Abstract
Moraxella catarrhalis is an important human mucosal pathogen causing otitis media in children and lower respiratory tract infection in adults with chronic obstructive pulmonary disease (COPD). Little is known about the mucosal antibody response to M. catarrhalis in adults with COPD. In this study, 10 pairs of well-characterized sputum supernatant samples from adults with COPD who had acquired and subsequently cleared M. catarrhalis from their respiratory tracts were studied in detail in an effort to begin to elucidate potentially protective immune responses. Flow cytometry analysis was used to study the distribution of immunoglobulin isotypes in paired preacquisition and postclearance sputum samples. The results showed that immunoglobulin A (IgA) is the predominant M. catarrhalis-specific immunoglobulin isotype and that the sputum IgA contains a secretory component, indicating that it is locally produced at the mucosal site. Most patients made new sputum IgA responses to the adhesins UspA1 and Hag, along with the surface protein UspA2. A smaller proportion of patients made new sputum IgA responses to the iron-regulated proteins TbpB and CopB and to lipooligosaccharide. These results have important implications in understanding the mucosal immune response to M. catarrhalis in the setting of COPD and in elucidating the elements of a protective immune response.
Chronic obstructive pulmonary disease (COPD) is a debilitating disease that is the fourth most common cause of death in the United States (2, 4). Bacteria play several potential roles in the course and pathogenesis of the disease (20). Selected bacteria colonize the lower airways of adults with COPD and release potent inflammatory molecules that contribute to the airway inflammation that is a hallmark of COPD (6, 7, 13, 21, 22). Patients with COPD acquire and clear bacteria from the respiratory tract continuously. Little is known about the immune responses that mediate this turnover of bacteria. The course of COPD is characterized by intermittent episodes of worsening of symptoms, known as exacerbations. It is estimated that approximately half of the exacerbations are caused by bacterial infection (20, 23, 24). Nontypeable Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae are the most frequent bacterial causes of exacerbations of COPD (19, 20).
Studies involving the molecular typing of isolates recovered from sputum samples collected prospectively have begun to elucidate the dynamics of colonization and infection by M. catarrhalis in the setting of COPD (15). M. catarrhalis likely causes approximately 10% of exacerbations of COPD, accounting for approximately 2 to 4 million episodes annually. When adults with COPD acquire M. catarrhalis, the organism is cleared efficiently from the respiratory tract after a relatively short duration of carriage (median time, 34 days). Patients then develop strain-specific protection against reacquisition of the same strain (15). This observation that humans develop apparent protective responses to M. catarrhalis after clearing it from the respiratory tract provides the opportunity to begin to understand protective immune responses. The majority of patients develop serum immunoglobulin G (IgG) and/or sputum IgA responses to their homologous infecting isolate of M. catarrhalis, as demonstrated by whole-cell enzyme-linked immunosorbent assay and flow cytometry assays (3, 15). Further analysis of serum immunoglobulins in this cohort has revealed that selected outer membrane antigens are the targets of serum IgG responses following the clearance of M. catarrhalis (14). These antigens include UspA1, UspA2, Hag, TbpB, and outer membrane protein CD (14).
Respiratory tract infections in the setting of COPD are mucosal infections, suggesting that mucosal immune responses likely participate in protective immune responses. Indeed, in previous work, we showed that asymptomatic colonization was associated with a greater frequency of sputum IgA response than exacerbation, suggesting that IgA may be protective from clinical signs of infection (14). IgA is the predominant immunoglobulin in most external secretions, and previous work has demonstrated the presence of IgA to surface antigens of M. catarrhalis in sputum samples (15). However, comparative studies of immunoglobulin isotypes in various human secretions reveal a high degree of heterogeneity in the relative levels of immunoglobulins (12). Little is known about the relative distribution of the isotypes of antigen-specific immunoglobulins in sputum from adults with COPD. Furthermore, the surface antigens of M. catarrhalis to which antibodies in sputum samples are directed in the setting of COPD are an area that is unexplored.
The goals of the present study were to characterize the distribution of M. catarrhalis-specific immunoglobulin isotypes in sputum and to identify the antigens to which potentially protective mucosal antibodies develop in patients who have cleared M. catarrhalis from the respiratory tract.
MATERIALS AND METHODS
COPD Study Clinic.
