Abstract
It has been proposed that long-lived memory T cells generated by vaccination or infection reside within a memory compartment that has a finite size. Consequently, in a variety of acute infection models interclonal competition has been shown to lead to attrition of preexisting memory CD8+ T cells. Contrary to expectations, therefore, we found that chronic Leishmania donovani infection of Listeria-immune mice results in heightened protection against subsequent Listeria challenge. This protection was associated with bystander expansion of Listeria-specific CD8+ T cells and a bias in these cells toward a central memory T-cell phenotype with an enhanced capacity for gamma interferon production. We propose that splenomegaly, which is characteristic of visceral leishmaniasis and other tropical infections, may help promote heterologous immunity by resetting the size of the memory compartment during chronic infection.
Memory CD8+-T-cell responses play an essential role in mediating natural and vaccine-induced immunity to a range of important human pathogens, and the dynamics of the CD8+-T-cell response to a number of prototypic microbial infections has been extensively studied. Activation of naïve CD8+ T cells results in rapid and extensive clonal expansion, followed by a clonal contraction phase. Subsequently, memory CD8+ T cells persist at a higher frequency than their naïve precursors (at approximately 5% of the maximal clone size) and occupy space in a finite memory compartment (1, 7). The mechanisms regulating memory cell persistence in this memory compartment are varied. For example, whereas cross-reactivity can promote the survival of preexisting memory CD8+ T cells (18-20, 22), clonal competition in the absence of cross-reactivity can promote attrition of preexisting memory CD8+ T cells (4, 19). In addition, inflammatory cytokines are also thought to play an important role in regulating memory CD8+-T-cell persistence (21). In the latter case, recent mathematical modeling suggests that clonal contraction should occur rapidly to maintain homeostasis (1).
In contrast to these studies with acute infection models, memory cell behavior during chronic infection is less well understood. A recent study demonstrated that in contrast to mice with acute lymphocytic choriomeningitis virus infection, mice with a chronic infection do not develop memory CD8+ T cells with the capacity for long-term antigen-independent persistence. Memory CD8+ cells in these mice exhibit reduced expression of interleukin-7 (IL-7) and IL-15 receptors and fail to undergo homeostatic proliferation (24). However, the impact of chronic infection on the persistence of preexisting host-protective memory CD8+ T cells has not been described previously. Here, we demonstrate that Leishmania donovani infection of Listeria-immune mice results in significantly enhanced protection against lethal challenge and long-term bystander expansion of Listeria-specific memory CD8+ T cells. We propose that infection-associated splenomegaly may reset the size of the memory compartment to avoid interclonal competition and allow the maintenance of preexisting immunity.
MATERIALS AND METHODS
Mice and pathogens.
BALB/c mice were obtained from Charles River U.K. (Margate, United Kingdom) and were maintained under barrier conditions at the Biological Services Unit, London School of Hygiene and Tropical Medicine. Female mice that were 8 to 12 weeks old were used in all experiments. L. donovani amastigotes (LV9) were obtained from the spleens of infected Syrian hamsters and were isolated as described elsewhere (6). Mice were infected intravenously via the lateral tail vein with 2 × 107 amastigotes. Listeria monocytogenes was grown overnight in static culture at 37°C in tryptone soya agar (TSA) broth (Oxoid, United Kingdom). Bacteria were then washed twice in phosphate-buffered saline before resuspension in 30% glycerol-phosphate-buffered saline for storage at −70°C. For vaccination and challenge, bacterial stocks were freshly thawed and serially diluted in sterile saline. The injected dose was confirmed by plating on TSA plates. All animal procedures were approved by the London School of Hygiene and Tropical Medicine Animal Procedures Ethical Committee and were subject to United Kingdom Home Office Regulations.
Vaccination and challenge.
Mice were inoculated with a vaccinating nonlethal dose containing ∼5 × 104 CFU Listeria, after which bacilli were cleared within 2 weeks (9; data not shown). At week 6 after vaccination, groups of mice (n = 12) were infected with L. donovani or retained as controls. Approximately 10 weeks later, groups of mice were killed to assess the fate of Listeria-specific CD8+ T cells or were challenged with 6 ×105 to 8 ×105 CFU Listeria, and the levels of protection were determined on day 3 after challenge. Naïve mice infected with this dose did not survive beyond day 3 (data not shown). Livers and spleens were removed and homogenized in fixed volumes of sterile saline and RPMI, respectively, and serial dilutions were plated in triplicate on TSA plates. After incubation for 24 h at 37°C, the number of colonies was determined, and total number of organ CFU was calculated.
