro.
To study CPE effects on human intestinal water and ion transport, net water flux and electrophysiological parameters were measured in parallel using modified Ussing chambers. After informed consent was obtained, sections of macroscopically unaffected margins of human ileum and colon specimens were obtained from organs surgically removed from eight adult intestinal-cancer patients. The study protocol was approved by the Institutional Review Board of the University of Buenos Aires (Argentina) and the University of California Committee for Research with Human Tissues (no. 200513194-1).
Transepithelial net water flux (Jw) was recorded automatically in a modified Ussing chamber connected to a special electro-optical device (5). The spontaneous potential difference (PD) was recorded in the other chamber across Ag/AgCl electrodes, via agar bridges placed adjacent to the epithelium under open-circuit conditions. The short-circuit current (Isc) was measured with an automatic voltage clamp system that kept the potential difference at 0 mV. Transepithelial resistance (Rt) was calculated using Ohm's law from the open-circuit PD and Isc. Variations in electric parameters were continuously measured for a minimum of 60 min. The effects of CPE on intestinal electrophysiology were compared using PD, ΔIsc, and ΔRt, where ΔIsc = (Isc at time t) − (Isc at time zero) and where ΔRt = (Rt at time t) − (Rt at time zero). Samples of each experimental specimen were obtained immediately after collection from the patients and also after treatment in the Ussing chambers, fixed by immersion in 10% buffered (pH 7.2) formalin for 24 h, embedded in paraffin wax, sectioned at 4 μm, stained with hematoxylin and eosin, and examined by use of light microscopy. Sections from selected cases were also processed by an indirect immunoperoxidase technique to detect CPE (3). This technique was performed using a monoclonal antibody against CPE (25) and the Dako EnVision kit (Dako Corporation, Carpinteria, CA). CPE was purified to homogenity from C. perfringens strain NCTC8239 (13).
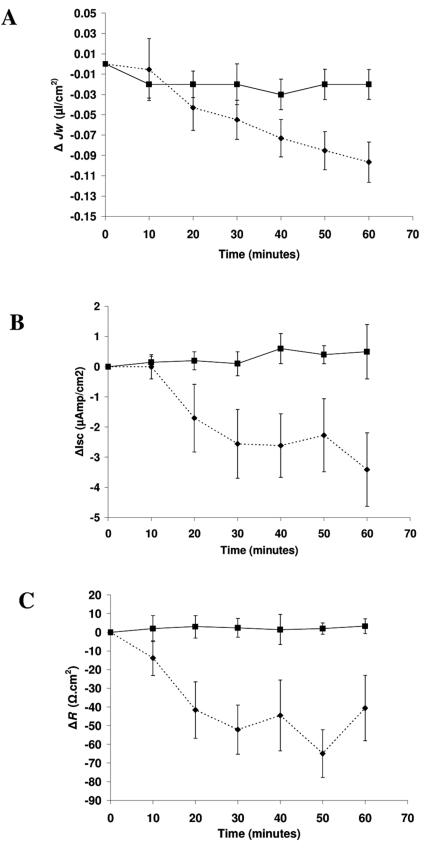
Under basal conditions, a net absorptive Jw was observed when human ileum or colon was placed between two identical Ringer solutions in the Ussing chamber. Table 1 shows the mean Jw values and electric parameters measured simultaneously before toxin treatment. Normal ileal (Fig. 1) and colonic (not shown) mucosa segments incubated for 60 min with Ringer solution alone showed stable Jw and electrophysiological values. Addition of 10 μg/ml of purified CPE to the mucosal side (t = 0) of the human ileum inhibited Jw (Fig. 1A), concomitant with a decreased Isc (Fig. 1B) and Rt (Fig. 1C). These effects depended upon the incubation time and became statistically significant 20 min after toxin exposure, continuing to decrease throughout the 60-min observation period. In the colon, purified CPE (10 μg/ml) altered both Jw and electrophysiological parameters (Isc and Rt), although the changes were not statistically significant after 60 min of toxin treatment (Table 2).
TABLE 1.
Net absorptive water flux and electric parameters in human intestine under basal conditions
| Parameter | Value
|
|
|---|---|---|
| Ileum | Colon | |
| Jw (μl/min/cm2) | 0.28 ± 0.09 | 0.35 ± 0.10 |
| PD (mV) | −1.5 ± 0.3 | −3.5 ± 1.0 |
| Isc (μA/cm2) | 10.3 ± 2.1 | 13.4 ± 3.4 |
| Rt (Ω · cm2) | 180 ± 23 | 257 ± 38 |
FIG. 1.