This prospective study at the Buffalo Veterans Affairs Medical Center has been described previously (15, 19). A total of 104 patients were enrolled between March 1994 and December 2000. Inclusion criteria were the presence of chronic bronchitis (2), the absence of other lung disease on the basis of a clinical assessment, the absence of immunosuppressive or life-threatening disorders, and the willingness to make monthly clinic visits. Patients were seen at the Buffalo Veterans Affairs Medical Center monthly and whenever they had symptoms suggestive of an exacerbation. At each clinic visit, clinical information and sputum and serum samples were obtained. A clinical evaluation was performed at each visit to determine whether the patient had stable disease or an exacerbation as previously described (19). This determination was made by one of two examiners (T. F. Murphy or S. Sethi) before the results of sputum cultures were available.
Bacteriological methods.
Study personnel who processed the sputum samples were unaware of the clinical status of the patients. Sputum samples that were spontaneously expectorated on the morning of the clinic visit were homogenized, diluted, and plated in a quantitative manner as previously described (19). Bacterial pathogens were identified with the use of standard techniques. The identity of an isolate as M. catarrhalis was confirmed by colony morphology and the presence of butyrate esterase.
Bacterial strains.
Isolates of M. catarrhalis were recovered from the sputum of the adults followed in the COPD Study Clinic. The isolates were subjected to molecular typing by pulsed-field gel electrophoresis as part of previously described studies (19). An exacerbation strain was defined as a newly acquired strain isolated from sputum during symptoms of an exacerbation.
The nomenclature of strains isolated from the COPD Study Clinic is as follows: the first number is the patient identification number, the letter “P” indicates a sputum isolate, the number following the “P” is the clinic visit number, the letter “B” indicates that the isolate is Moraxella (Branhamella) catarrhalis, and the number following the “B” is the colony number from the original plate. For example, strain 3P67B1 is from patient 3 and was isolated from sputum at clinic visit 67.
The characteristics of M. catarrhalis O35E and its isogenic knockout mutants are shown in Table 1 and were described previously (10, 11, 14).
TABLE 1.
M. catarrhalis mutants used in this study
| Strain | Description | Antibiotic resistance marker(s) |
|---|---|---|
| O35E | Parent strain | None |
| O35E.1 | uspA1 mutant | Kanamycin |
| O35E.2 | uspA2 mutant | Kanamycin |
| O35E.12 | uspA1 uspA2 double mutant | Kanamycin, chloramphenicol |
| O35E.copB | copB mutant | Kanamycin |
| O35E.tbpB | tbpB mutant | Kanamycin |
| O35E.hag | hag mutant | Kanamycin |
| O35E.12-hag | uspA1 uspA2 hag triple mutant | Kanamycin, chloramphenicol, erythromycin |
M. catarrhalis was grown in brain heart infusion (BHI) broth at 37°C with shaking or on BHI agar plates in an atmosphere of 5% CO2. When mutants were grown, the media were supplemented with kanamycin, chloramphenicol, or erythromycin as appropriate at a concentration of 20 mg/liter.
Purification of outer membranes.
Bacteria were grown on BHI agar overnight and harvested by suspension in 0.01 M HEPES, pH 7.4. Cells were disrupted by sonication on ice with four 15-second periods of sonication at 100 W. Unbroken cells and debris were removed by centrifugation at 10,000 × g for 2 min at 4°C. The suspension was centrifuged at 100,000 × g for 45 min at 4°C to collect cell envelopes. The pellets were suspended in 1% sarcosyl in 0.01 M HEPES and incubated at room temperature for 1 h to solubilize the cytoplasmic membranes. The sarcosyl-insoluble fraction was obtained by centrifugation at 100,000 × g for 45 min at 4°C.
Purification of LOS.
Lipooligosaccharide (LOS) of isolates of M. catarrhalis was prepared using a microphenol method as described previously (3).
Sputum supernatant samples.
After an aliquot of homogenized sputum was removed for culture as described above, sputum supernatants were obtained by centrifugation at 27,000 × g for 30 min at 4°C. The supernatants were saved by storage at −80°C. Preacquisition sputum samples were obtained approximately 4 weeks prior to a patient's acquisition of a strain of M. catarrhalis based on monthly sputum cultures. Postclearance sputum samples were obtained 4 to 8 weeks following clearance of the strain.
The nomenclature of the sputum supernatants is as follows: the first number is the patient identification number, “PS” indicates that the sample is a sputum supernatant, and the number following “PS” is the clinic visit number. For example, sample 3PS68 is a sputum supernatant from patient 3 obtained at clinic visit 68.
Flow cytometry.
Sputum supernatant samples were subjected to flow cytometry with the patients' homologous strain to detect antibodies that bound to the bacterial surface using previously described methods (3). Fluorescein-labeled goat anti-human IgA, fluorescein-labeled goat anti-human IgG, and fluorescein-labeled goat anti-human IgM (Kirkegaard & Perry, Gaithersburg, Maryland) were used to detect IgA, IgG, and IgM, respectively. Fluorescein-labeled goat anti-human secretory component (Autogen Bioclear, Wiltshire, United Kingdom) was used to detect IgA with a secretory component.