In vitro restimulation of Listeria-immune CD8+ T cells.
Dendritic cells (DC) were obtained by culturing BALB/c or C57BL/6 bone marrow cells in granulocyte-macrophage colony-stimulating factor for 7 days by using conventional protocols. Dendritic cells were plated in six-well plates and were infected at a multiplicity of infection of 10:1 with either Listeria or amastigotes of L. donovani OVA, a transgenic line of L. donovani expressing chicken ovalbumin (OVA) (15a). When appropriate, control cultures received 5 μg/ml LLO91-99 or OVA257-264. Listeria infection was terminated using 50 μg/ml gentamicin. After 4 h, DC were irradiated (3,000 R) and then plated overnight. CD8+ T cells were isolated from spleens of Listeria-immune mice by using magnetic sorting and were added to DC at a 10:1 ratio. In control experiments, OVA-specific OT-1 T cells were used. Proliferative responses were assessed at day 3 using thymidine incorporation, and gamma interferon (IFN-γ) production by bulk CD8+ T cells and by tetramer-positive CD8+ T cells was determined on day 3 following incubation for 5 h in the presence of brefeldin A as described below.
Flow cytometry.
CD8+ cells were positively selected from spleen cell suspensions by magnetic sorting using anti-CD8α (Ly-2) MicroBeads (Miltenyi Biotec). Four-color flow cytometry was performed with spleen cell populations using fluorescein isothiocyanate-conjugated CD8 (Caltag, Burlingame, CA), phycoerythrin-conjugated anti-CD62L (MEL-14), biotinylated anti-CD44 (Pgp-1; detected with streptavidin-PerCP), allophycocyanin (APC)-conjugated anti-IFN-γ (XMG1.2), or APC-conjugated rat immunoglobulin G1 as an isotype control (all obtained from BD Pharmingen, Oxon, United Kingdom). H2Kd/LLO91-99 tetramers were obtained from Proimmune (Oxford, United Kingdom) and used to detect LLO91-99-specific CD8+ T cells. For intracellular detection of IFN-γ, spleen cells (1 × 107 cells/ml) were restimulated in the presence or absence of 5 μg/ml of LLO91-99 (Proimmune) for 1 h, followed by 4 h in the presence of 10 μg/ml brefeldin A (Sigma). Data were collected for >50,000 events using a FACSCalibur and were analyzed using the Cell Quest II software (BD). All mice (four to six mice per group per experiment) were analyzed individually.
Statistical analysis.
Statistical analysis was performed using Student's t test, and a P value of <0.05 was considered significant. Each experiment was repeated independently at least twice, with similar results.
RESULTS AND DISCUSSION
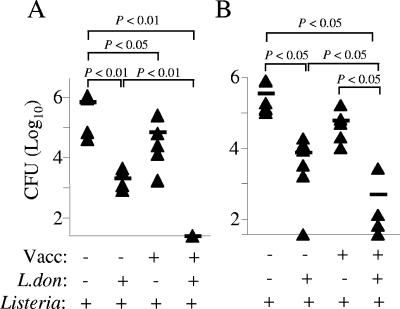
To determine how L. donovani infection affected established CD8+-T-cell-dependent protective immunity to a heterologous pathogen, we first vaccinated mice with a sublethal dose of L. monocytogenes, prior to L. donovani infection. When chronic L. donovani infection was well established, mice were challenged with an otherwise lethal dose of Listeria. Control vaccinated mice were significantly, although moderately, protected in terms of tissue CFU (Fig. 1A). Similar levels of protection were also observed in control unvaccinated mice infected with L. donovani (Fig. 1A and B), most likely as a result of enhanced local macrophage effector function (14) or bystander cytokine-dependent activation of irrelevant memory CD8+ T cells (2, 12). In contrast, Listeria-immune mice infected with L. donovani showed remarkably high levels of protection. In the spleen, the number of CFU was reduced to undetectable levels (Fig. 1A), whereas in the liver, the protection was ∼1.5 log greater than that seen either in L. donovani-infected, Listeria-naïve mice or in control vaccinated mice (Fig. 1B). These data demonstrate that L. donovani infection both enhanced innate immunity to Listeria challenge and also significantly augmented vaccine-induced protection.
FIG. 1.