Time course effect of CPE on (A) transepithelial net water flux (ΔJw), (B) short-circuit current (ΔIsc), and (C) transepithelial resistance (ΔRt) in human ileum. After an equilibration period, intestinal mucosal sheets placed in Ussing chambers were incubated at the mucosal side with either Ringer solution alone (solid line) or Ringer solution containing 10 μg/ml of CPE (dotted line) and monitored for 60 min. Each point indicates the mean ± standard error of the mean of four experiments.
TABLE 2.
Effects of purified CPE (10 μg/ml) on large human intestine
| Parameter | Valuea
|
|
|---|---|---|
| Control | CPE | |
| ΔJw (μl/min/cm2) | −0.025 ± 0.02 | −0.03 ± 0.01 |
| ΔIsc (μA/cm2) | 1.9 ± 1.4 | −1.9 ± 1.0 |
| ΔRt (Ω · cm2) | −10 ± 6 | −23 ± 7 |
Data are reported as means ± standard errors of the mean (n = 4).
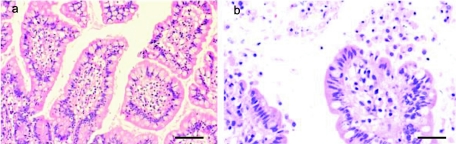
Without CPE treatment, the human colonic and ileal tissue showed no significant histological changes (Fig. 2A). Ileal tissues (n = 4) treated for 60 min in vitro with purified CPE (10 μg/ml) showed similar morphological changes, including shortening and blunting of villi. The villus tips showed enterocyte necrosis, with disruption of the basement membrane and extrusion of epithelial, goblet, and lamina propia cells into the intestinal lumen. The necrotic enterocytes showed pyknotic, condensed nuclei or figures of karyorrhexis and karyolysis. These cells had a rounded, homogeneous, and acidophilic cytoplasm, which was frequently vacuolated and/or disrupted. A basophilic layer of mucus, mixed with desquamated epithelial cells, covered the intestinal mucosa (Fig. 2B). Colonic mucosa from all patients (n = 4) similarly treated with purified CPE showed mild effects, consisting only of flattening of superficial epithelial cells, individual epithelial cell necrosis, and sloughing of a few enterocytes.
FIG. 2.
Morphological effect of CPE on human ileal mucosa. (a) Human ileum after 60 min of incubation with Ringer solution (control) in Ussing chamber. No significant histological abnormalities were observed. Hematoxylin and eosin, 400×. (b) Human ileum treated with CPE in an Ussing chamber for 60 min. Observe the necrosis and desquamation of the superficial epithelium at the tips of the villi. Hematoxylin and eosin, 400×. Bars, 40 μm.
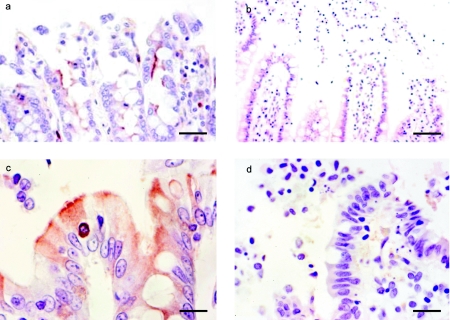
Immunohistochemistry showed intense CPE staining along the brush border of the ileal epithelium, which sometimes extended to the intercellular lateral borders of the enterocytes. Desquamated epithelial cells in the intestinal lumen were surrounded by thin CPE staining (Fig. 3A). Clearly positive CPE staining was observed in only one colonic segment after CPE treatment and consisted of a fine brown line over the apical and basolateral enterocyte surface (Fig. 3C). Two other colonic segments gave similar but weaker staining. No CPE staining was observed in any tissues treated with CPE and stained using normal rabbit serum (negative control) (Fig. 3B and C).
FIG. 3.
Binding of CPE on human intestine. (a) Human ileum treated with CPE in an Ussing chamber for 60 min. Observe positively stained CPE on the apical borders of the enterocytes. Avidin biotin peroxidase, anti-CPE primary antibody, 400×. (b) Human ileum treated with CPE in an Ussing chamber for 60 min. Avidin biotin peroxidase, normal rabbit serum (negative control), 400×. (c) Human colon treated with CPE in an Ussing chamber for 60 min. Observe positively stained CPE on the apical and basolateral borders of the enterocytes. Avidin biotin peroxidase, anti-CPE primary antibody, 400×. (d) Human colon treated with CPE in an Ussing chamber for 60 min. Avidin biotin peroxidase, normal rabbit serum (negative control), 400×. Bars, 40 μm (a and b) and 20 μm (c and d).