Purification of sputum IgA.
IgA was purified from sputum supernatant samples by affinity chromatography with a streptococcal IgA binding peptide (Sap) (18). A total of 5 mg of the 50-residue synthetic peptide (purchased from Sigma-Genosys, Woodland, Texas) was immobilized on a 1-ml HiTrap NHS- activated high-performance column (Amersham Pharmacia Biotech) according to the instructions of the manufacturer. Before purification of the IgA, sputum supernatants were centrifuged at 16,000 × g for 30 min at 4°C and filtered through a 0.45-μm-pore-size filter. A volume of 1 ml of sputum supernatant was applied to the column, which was then washed with phosphate-buffered saline. Bound proteins were eluted with 0.1 M acetate buffer, pH 4, in fractions of 0.32 ml. The pH of the fractions was adjusted immediately by adding 0.32 ml of 1 M Tris, pH 8.3. Fractions were assayed for the presence of IgA by dotting 1 μl of each fraction onto nitrocellulose and probing with peroxidase-conjugated goat anti-human IgA. The fractions that contained IgA (generally the first eight fractions) were pooled and stored at 4°C. The protein concentration was determined by the method of Lowry (Sigma). After use, the column was regenerated with 3 M KSCN, washed with phosphate-buffered saline, and stored at 4°C in 0.05 M Na2HPO4, 0.1% NaN3, pH 7.
Immunoblot assays.
Purified outer membranes and LOS of infecting strains of M. catarrhalis were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot assays. Purified LOS was subjected to SDS-PAGE and immunoblot assay with sputum supernatants diluted 1:200. The outer membranes of homologous strains were probed with IgA purified from preacquisition and postclearance sputum samples using the same concentration of IgA in the paired samples. M. catarrhalis mutants were probed with IgA purified from postclearance sputum samples. IgA was detected with peroxidase-conjugated rabbit anti-human IgA (Dako, Carpinteria, California).
Immunoblot assays were analyzed by densitometry using an Alpha Innotech imaging system. The integrated density values were determined for the individual bands detected by purified IgA from preacquisition and postclearance sputum samples run at identical concentrations in adjacent lanes. The results of densitometric analysis were expressed as a percentage based on the relative integrated density value for each band pair analyzed.
RESULTS
Identification of sputum samples.
All sputum samples were from adults with COPD who were followed in the COPD Study Clinic at the Buffalo Veterans Affairs Medical Center as part of a prospective study. In previous work, paired preacquisition and postclearance sputum samples were analyzed by flow cytometry with the homologous infecting strain of M. catarrhalis, allowing us to identify new sputum IgA antibodies that bound to epitopes on the bacterial surface (15). To identify the surface antigens to which new sputum IgA antibodies were directed, the 10 pairs of sputum supernatant samples that yielded the largest increases in the levels of new IgA from preacquisition of M. catarrhalis to postclearance were chosen for detailed study. Four pairs of samples were associated with clinical symptoms of exacerbation, and six pairs of samples were associated with episodes of asymptomatic colonization. Based on the results of monthly sputum cultures, 8 of the 10 episodes of carriage of M. catarrhalis were 1 month in duration, and all were 3 months or less (Table 2).
TABLE 2.