L. donovani infection enhances protection against Listeria. Listeria-naïve or Listeria-immune BALB/c mice were infected with L. donovani and at 68 days after infection were challenged with 6 × 105 CFU Listeria. The bacterial burdens in the spleens (A) and livers (B) were determined by colony counting as described in Materials and Methods. Each symbol represents an individual mouse (five or six mice per group), and statistical significance between groups was determined using Student's t test. Vacc, vaccination; L.don, L. donovani.
During chronic L. donovani infection, mice exhibited marked splenomegaly (Fig. 2A) that was accompanied by an increase in the number of total spleen memory CD44hi CD8+ T cells (2.6 × 105 ± 0.3 × 105 and 21 × 105 ± 8 × 105 cells in naïve and chronically infected mice, respectively; P < 0.005). To determine whether this expansion of memory CD8+ T cells had an effect on preestablished CD8+ Listeria-specific memory CD8+ T cells, we determined the numbers of H2Kd/LLO91-99 tetramer-positive cells (3). Listeria-immune mice had significantly greater numbers of tetramer-positive cells than Listeria-naïve mice (Fig. 2B). More importantly, almost fivefold more tetramer-positive cells could be recovered from L. donovani-infected, Listeria-immune mice than from mice that were not infected with L. donovani (Fig. 2B). Thus, L. donovani infection, rather than causing attrition of preexisting CD8+ memory T cells, appeared to favor bystander expansion. To rule out the possibility that tetramer-positive cells in L. donovani-infected mice merely had a greater potential for proliferation than the cells in noninfected mice, we also determined the number of tetramer-positive cells in mice before challenge with Listeria. The spleens of control Listeria-immune mice contained 10.3 × 103 ± 2.24 × 103 tetramer-positive cells, whereas the spleens of L. donovani-infected, Listeria-immune mice contained 44 × 103 ± 10.16 ×103 tetramer-positive cells (P < 0.05; n = 4). Together, these experiments demonstrated for the first time that L. donovani infection expands a population of memory CD8+ T cells specific for a heterologous pathogen.
FIG. 2.
L. donovani infection causes splenomegaly and expansion of LLO-specific CD8+ T cells. Spleen cells were isolated from groups of mice that were treated 3 days after Listeria challenge, enriched for CD8+ T cells by magnetic sorting, and then analyzed for binding of the H2Kd/LLO91-99 tetramer. The degree of splenomegaly (A) was calculated relative to body weight. The dashed line indicates the mean value for control uninfected and nonvaccinated mice. The tetramer data are expressed as the absolute number of tetramer-positive CD8+ T cells in the spleen (B). Each symbol represents an individual mouse (five or six mice per group), and statistical significance between groups was determined using Student's t test. Vacc, vaccination; L.don, L. donovani.
Although the number of tetramer-positive cells was not increased by L. donovani infection of naïve mice, it was still possible that LLO91-99-specific memory CD8+ T cells might show some cross-reactivity with L. donovani that was more readily expressed due to the lower threshold for activation of memory cells than for activation of naïve T cells. To address this possibility, we assessed the capacity of CD8+ T cells from Listeria-immune mice to recognize either Listeria- or L. donovani-infected dendritic cells in vitro. Whereas bulk populations of CD8+ T cells proliferated (Fig. 3A) and secreted IFN-γ (Fig. 3B) in response to Listeria-infected dendritic cells and to LLO91-99-pulsed dendritic cells, no such response was evident with dendritic cells infected with a transgenic line of L. donovani expressing OVA. Furthermore, tetramer-positive cells also failed to respond to these L. donovani-infected dendritic cells (Fig. 3C). The lack of response in these cultures was not due to a failure in class I presentation, as DC infected with these transgenic L. donovani cells were capable of stimulating responses in OVA-specific class I-restricted OT-1 cells (data not shown). Based on these findings together with the in vivo data described above, we concluded that there is not significant recognition of L. donovani either by bulk Listeria-specific CD8+ T cells or by the LLO91-99-specific tetramer-positive cells that expand during chronic L. donovani infection of Listeria-immune mice.
FIG. 3.
Listeria-specific CD8+ T cells do not recognize L. donovani-infected dendritic cells. CD8+ T cells were isolated from Listeria-immune BALB/c mice and stimulated with dendritic cells infected with either Listeria or L. donovani OVA (multiplicity of infection, 10:1). Proliferation (A) and IFN-γ secretion (B) of CD8+ T cells were determined on day 3. (C) CD8+ T cells gated on tetramer staining prior to analysis. Unstim, unstimulated; L.don, L. donovani.