Previous rabbit studies (15, 21) suggested that tissue damage is important for triggering fluid and electrolyte transport changes in CPE-treated ileum. Similarly, our results indicate that purified CPE also impairs ion and water transport in human ileum and that those effects are related to ileal tissue damage, i.e., villus damage was concurrent with decreases in ileal transepithelial net water absorption and Rt.
Caco-2 cell experiments suggested that CPE interactions with enterocytes disrupt intercellular tight junctions, which could alter paracellular permeability (23). Tight junctions of the small intestine are the low-resistance type, and the paracellular route is considered the main route for transport-associated water transfer (19). Therefore, CPE-induced tight-junction disruptions could explain the changes in Jw, Isc, and Rt observed in human ileum. However, the histological damage to villus cells caused by 60-min treatment with 10 μg/ml CPE was very severe, indicating the occurrence of more than tight-junction disruption and suggesting that CPE induces diarrhea by altering the normal intestinal absorption-secretion balance toward net secretion. Since tight junctions of villus enterocytes have higher resistance than those of the crypts, tight-junction damage at the villi could fundamentally impact total mucosal electrical resistance.
The diarrhea observed in humans infected with CPE-producing C. perfringens could result (at least partially) from impairment of small-intestine water absorption, which leads to a decreased intestinal absorptive capacity and increased stool mass.
Acknowledgments
This work was supported by grants from the Universidad de Buenos Aires to C.I. and by U.S. Public Health Service Grant AI19844 from the National Institute of Allergy and Infectious Diseases. V. P.-C. has fellowships from the National Council of Research (CONICET). C.I, is a member of CONICET.
We thank Mercedes Pistone Creydt from Hospital Bernardino Rivadavia and Patricia Faciano from Hospital de Gastroenterología Bonorino Udaondo for providing human intestine tissues. We gratefully acknowledge E. J. Hurley, S. Kwiek, and R. Crespo for excellent technical assistance.
Editor: J. T. Barbieri
REFERENCES
- 1.Bartholomew, B. A., M. F. Stringer, G. N. Watson, and R. J. Gilbert. 1985. Development and application of an enzyme linked immunosorbent assay for Clostridium perfringens type A enterotoxin. J. Clin. Pathol. 38:222-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birkhead, G., R. L. Vogt, E. M. Heun, J. T. Snyder, and B. A. McClane. 1988. Characterization of an outbreak of Clostridium perfringens food poisoning by quantitative fecal culture and fecal enterotoxin measurement. J. Clin. Microbiol. 26:471-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bos, J., L. Smithee, B. A. McClane, R. F. Distefano, F. A. Uzal, G. Songer, S. Mallonee, and, J. M. Crutcher. 2005. Fatal necrotizing colitis following a foodborne outbreak of enterotoxigenic Clostridium perfringens type A infection. J. Clin. Infect. Dis. 40:e78-e83. [DOI] [PubMed] [Google Scholar]
- 4.Chakrabarti, G., X. Zhou, and B. A. McClane. 2003. Death pathways activated in CaCo-2 cells by Clostridium perfringens enterotoxin. Infect. Immun. 71:4260-4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorr, R. A., A. Kierbel, J. Vera, and M. Parisi. 1997. A new data-acquisition system for the measurement of the net water flux across epithelia. Comput. Methods Programs Biomed. 53:9-14. [DOI] [PubMed] [Google Scholar]
- 6.Fujita, K., J. Katahira, Y. Horiguchi, N. Sonoda, M. Furuse, and S. Tsukita. 2000. Clostridium perfringens enterotoxin binds to the second extracellular loop of claudin-3, a tight junction integral membrane protein. FEBS Lett. 476:258-261. [DOI] [PubMed] [Google Scholar]
- 7.Hardy, S. P., M. Denmead, N. Parekh, and P. E. Granum. 1999. Cationic currents induced by Clostridium perfringens type A enterotoxin in human intestinal CaCO-2 cells. J. Med. Microbiol. 48:235-243. [DOI] [PubMed]
- 8.Katahira, J., H. Sugiyama, N. Inoue, Y. Horiguchi, M. Matsuda, and N. Sugimoto. 1997. Clostridium perfringens enterotoxin utilizes two structurally related membrane proteins as functional receptors in vivo. J. Biol. Chem. 272:26652-26658. [DOI] [PubMed] [Google Scholar]
- 9.McClane B. A. 2001. Clostridium perfringens, p. 351-372. In M. P. Doyle, L. R. Beuchat, and T. J. Montville (ed.), Food microbiology: fundamentals and frontiers. ASM Press, Washington, D.C.