Summary of outer membrane antigens to which sputum IgA is directed following M. catarrhalis carriage in adults with COPD
| Patient visit IDb no. | Clinical episodec | Duration of carriage (mo)d | Presence of IgA band and relative densities of bands in preacquisition/postclearance sputum samples fora:
|
|||||
|---|---|---|---|---|---|---|---|---|
| UspA1 | UspA2 | Hag | TbpB | CopB | LOS | |||
| 1PS45 | Col | 3 | +, 46.8/53.2 | +, 46.4/53.6 | ||||
| 3PS68 | Col | 1 | +, 37.6/62.4 | +, 27.7/72.3 | ||||
| 7PS95 | Exac | 1 | +, 34.8/65.2 | +, 33.5/66.5 | +, 44.5/55.5 | + | ||
| 10PS92 | Exac | 1 | +, 53.0/47.0 | +, 52.9/47.1 | +, 56.4/43.6 | |||
| 12PS75 | Exac | 2 | +, 19.1/80.9 | +, 13.0/87.0 | +, 19.4/80.6 | +, 42/58 | ||
| 19PS8 | Col | 1 | +e | +e | +, 54.1/45.9 | |||
| 25PS26 | Col | 1 | +, 14.8/85.2 | +, 32.9/67.1 | +, 37.5/62.5 | |||
| 29PS25 | Col | 1 | +, 54.0/46.0 | +, 54.0/46.0 | +f | |||
| 44PS24 | Col | 1 | +, 30.1/69.9 | +, 28.1/71.9 | +, 31.6/68.4 | |||
| 66PS6 | Exac | 1 | +, 30.9/69.1 | +, 30.9/69.1 | +, 33.1/66.9 | +, 27.1/72.9 | ||
| Total | 9/10 | 7/10 | 10/10 | 3/10 | 1/10 | 1/10 | ||
A “+” indicates the presence of a band in the immunoblot assay with IgA purified from the postclearance sample. Numbers after the symbol indicate the relative densities of bands in the immunoblot assay based on the integrated density values of IgA purified from preacquisition/postclearance sputum samples at identical concentrations and assayed with the homologous infecting strain. Bands with relative band densities of ∼52/48 or greater are different in intensity by visual inspection. Values of ∼70/30 or greater are essentially the development of a new band in the postclearance sample compared to the bands in the preacquisition sample by visual inspection.
ID, identification.
Col, colonization defined by sputum that is culture positive for a newly acquired isolate of M. catarrhalis with no change in symptoms; Exac, exacerbation defined by sputum that is culture positive for a newly acquired isolate of M. catarrhalis from a patient with clinical symptoms of an exacerbation.
Determined by monthly sputum cultures and molecular typing of isolates of M. catarrhalis.
Antibody to the antigen producing the band is present in postclearance sputum IgA, but there was no increase from the level in preacquisition sputum IgA.
Recognizes the band in strain O35E but not in the homologous isolate.
Immunoglobulin isotypes in sputum.
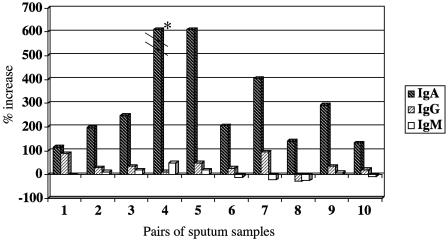
To characterize the isotypes of antigen-specific immunoglobulins in sputum samples, paired preacquisition and postclearance sputum supernatants were subjected to flow cytometry with homologous infecting strains of M. catarrhalis. The results were expressed as the percent increase in fluorescence from preacquisition to postclearance samples, allowing the detection of antibody made specifically during the episode of carriage of M. catarrhalis, as previously described (3, 15). Figure 1 shows that the predominant immunoglobulin isotype made following the clearance of M. catarrhalis from the respiratory tract was IgA. A small amount of IgG was detected, suggesting transudation into the airways. Little or no new IgM bound to surface epitopes was detected.
FIG. 1.
Distribution of M. catarrhalis-specific immunoglobulin isotypes determined by flow cytometry using preacquisition and postclearance sputum supernatants with the homologous infecting strain. The x axis shows samples from 10 individual patients. The y axis indicates the percent increase of antibody in paired preacquisition and postclearance sputum samples determined by flow cytometry. Immunoglobulin isotypes are noted in the legend on the right. *, the percent increase of IgA in sample 4 was actually 1,425%.
To assess whether IgA in sputum was locally produced, flow cytometry was performed on paired sputum supernatants, and IgA was detected with a fluorescein-conjugated antibody to human secretory component. Nine of 10 pairs of sputum supernatants demonstrated the development of new IgA with a secretory component (IgA-SC). Therefore, we conclude that the sputum samples contain locally produced IgA to M. catarrhalis, but it is difficult to determine the precise proportion of total IgA that is IgA-SC because different fluorescein-conjugated secondary antibodies have different binding characteristics.
Immunoblot assays with paired preacquisition and postclearance sputum samples.
To identify the antigens to which the new IgA antibodies in the sputum supernatants were directed, IgA was purified from preacquisition and postclearance samples and tested in immunoblot assays with the purified outer membranes of a patient's homologous infecting strain of M. catarrhalis. Purified IgA rather than the whole-sputum supernatant was used in immunoblot assays to minimize the background reactivity seen with sputum samples and to allow standardization by IgA concentration rather than by the dilution of heterogeneous expectorated sputum samples.