Recently, memory T cells have been subdivided on the basis of CD44 and CD62L expression into two subsets. CD44hi CD62Llo T effector memory (TEM) cells represent cells that traffic to inflammatory sites and act as rapid effectors after reexposure to antigen (15, 23). In contrast, CD44hi CD62Lhi T central memory (TCM) cells have the capacity to migrate into peripheral lymphoid tissues (16). The responsiveness of these two subsets of memory cells to cytokine-driven bystander proliferation has been partially characterized in vitro (8, 25). However, the impact of chronic infection on the relative frequencies of heterologous antigen-specific TEM and TCM cells populations in vivo has not been documented previously. Examination of tetramer-positive cells in Listeria-immune mice indicated that approximately 60% were TCM cells, as defined by CD44 and CD62L staining. However, in Listeria-immune mice infected with L. donovani, the frequency of TCM cells was almost 80%, and reciprocal changes were observed in the TEM-cell population (Fig. 4A and B). In absolute terms, the number of tetramer-positive TCM cells was approximately sevenfold greater in L. donovani-infected, Listeria-immune mice than in control Listeria-immune mice (102 ×103 ± 27 × 103 and 14.9 ×103 ± 6.4 ×103 cells, respectively; P < 0.05; n = 5 or 6). Thus, L. donovani infection significantly skewed the LLO91-99-specific memory CD8+-T-cell pool toward a TCM phenotype. A number of possibilities could explain this finding: the greater potential for homeostatic proliferation of TCM cells than of TEM cells (25) may be maintained in response to cytokines present in the inflammatory environment caused by L. donovani infection (6); the rate of conversion from TEM cells to TCM cells (25) might be enhanced in the environment of the L. donovani-infected spleen, by factors independent of the turnover rate; or tetramer-positive TEM cells may have been preferentially recruited from the spleen to other sites of L. donovani infection (e.g., the liver). Further studies are required to differentiate between these possibilities.
FIG. 4.
L. donovani infection alters the balance of LLO-specific TEM and TCM cells. CD8+ T cells from groups of mice were isolated before and after Listeria challenge, stained with the H2Kd/LLO91-99 tetramer, and analyzed for expression of CD44 and CD62L. (A) Representative dot plots. (B) Data for each group of mice. Each symbol represents an individual mouse (five or six mice per group), and statistical significance between groups was determined using Student's t test. Vacc, vaccination; L.don, L. donovani.
To assess the functional capacity of tetramer-positive cells expanded as a consequence of L. donovani infection, we restimulated spleen cells with 5 μg/ml LLO91-99 and then determined the frequency of IFN-γ-producing cells by flow cytometry. In nonimmune mice, very few LLO91-99-specific CD8+ T cells were detectable after Listeria challenge, whether the mice had been infected with L. donovani or not (Fig. 5A and B). However, in Listeria-immune mice, LLO91-99-specific CD8+ T cells were readily stimulated in vitro to produce IFN-γ. For spleen cells from L. donovani-infected, Listeria-immune mice, but not for spleen cells from L. donovani-infected, Listeria-naïve mice, there was a marked increase in the frequency of LLO91-99-specific CD8+ T cells able to make IFN-γ (Fig. 5A). The impact of L. donovani infection on the total capacity of the mice to make IFN-γ in response to LLO91-99 was even more evident after we adjusted for the total number of CD8+ T cells in the spleen (Fig. 5B). The levels of IFN-γ produced by CD8+ T cells in response to restimulation with LLO91-99 were also different in these groups of mice. CD8+ T cells from control Listeria-immune mice produced significantly more IFN-γ when they were directly restimulated in vitro than CD8+ T cells produced when they were restimulated 3 days after challenge infection (Fig. 5C and D). These data may reflect down-regulation of the T-cell receptor and associated molecules on the recently antigen-reactivated memory cells (10). More strikingly, in mice infected with L. donovani, LLO91-99-specific CD8+ T cells produced higher levels of IFN-γ than the cells in control Listeria-immune mice produced (Fig. 5C and D). Hence, not only does L. donovani infection increase the numbers of LLO91-99-specific CD8+ T cells in the spleens of Listeria-immune mice, but these cells have a heightened capacity to produce IFN-γ when they are reexposed to antigen.
FIG. 5.