- 10.McClane, B. A. 1984. Osmotic stabilizers differentially inhibit permeability alterations induced in Vero cells by Clostridium perfringens enterotoxin. Biochim. Biophys. Acta 777:99-106. [DOI] [PubMed] [Google Scholar]
- 11.McClane, B. A., and J. L. McDonel. 1981. Protective effects of osmotic stabilizers on morphological and permeability alterations induced in Vero cells by Clostridium perfringens enterotoxin. Biochim. Biophys. Acta 641:401-419. [DOI] [PubMed] [Google Scholar]
- 12.McDonel, J. L. 1974. In vivo effects of Clostridium perfringens enteropathogenic factors on the rat ileum. Infect. Immun. 10:1156-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonel, J. L., and B. A. McClane. 1988. Production, purification, and assay of Clostridium perfringens enterotoxin. Methods Enzymol. 165:94-103. [DOI] [PubMed] [Google Scholar]
- 14.McDonel, J. L., and C. L. Duncan. 1975. Histopathological effect of Clostridium perfringens enterotoxin in the rabbit ileum. Infect. Immun. 12:1214-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonel, J. L., and C. L. Duncan. 1977. Regional localization of activity of Clostridium perfringens type A enterotoxin in the rabbit ileum, jejunum, and duodenum. J. Infect. Dis. 136:661-666. [DOI] [PubMed] [Google Scholar]
- 16.McDonel, J. L., and G. W. Demers. 1982. In vivo effects of enterotoxin from Clostridium perfringens type A in the rabbit colon: binding vs. biologic activity. J. Infect. Dis. 145:490-494. [DOI] [PubMed] [Google Scholar]
- 17.McDonel, J. L., and T. Asano. 1975. Analysis of unidirectional fluxes of sodium during diarrhea induced by Clostridium perfringens enterotoxin in the rat terminal ileum. Infect. Immun. 11:526-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonel, J. L., L. W. Chang, J. G. Pounds, and C. L. Duncan. 1978. The effects of Clostridium perfringens enterotoxin on rat and rabbit ileum: an electron microscopic study. Lab. Investig. 39:210-218. [PubMed] [Google Scholar]
- 19.Parisi, M., and C. Ibarra. 1996. Aquaporins and water transfer across epithelial barriers. Braz. J. Med. Biol. Res. 29:933-939. [PubMed] [Google Scholar]
- 20.Sarker, M. R., R. J. Carman, and B. A. McClane. 1999. Inactivation of the gene (cpe) encoding Clostridium perfringens enterotoxin eliminates the ability of two cpe-positive C. perfringens type A human gastrointestinal disease isolates to affect rabbit ileal loops. Mol. Microbiol. 33:946-958. [DOI] [PubMed] [Google Scholar]
- 21.Sherman, S., E. Klein, and B. A. McClane. 1994. Clostridium perfringens type A enterotoxin induces tissue damage and fluid accumulation in rabbit ileum. J. Diarrhoeal Dis. Res. 12:200-207. [PubMed] [Google Scholar]
- 22.Singh, U., C. M. Van Itallie, L. L. Mitic, J. M. Anderson, and B. A. McClane. 2000. CaCo-2 cells treated with Clostridium perfringens enterotoxin form multiple large complex species, one of which contains the tight junction protein occludin. J. Biol. Chem. 275:18407-18417. [DOI] [PubMed] [Google Scholar]
- 23.Singh, U., L. L. Mitic, E. U. Wieckowski, J. M. Anderson, and B. A. McClane. 2001. Comparative biochemical and immunocytochemical studies reveal differences in the effects of Clostridium perfringens enterotoxin on polarized CaCo-2 cells versus Vero cells. J. Biol. Chem. 276:33402-33412. [DOI] [PubMed] [Google Scholar]
- 24.Skjelkvale, R., and T. Uemura. 1977. Experimental diarrhoea in human volunteers following oral administration of Clostridium perfringens enterotoxin. J. Appl. Bacteriol. 3:281-286. [DOI] [PubMed] [Google Scholar]
- 25.Wnek, A. P., R. J. Strouse, and B. A. McClane. 1985. Production and characterization of monoclonal antibodies against Clostridium perfringens type A enterotoxin. Infect. Immun. 50:442-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wieckowski, E. U., A. P. Wnek, and B. A. McClane. 1994. Evidence that an approximately 50-kDa mammalian plasma membrane protein with receptor-like properties mediates the amphiphilicity of specifically bound Clostridium perfringens enterotoxin. J. Biol. Chem. 269:10838-10848. [PubMed] [Google Scholar]