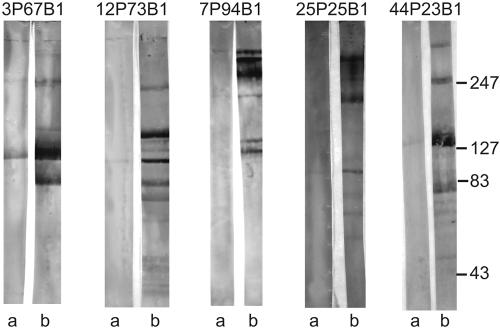
Paired preacquisition and postclearance samples were subjected to immunoblot assays with the purified outer membranes of the patients' homologous strains in adjacent lanes of the immunoblot with identical concentrations of purified IgA. Figure 2 shows the results with purified IgA from five pairs of preacquisition and postclearance sputum supernatant samples. We noted the development of new bands in the postclearance sample compared to essentially absent bands in the corresponding preacquisition sample when IgA from each was assayed at identical concentrations (Fig. 2). In other samples, bands were present in both the preacquisition and postclearance samples; in many of these samples, the intensity of the bands increased from the preacquisition to the postclearance sample, indicating the development of increased IgA above the level of the preexisting antibody. Table 2 shows a summary of the results of densitometric analysis of each of the immunoblot assays of IgA purified from paired preacquisition and postclearance samples and tested at identical concentrations. The majority of bands observed in the immunoblot assay were 80 kDa or greater in molecular mass. The identities of the bands noted in Table 2 were determined by subjecting the purified IgA samples to immunoblot assays with a series of well-defined mutants (see below). The limited number bands in the immunoblot assays (Fig. 2 and 4) allowed for the accurate identification of bands in homologous strains by comparison with the bands from immunoblot assays of mutants. The densitometric analyses were performed on immunoblots using homologous infecting strains.
FIG. 2.
Immunoblot assay of the purified outer membranes of M. catarrhalis strains (noted at the tops of lanes). Immunoblots were probed with purified IgA from homologous preacquisition sputum supernatants (lanes a) and postclearance sputum supernatants (lanes b) at identical concentrations. Antibodies were detected with peroxidase-conjugated rabbit anti-human IgA. Molecular masses are noted in kilodaltons on the right.
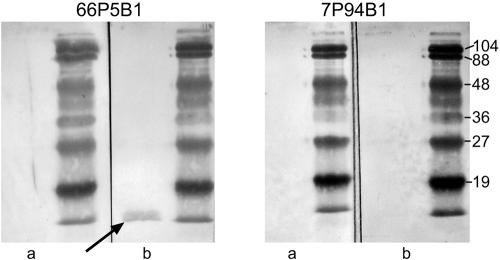
FIG. 4.
Immunoblot assays with purified IgA from postclearance sputum supernatant samples corresponding to strain 7P94B1 (top panel) and strain 44P23B1 (bottom panel). Lanes contain purified outer membrane preparations of the following strains: the homologous strain (lane a), O35E (lane b), the UspA1 mutant (lance c), the UspA2 mutant (lane d), the UspA1 UspA2 double mutant (lane e), the CopB mutant (lane f), the TbpB mutant (lane g), the Hag mutant (lane h), and the UspA1 UspA2 Hag triple mutant (lane i). Antibodies were detected with peroxidase-conjugated anti-human IgA. Molecular mass markers are noted on the left in kilodaltons. Arrows denote the locations of UspA2, Hag, UspA1, and UspA2 monomers.
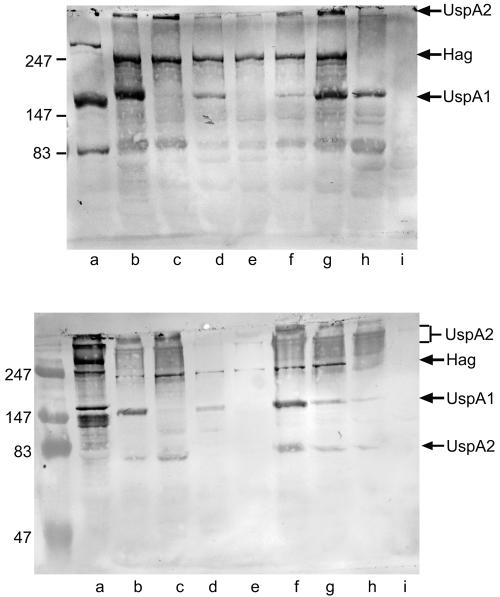
To more carefully evaluate sputum samples for the development of IgA to LOS, purified LOS from each of the 10 homologous isolates was subjected to immunoblot assays with preacquisition and postclearance sputum supernatant samples. One of the 10 episodes of acquisition and clearance of M. catarrhalis showed the development of IgA to homologous LOS (Fig. 3).
FIG. 3.