IFN-γ production by LLO-specific CD8+ T cells. Bulk splenocytes were purified from individual mice in groups and restimulated in vitro with LLO91-99 in the presence of brefeldin A. The frequency (A) and absolute number (B) of IFN-γ-positive CD8+ T cells are shown. (C) Representative dot plots showing the IFN-γ levels produced by CD8+ T cells taken from mice before challenge (top panels) and after challenge (bottom panels) with Listeria. Data for all mice analyzed in this way before challenge (open bars) and after challenge (solid bars) are shown in panel D. The bars indicate IFN-γ production expressed as the mean fluorescence intensity (MFI), and the error bars indicate standard deviations (four to six mice per group). Data were analyzed using Student's t test. Vacc, vaccination; L.don, L. donovani.
Concluding remarks.
Our results identified a number of features associated with in vivo bystander CD8+-T-cell expansion that have not been reported previously. First, we found that infection with L. donovani augments vaccine-induced immunity to Listeria, expanding and enhancing the effector function of a host-protective LLO91-99-specific memory CD8+-T-cell population. The in vivo regulation of bystander expansion is likely to be both complex and highly dynamic and controlled by cytokines and costimulatory receptor-ligand interactions (4, 5, 11, 13, 26), and many of these parameters remain to be fully evaluated in our model. However, it is known that there is increased accumulation of IL-2 mRNA in mice with chronic L. donovani infections (6), and more recent preliminary studies indicate that IL-7 mRNA accumulation was not altered, while IL-15 mRNA accumulation was reduced in the spleens of infected mice compared to the spleens of naïve mice (Polley, unpublished data). Second, our data indicate that as a consequence of L. donovani infection, LLO91-99-specific memory CD8+ T cells acquire an enhanced functional capacity, as measured at the single-cell level by intracellular IFN-γ production. The basis for this enhanced responsiveness remains to be determined, but the responsiveness is consistent with previous findings which showed that there was bystander enhancement of IFN-γ production (12). Finally, the increase in heterologous memory CD8+ T cells that accompanies L. donovani infection was contrary to our expectations based on the concept of a finite size for the memory pool (7) and evidence from viral infection models indicating that non-cross-reactive infections can cause attrition of preexisting memory (17, 18). In this respect, it is tempting to speculate that the splenomegaly associated with L. donovani infection, and indeed with other chronic parasitic infections, such as malaria and schistosomiasis, may override some of the constraints normally imposed on the memory compartment under steady-state conditions, resetting a new level of homeostasis in the enlarged spleen. Given the global burden of these infections, the extent to which chronic or recurrent splenomegaly and/or lymphadenopathy may affect the memory CD8+-T-cell compartment clearly warrants further experimental and clinical study.
Acknowledgments
We thank the staff of the Biological Services Facility for animal husbandry and G. Bancroft and G. Lertmemongkolchai for comments on the manuscript.
This work was supported by grants from the British Medical Research Council and The Wellcome Trust. R.P. and S.L.S. were recipients of MRC postgraduate training awards. S.P. was a recipient of an Imperial College Biochemistry Department postgraduate bursary.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Antia, R., V. V. Ganusov, and R. Ahmed. 2005. The role of models in understanding CD8+ T-cell memory. Nat. Rev. Immunol. 5:101-111. [DOI] [PubMed] [Google Scholar]
- 2.Berg, R. E., E. Crossley, S. Murray, and J. Forman. 2003. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J. Exp. Med. 198:1583-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busch, D. H., I. M. Pilip, S. Vijh, and E. G. Pamer. 1998. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity 8:353-362. [DOI] [PubMed] [Google Scholar]
- 4.Chapdelaine, Y., D. K. Smith, J. A. Pedras-Vasconcelos, L. Krishnan, and S. Sad. 2003. Increased CD8+ T cell memory to concurrent infection at the expense of increased erosion of pre-existing memory: the paradoxical role of IL-15. J. Immunol. 171:5454-5460. [DOI] [PubMed] [Google Scholar]
- 5.Eberl, G., P. Brawand, and H. R. MacDonald. 2000. Selective bystander proliferation of memory CD4+ and CD8+ T cells upon NK T or T cell activation. J. Immunol. 165:4305-4311. [DOI] [PubMed] [Google Scholar]
