Abstract
Twelve cases of Mycobacterium bovis subsp. caprae infection have occurred in four humans, three cattle, and five red deer in western Austria since 1994. DNA-fingerprinting of the isolates suggested transmission in and between these species over several years. Contact with cattle, but not with goats, was found to be associated with three of four human cases.
Tuberculosis in cattle, goats, and captive deer is still a source of economic losses in many countries. Transmission between livestock and some wild animals, e.g., the Eurasian badger (Meles meles) (7) and the Australian brush-tailed possum (Trichosurus vulpecula) (20), contributes to its endemicity (14), whereas others, like free-living deer, are primarily considered “spillover hosts” (2, 3).
The vast majority of animal infections are caused by Mycobacterium bovis. Strains isolated from goats, however, show genetic features not found with other M. bovis strains: they lack characteristic spacers in the spoligotyping fingerprinting method (2, 9) and show the wild-type allele of the pncA gene as in Mycobacterium tuberculosis (1, 6, 11). The new taxon M. tuberculosis subsp. caprae Aranaz et al. 1999 originally proposed for these caprine strains (1) was recently transferred to Mycobacterium bovis Karlson and Lessel 1970 as Mycobacterium bovis subsp. caprae comb. nov. (12). In western Austria, a region virtually free of animal tuberculosis (16), outbreaks of M. bovis subsp. caprae infections in cattle and red deer have caused investigations of the prevalence of this subspecies in animals and humans, the results of which are presented here.
Mycobacterial isolates and strain typing methods.
Since 1994, 640 M. tuberculosis complex isolates have been obtained from patients in western Austria (population, 1,100,000) and fingerprinted, corresponding to 80% of the regionally reported culture-positive cases. Specimens taken from granulomatous lesions or abscesses in animal carcasses were cultured on standard solid and liquid media. Identification of M. tuberculosis complex was routinely done by hybridization (AccuProbe system; Gen-Probe, San Diego, Calif.).
Spoligotyping (10) was carried out on membranes with covalently bound, spacer sequence-specific oligonucleotides purchased from Isogen Bioscience BV (Maarsen, The Netherlands). Approximately 10 ng of mycobacterial DNA was used for amplification of spacer sequences in the direct repeat (DR) region by PCR. For amplification of DR sequences from paraffin wax-embedded tissue, DNA was extracted from 14-μm-thick sections by the method of van der Zanden et al. (21). IS6110 restriction fragment length polymorphism (RFLP) patterns were generated by the standardized method (22) and analyzed with Gelcompar software, version 4.1 (Applied Math, Sint-Martens-Latem, Belgium) as described previously (15). The presence of M. bovis subsp. caprae was confirmed by an allele-specific PCR method (6) detecting DNA polymorphisms described for the pncA gene (17) and the oxyR gene (19).
Two outbreaks of tuberculosis among cattle and free-living red deer.
In 1999, tuberculosis was detected in wild and livestock animals in two geographically distant western Austrian regions (regions I and II). In region I, M. tuberculosis complex isolates were obtained from three cattle originating from one farm. A fourth isolate came from pulmonary lesions in a wild red deer (Cervus elaphus hippelaphus) fawn found 20 km away from the afflicted farm. In region II, a natural park area comprising both Austrian and German territory and more than 70 km apart from region I, four cases of pulmonary tuberculosis in adult red deer occurred in 1999 and 2000.
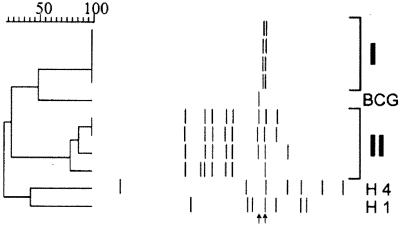
IS6110 RFLP analysis of the isolates showed two clusters, one for each region (Fig. 1). The region I isolates showed an identical double band pattern, whereas region II isolates had six of seven or eight bands in common. Spoligotyping revealed a deletion of spacers 1 and 3 through 16 in all isolates (Fig. 2), characteristic of M. bovis subsp. caprae. Results of an allele-specific PCR for pncA and oxyR corroborated the presence of M. bovis subsp. caprae for all isolates; the wild-type sequence was present at position 169 in pncA, and the G/A transition was found in oxyR at position 295.
FIG. 1.
IS6110 RFLP patterns for 10 M. bovis subsp. caprae isolates from animals and humans. The dendrogram was constructed by the unweighted pair-group method using arithmetic averages with the dendrogram software Gelcompar 4.1. The Dice index of band position similarity is given on top. Band positions are shown (top to bottom) for eight animal isolates from regions I (3 cattle, 1 deer) and II (4 deer), for the human isolates H1 and H4, and for M. bovis BCG. The left arrow points to the single 1.9-kb band typical of M. bovis isolates from cattle (5, 23). The right arrow points to a band of 1.75 to 1.8 kb observed with all M. bovis subsp. caprae in this study.