Immunoblot assays with purified lipooligosaccharide of M. catarrhalis strains (noted at the top). Immunoblots were probed with homologous preacquisition sputum supernatants (lanes a) and postclearance sputum supernatants (lanes b). Antibodies were detected with peroxidase-conjugated anti-human IgA. Molecular masses are noted in kilodaltons on the right. The arrow denotes the presence of IgA binding to lipooligosaccharide in the postclearance sputum sample.
Analysis of purified sputum IgA with mutants.
To determine the identities of some of the antigens to which human IgA antibodies were directed, a series of isogenic knockout mutants of M. catarrhalis were studied in immunoblot assays with purified IgA from postclearance sputum samples. The characteristics of the mutants are noted in Table 1 and have been described previously (10, 11, 14). Purified outer membranes of the homologous infecting strains, each of the knockout mutants, and the isogenic parent strain used to make the mutants were subjected to immunoblot assay with purified IgA. Figure 4, shows that the banding patterns of the mutant strains allow one to identify the outer membrane proteins to which IgA antibodies are directed. Table 2 summarizes the results. UspA1, UspA2, and Hag were the major targets of new IgA antibodies in the sputum supernatants. A much smaller proportion of samples showed the development of IgA antibodies to TbpB, CopB, and LOS.
DISCUSSION
In this study, expectorated sputum samples from a well-characterized cohort of patients with COPD who cleared M. catarrhalis from the respiratory tract were studied to identify the distribution of immunoglobulin isotypes in sputum and to identify the antigens to which sputum IgA antibodies were directed. The results demonstrated that IgA is by far the predominant M. catarrhalis-specific immunoglobulin isotype in the sputum of patients with COPD and that IgA is produced at the mucosal site as indicated by the presence of a secretory component. The study further showed that IgA in sputum recognizes specific outer membrane antigens of M. catarrhalis with the adhesins UspA1 and Hag, as well as UspA2 as the targets of mucosal IgA antibodies in a majority of patients studied. The outer membrane antigens TbpB, CopB, and lipooligosaccharide are recognized by a smaller proportion of patients.
UspA1, UspA2, and Hag are expressed on the bacterial surface and are involved in the formation of surface projections (17). UspA1 and Hag are adhesins, mediating the adherence of M. catarrhalis to human respiratory epithelial cells (8, 9). Sera from healthy adults and children contain antibodies to UspA1 and UspA2 (5). In a previous study (14), analysis of IgG in the sera of patients from this cohort of adults with COPD revealed that UspA1, UspA2, and Hag were important targets of serum IgG, but interesting differences from the targets of sputum IgA were observed in the present study. For example, all 10 patients made mucosal IgA antibodies to Hag (Table 2), whereas only 7 of 12 made serum IgG to Hag (14). Nine of 12 patients made new serum IgG to the iron-regulated protein TbpB, whereas only 3 of 10 patients made sputum IgA to TbpB (Table 2). In general, there was more heterogeneity in serum IgG responses among patients than in sputum IgA responses. The observation that both UspA1 and Hag are adhesins that mediate adherence to respiratory tract cells leads to the speculation that, as adhesins, they are highly expressed by M. catarrhalis while it is present in the airway, contributing to the prominent mucosal antibody response to these surface antigens.
An important feature of the present study is that the sputum samples were obtained from patients followed prospectively with monthly sputum cultures. Long-term follow-up has established that patients efficiently clear M. catarrhalis from the respiratory tract and do not reacquire the same strain, indicating that a protective response occurs (15). Of interest, analysis of the serum IgG and sputum IgA responses to the homologous infecting strains revealed that the development of a sputum IgA response was associated more often with asymptomatic colonization than with clinical exacerbation, suggesting that mucosal antibody responses may mediate protection from clinical infections (15). The protective response appears to be strain specific, because while reacquisition of the same strain did not occur, patients acquired new strains after clearing previous strains (15). The sputum samples will be valuable in identifying potentially protective immune responses; however, one cannot yet conclude that the antibodies detected in the present study mediate protection.