- 6.Engwerda, C. R., S. C. Smelt, and P. M. Kaye. 1996. An in vivo analysis of cytokine production during Leishmania donovani infection in SCID mice. Exp. Parasitol. 84:195-202. [DOI] [PubMed] [Google Scholar]
- 7.Freitas, A. A., and B. Rocha. 2000. Population biology of lymphocytes: the flight for survival. Annu. Rev. Immunol. 18:83-111. [DOI] [PubMed] [Google Scholar]
- 8.Geginat, J., F. Sallusto, and A. Lanzavecchia. 2001. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4+ T cells. J. Exp. Med. 194:1711-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harty, J. T., L. L. Lenz, and M. J. Bevan. 1996. Primary and secondary immune responses to Listeria monocytogenes. Curr. Opin. Immunol. 8:526-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kambayashi, T., E. Assarsson, B. J. Chambers, and H. G. Ljunggren. 2001. IL-2 down-regulates the expression of TCR and TCR-associated surface molecules on CD8+ T cells. Eur. J. Immunol. 31:3248-3254. [DOI] [PubMed] [Google Scholar]
- 11.Koschella, M., D. Voehringer, and H. Pircher. 2004. CD40 ligation in vivo induces bystander proliferation of memory phenotype CD8 T cells. J. Immunol. 172:4804-4811. [DOI] [PubMed] [Google Scholar]
- 12.Lertmemongkolchai, G., G. Cai, C. A. Hunter, and G. J. Bancroft. 2001. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J. Immunol. 166:1097-1105. [DOI] [PubMed] [Google Scholar]
- 13.Li, X. C., G. Demirci, S. Ferrari-Lacraz, C. Groves, A. Coyle, T. R. Malek, and T. B. Strom. 2001. IL-15 and IL-2: a matter of life and death for T cells in vivo. Nat. Med. 7:114-118. [DOI] [PubMed] [Google Scholar]
- 14.Mackaness, G. B. 1964. The immunological basis of acquired cellular resistance. J. Exp. Med. 120:105-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masopust, D., V. Vezys, A. L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413-2417. [DOI] [PubMed] [Google Scholar]
- 15a.Polley, R., S. Stager, S. Prickett, A. Maroof, S. Zubairi, D. F. Smith, and P. M. Kaye. Adoptive immunotherapy against experimental visceral leishmaniasis with CD8+ T cells requires the presence of cognate antigen, Infect. Immun., in press. [DOI] [PMC free article] [PubMed]
- 16.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708-712. [DOI] [PubMed] [Google Scholar]
- 17.Schluns, K. S., and L. Lefrancois. 2003. Cytokine control of memory T-cell development and survival. Nat. Rev. Immunol. 3:269-279. [DOI] [PubMed] [Google Scholar]
- 18.Selin, L. K., M. Y. Lin, K. A. Kraemer, D. M. Pardoll, J. P. Schneck, S. M. Varga, P. A. Santolucito, A. K. Pinto, and R. M. Welsh. 1999. Attrition of T cell memory: selective loss of LCMV epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity 11:733-742. [DOI] [PubMed] [Google Scholar]
- 19.Selin, L. K., K. Vergilis, R. M. Welsh, and S. R. Nahill. 1996. Reduction of otherwise remarkably stable virus-specific cytotoxic T lymphocyte memory by heterologous viral infections. J. Exp. Med. 183:2489-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith, D. K., R. Dudani, J. A. Pedras-Vasconcelos, Y. Chapdelaine, H. van Faassen, and S. Sad. 2002. Cross-reactive antigen is required to prevent erosion of established T cell memory and tumor immunity: a heterologous bacterial model of attrition. J. Immunol. 169:1197-1206. [DOI] [PubMed] [Google Scholar]
- 21.Tough, D. F., X. Zhang, and J. Sprent. 2001. An IFN-gamma-dependent pathway controls stimulation of memory phenotype CD8+ T cell turnover in vivo by IL-12, IL-18, and IFN-gamma. J. Immunol. 166:6007-6011. [DOI] [PubMed] [Google Scholar]
- 22.Welsh, R. M., and L. K. Selin. 2002. No one is naive: the significance of heterologous T-cell immunity. Nat. Rev. Immunol. 2:417-426. [DOI] [PubMed] [Google Scholar]
- 23.Weninger, W., M. A. Crowley, N. Manjunath, and U. H. von Andrian. 2001. Migratory properties of naive, effector, and memory CD8+ T cells. J. Exp. Med. 194:953-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wherry, E. J., D. L. Barber, S. M. Kaech, J. N. Blattman, and R. Ahmed. 2004. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl. Acad. Sci. USA 101:16004-16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wherry, E. J., V. Teichgraber, T. C. Becker, D. Masopust, S. M. Kaech, R. Antia, U. H. Von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225-234. [DOI] [PubMed] [Google Scholar]
- 26.Wong, P., and E. G. Pamer. 2001. Cutting edge: antigen-independent CD8 T cell proliferation. J. Immunol. 166:5864-5868. [DOI] [PubMed] [Google Scholar]