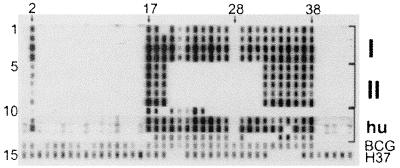
FIG. 2.
Spoligotypes obtained from 13 cases of M. bovis subsp. caprae infection in animals and humans in western Austria. Salient spacer positions are marked on top with arrows and spacer numbers according to reference 10. Patterns obtained in independent experiments were scanned and compiled. Lanes 1 to 4, spoligotypes from region I isolates (3 cattle, 1 deer); lanes 5 to 9, spoligotypes from four isolates and one paraffin wax-embedded tissue from five deer from region II; lanes 10 to 13, four human isolates (H1 to H4, respectively); lane 14, M. bovis BCG; lane 15, M. tuberculosis strain H37Rv. Note the absence of spacers 1, 3 to 16, and 28 in lanes 1 to 13, which is characteristic of M. bovis subsp. caprae.
As tuberculosis in red deer had not been found in the region for decades, histopathological specimens taken from deer in the previous years were reexamined. One deer shot in region II in 1998 had shown large abscesses containing neutrophils in several organs, a picture compatible with tuberculosis, although no acid-fast bacilli were detected. Sections from paraffin wax-embedded tissue from that animal yielded the region II spoligotyping pattern (Fig. 2, lane 9), proving that M. bovis subsp. caprae had been present in deer for at least 3 years.
M. bovis subsp. caprae has been identified in Spanish (2, 8) and German (11) studies on animal tuberculosis but was not detected in larger spoligotyping analyses of M. bovis isolates from Italy (18), Ireland (4, 5), Cameroon (13), Argentina (24), or Australia, Canada, and Iran (5). Here, we report the first isolation of M. bovis subsp. caprae from wild animals. Wild animal species are recognized sources of tuberculosis in cattle (3, 14), and this may have been the case in the region I outbreak (cattle skin testing in region I failed to reveal further infections except in another 15 of 30 cattle at the afflicted farm). However, no further tuberculosis cases in wild animals were found in region I, and no epidemiological connection between the cattle and deer cases was established.
In contrast to the single outbreak strain in region I, clustered but not identical isolates were obtained from region II. This suggests that M. bovis subsp. caprae has been circulating in the region for some years, either among deer alone or between deer and other animals, e.g., cattle. However, no tuberculosis infections among region II cattle were revealed by skin testing, suggesting that M. bovis subsp. caprae transmission has occurred primarily among deer. The earlier introduction of a founder strain into the deer population from infected livestock cannot be excluded.
M. bovis subsp. caprae infections in humans.
To examine whether caprine genotype strains have affected humans, a database comprising 640 fingerprints from human M. tuberculosis complex isolates was examined. Four isolates (H1 to H4) showed spoligotyping patterns compatible with M. bovis subsp. caprae (Fig. 2, lanes 10 to 13). H2 and H3 presented a spoligotype identical or almost identical to the region I strain. H1 and H4 showed larger deletions within the DR region (upstream from spacer 25 and downstream from spacer 18, respectively) but had characteristics of M. bovis subsp. caprae in the remaining part, i.e., lack of spacers 1, 3 to 16, and 28. The identity of H1 through H4 as M. bovis subsp. caprae was confirmed by the allele-specific PCR for pncA and oxyR described above. Unfortunately, IS6110 RFLP fingerprints were only available for H1 and H4 (Fig. 1), but no isolate with >55% similarity to H1 or H4 was present in the RFLP database.
All four patients suffered from pulmonary tuberculosis, and all but one recovered (see Table 1 for details). Patient H1 made a protracted recovery, also due to acquired multidrug resistance. Although patient H1 had smear-positive cavitating disease for several years, no indication of secondary human-to-human transmission was evident from the database. Transmission between H2 and H3 was unlikely from the epidemiological data. The circumstance, however, that two patients out of four were cattle farmers and a third lived close to cattle farms is interesting, the more so as handling of or contact with goats could not be ascertained for these patients (Table 1).
TABLE 1.
Epidemiological data for human tuberculosis cases caused by M. bovis subsp. caprae occurring in western Austria, 1994 to 2000
| Patient no. (yr of diagnosis) | Age (yr), sex | Drug resistancea | Farming area resident | Contact with stock animals (profession) | Clinical outcome of treatment |
|---|---|---|---|---|---|
| H1 (1995) | 39, male | HR | Yes | Yes (farmer) | Prolonged recovery, strain developed multidrug resistance |
| H2 (1995) | 66, male | None | No | No (physician) | Lethal due to miliary course after transplantation |
| H3 (1998) | 54, male | None | Yes | No (worker) | Complete uneventful recovery |
| H4 (1998) | 72, male | None | Yes | Yes (farmer) | Complete uneventful recovery |
Pyrazinamide susceptibility was not tested biochemically but by PCR analysis of the pncA gene (17). HR, isoniazid and rifampin.