Limitations of the approach used in this study should be considered. The present study employed immunoblot assays exclusively to identify antigens to which sputum IgA is directed. Therefore, antibodies to conformational epitopes that are denatured in SDS-PAGE will not be detected. Indeed, in other work using quantitative enzyme-linked immunosorbent assays with purified recombinant protein antigens, we have demonstrated the development of sputum IgA to other surface proteins of M. catarrhalis, including outer membrane proteins CD and G1a (1, 16). Sputum IgA to these antigens was not detected by immunoblot assay in the present study, indicating the development of antibodies to conformational epitopes on the proteins. A second consideration is that the present study detected antibodies to antigens that are expressed when M. catarrhalis is grown on laboratory media. Surface molecules that are expressed during human infection but that are not expressed during in vitro growth will not be detected. Therefore, the results of this study are highly specific, demonstrating that the antigens noted in Table 2 are unequivocally the targets of a sputum IgA response. However, the sensitivity of the approach is limited by the methods, indicating that antibodies to other antigens are present, and alternative experimental approaches will be needed to more fully characterize the mucosal antibody response to M. catarrhalis. Finally, because the results of the present study are based on a subset of samples selected based on the demonstration of the development of a sputum IgA response to whole bacteria (15), one must use caution in applying these conclusions to all episodes of M. catarrhalis infection.
IgA is the most abundant immunoglobulin in most external secretions. However, comparative studies of immunoglobulin levels in secretions of human and experimental animals reveal that in some secretions the relative amount of IgG may be quite high (12). Indeed, one might expect that in the presence of a lower respiratory tract infection, substantial transudation of serum IgG into respiratory secretions may occur. The present study shows that the predominant M. catarrhalis-specific immunoglobulin in the 10 selected pairs of sputum samples studied is IgA. While it is difficult to determine with precision the proportion of the IgA that has a secretory component from our assays, the observation that the secretory component is detected in the new IgA from 9 of 10 samples indicates production at the mucosal site. IgA-SC is relatively protected from proteolytic degradation, and this may be important in view of the abundant proteases in the human respiratory tract. It should be emphasized that the sputum immunoglobulin responses shown in Fig. 1 represent the development of new immunoglobulin specifically directed toward the surface epitopes of the homologous infecting strain, rather than absolute levels of immunoglobulin.
The results of the present study, which identifies UspA1, UspA2, and Hag as major targets of human sputum IgA, are consistent with a previous study showing these same antigens as frequent targets of salivary IgA from healthy adults (11). Salivary IgA was also directed at TbpB, CopB, and outer membrane protein CD in each of the samples from 14 healthy adults. However, one must be cautious in comparing the two studies because the previous study of salivary antibodies identified the presence of antigen-specific IgA in saliva, whereas the current study has identified the development of an IgA response by expressing the results as an increase in levels over preacquisition levels.
In summary, analysis of IgA isolated from well-characterized sputum samples from adults with COPD who have cleared M. catarrhalis from the respiratory tract showed that the patients made new sputum IgA responses to the adhesins UspA1 and Hag, along with the surface protein UspA2. The results further show that IgA is the predominant M. catarrhalis-specific immunoglobulin in sputum and that sputum IgA has a secretory component, indicating that it is locally produced at the mucosal site. Future work should be directed at characterizing the function of these mucosal antibodies in an effort to elucidate the elements of a protective immune response.
Acknowledgments
This work was supported by NIH grants AI 46422 and AI 28304, by the Department of Veterans Affairs, and by grant 3100A0-102246 from the Swiss National Science Foundation (to C.A.).
We thank Karen Eschberger, Nancy Evans, Adeline Thurston, Catherine Wrona, Phyllis Lobbins, and Lori Grove for their work in the COPD Study Clinic.
Editor: D. L. Burns
REFERENCES
- 1.Adlowitz, D. G., S. Sethi, P. Cullen, B. Adler, and T. F. Murphy. 2005. Human antibody response to outer membrane protein G1a, a lipoprotein of Moraxella catarrhalis. Infect. Immun. 73:6601-6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Thoracic Society. 1995. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 152:S77-S121. [PubMed] [Google Scholar]
- 3.Bakri, F., A. L. Brauer, S. Sethi, and T. F. Murphy. 2002. Systemic and mucosal antibody response to Moraxella catarrhalis following exacerbations of chronic obstructive pulmonary disease. J. Infect. Dis. 185:632-640. [DOI] [PubMed] [Google Scholar]
- 4.Barnes, P. J. 2000. Chronic obstructive pulmonary disease. N. Engl. J. Med. 343:269-280. [DOI] [PubMed] [Google Scholar]
- 5.Chen, D., V. Barniak, K. R. VanDerMeid, and J. C. McMichael. 1999. The levels and bactericidal capacity of antibodies directed against the UspA1 and UspA2 outer membrane proteins of Moraxella (Branhamella) catarrhalis in adults and children. Infect. Immun. 67:1310-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill, A., S. Gompertz, and R. Stockley. 2000. Factors influencing airway inflammation in chronic obstructive pulmonary disease. Thorax 55:970-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill, A. T., E. J. Campbell, S. L. Hill, D. L. Bayley, and R. A. Stockley. 2000. Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. Am. J. Med. 109:288-295. [DOI] [PubMed] [Google Scholar]
- 8.Holm, M. M., S. L. Vanlerberg, D. D. Sledjeski, and E. R. Lafontaine. 2003. The Hag protein of Moraxella catarrhalis strain O35E is associated with adherence to human lung and middle ear cells. Infect. Immun. 71:4977-4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lafontaine, E. R., L. D. Cope, C. Aebi, J. L. Latimer, G. H. McCracken, Jr., and E. J. Hansen. 2000. The UspA1 protein and a second type of UspA2 protein mediate adherence of Moraxella catarrhalis to human epithelial cells in vitro. J. Bacteriol. 182:1364-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meier, P. S., S. Freiburghaus, A. Martin, N. Heiniger, R. Troller, and C. Aebi. 2003. Mucosal immune response to specific outer membrane proteins of Moraxella catarrhalis in young children. Pediatr. Infect. Dis. J. 22:256-262. [DOI] [PubMed] [Google Scholar]
- 11.Meier, P. S., N. Heiniger, R. Troller, and C. Aebi. 2003. Salivary antibodies directed against outer membrane proteins of Moraxella catarrhalis in healthy adults. Infect. Immun. 71:6793-6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mestecky, J., I. Moro, M. A. Kerr, and J. M. Woof. 2005. Mucosal immunoglobulins, p. 153-181. In J. Mestecky, M. E. Lamm, J. R. McGhee, J. Bienenstock, L. W. Mayer, and W. Strober (ed.), Mucosal immunology, 3rd ed., vol. 1. Academic Press, San Diego, Calif. [Google Scholar]
- 13.Monso, E., J. Ruiz, A. Rosell, J. Manterola, J. Fiz, J. Morera, and V. Ausina. 1995. Bacterial infection in chronic obstructive pulmonary disease. A study of stable and exacerbated outpatients using the protected specimen brush. Am. J. Respir. Crit. Care Med. 152:1316-1320. [DOI] [PubMed] [Google Scholar]
- 14.Murphy, T. F., A. L. Brauer, C. Aebi, and S. Sethi. 2005. Identification of surface antigens of Moraxella catarrhalis as targets of human serum antibody responses in chronic obstructive pulmonary disease. Infect. Immun. 73:3471-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy, T. F., A. L. Brauer, B. J. Grant, and S. Sethi. 2005. Moraxella catarrhalis in chronic obstructive pulmonary disease. Burden of disease and immune response. Am. J. Respir. Crit. Care Med. 172:195-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy, T. F., C. Kirkham, D.-F. Liu, and S. Sethi. 2003. Human immune response to outer membrane protein CD of Moraxella catarrhalis in adults with chronic obstructive pulmonary disease. Infect. Immun. 71:1288-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearson, M. M., E. R. Lafontaine, N. J. Wagner, J. W. St. Geme III, and E. J. Hansen. 2002. A hag mutant of Moraxella catarrhalis strain O35E is deficient in hemagglutination, autoagglutination, and immunoglobulin D-binding activities. Infect. Immun. 70:4523-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandin, C., S. Linse, T. Areschoug, J. M. Woof, J. Reinholdt, and G. Lindahl. 2002. Isolation and detection of human IgA using a streptococcal IgA-binding peptide. J. Immunol. 169:1357-1364. [DOI] [PubMed] [Google Scholar]
- 19.Sethi, S., N. Evans, B. J. B. Grant, and T. F. Murphy. 2002. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N. Engl. J. Med. 347:465-471. [DOI] [PubMed] [Google Scholar]
- 20.Sethi, S., and T. F. Murphy. 2001. Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art review. Clin. Microbiol. Rev. 14:336-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soler, N., S. Ewig, A. Torres, X. Filella, J. Gonzalez, and A. Zaubet. 1999. Airway inflammation and bronchial microbial patterns with stable chronic obstructive pulmonary disease. Eur. Respir. J. 14:1015-1022. [DOI] [PubMed] [Google Scholar]
- 22.White, A. J., S. Gompertz, D. L. Bayley, S. L. Hill, C. O'Brien, I. Unsal, and R. A. Stockley. 2003. Resolution of bronchial inflammation is related to bacterial eradication following treatment of exacerbations of chronic bronchitis. Thorax 58:680-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson, R. 2000. Evidence of bacterial infection in acute exacerbations of chronic bronchitis. Semin. Respir. Infect. 15:208-215. [DOI] [PubMed] [Google Scholar]
- 24.Wilson, R. 1998. The role of infection in COPD. Chest 113:242S-248S. [DOI] [PubMed] [Google Scholar]