Human cases of M. bovis subsp. caprae infection contracted from goats have been observed in Spain (9). A report from Germany also listed human cases, together with cases in cattle, but did not demonstrate transmission between humans and cattle (11). Spoligotypes found in this study for the region I strain and the H1 isolate have already been described in the German study, underlining the prevalence of genetically related M. bovis subsp. caprae in central Europe. In contrast to the German report, which included 15 M. bovis subsp. caprae versus 35 other human M. bovis cases, in our study population, four caprine genotype isolates but only two other M. bovis isolates were obtained from patients. We conclude that M. bovis subsp. caprae infections are not an insignificant part of human tuberculosis contracted from animals due to the continued presence of the pathogen in rural alpine regions.
Acknowledgments
This work was supported by grant 7599 from the Austrian National Bank, Jubilaeumsfonds, and by the European Commission, Concerted Action “New generation markers and techniques for the epidemiology and control of tuberculosis” (QLK2-CT-2000-00630).
We thank Eva Schaber and Martin Wurm for excellent technical support and Christian Messner for providing deer specimens.
REFERENCES
- 1.Aranaz, A., E. Liebana, E. Gomez Mampaso, J. Galan, D. Cousins, A. Ortega, J. Blazquez, F. Baquero, A. Mateos, G. Suarez, and L. Dominguez. 1999. Mycobacterium tuberculosis subsp. caprae subsp. nov.: a taxonomic study of a new member of the Mycobacterium tuberculosis complex isolated from goats in Spain. Int. J. Syst. Bacteriol. 49:1263-1273. [DOI] [PubMed] [Google Scholar]
- 2.Aranaz, A., E. Liebana, A. Mateos, L. Dominguez, D. Vidal, M. Domingo, O. Gonzolez, E. F. Rodriguez Ferri, A. E. Bunschoten, J. D. van Embden, and D. Cousins. 1996. Spacer oligonucleotide typing of Mycobacterium bovis strains from cattle and other animals: a tool for studying epidemiology of tuberculosis. J. Clin. Microbiol. 34:2734-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clifton Hadley, R. S., and J. W. Wilesmith. 1991. Tuberculosis in deer: a review. Vet. Rec. 129:5-12. [DOI] [PubMed] [Google Scholar]
- 4.Costello, E., D. O'Grady, O. Flynn, R. O'Brien, M. Rogers, F. Quigley, J. Egan, and J. Griffin. 1999. Study of restriction fragment length polymorphism analysis and spoligotyping for epidemiological investigation of Mycobacterium bovis infection. J. Clin. Microbiol. 37:3217-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cousins, D., S. Williams, E. Liebana, A. Aranaz, A. Bunschoten, J. van Embden, and T. Ellis. 1998. Evaluation of four DNA typing techniques in epidemiological investigations of bovine tuberculosis. J. Clin. Microbiol. 36:168-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espinosa de los Monteros, L. E., J. C. Galan, M. Gutierrez, S. Samper, J. F. Garcia Marin, C. Martin, L. Dominguez, L. de Rafael, F. Baquero, E. Gomez Mampaso, and J. Blazquez. 1998. Allele-specific PCR method based on pncA and oxyR sequences for distinguishing Mycobacterium bovis from Mycobacterium tuberculosis: intraspecific M. bovis pncA sequence polymorphism. J. Clin. Microbiol. 36:239-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallagher, J., and R. S. Clifton Hadley. 2000. Tuberculosis in badgers; a review of the disease and its significance for other animals. Res. Vet. Sci. 69:203-217. [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez, M., S. Samper, J. A. Gavigan, J. F. Garcia Marin, and C. Martin. 1995. Differentiation by molecular typing of Mycobacterium bovis strains causing tuberculosis in cattle and goats. J. Clin. Microbiol. 33:2953-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutierrez, M., S. Samper, M. S. Jimenez, J. D. van Embden, J. F. Marin, and C. Martin. 1997. Identification by spoligotyping of a caprine genotype in Mycobacterium bovis strains causing human tuberculosis. J. Clin. Microbiol. 35:3328-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamerbeek, J., L. Schouls, A. Kolk, M. van Agterveld, D. van Soolingen, S. Kuijper, A. Bunschoten, H. Molhuizen, R. Shaw, M. Goyal, and J. van Embden. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niemann, S., E. Richter, and S. Rusch Gerdes. 2001. Differentiation among members of the Mycobacterium tuberculosis complex by molecular and biochemical features: evidence for two pyrazinamide-susceptible subtypes of M. bovis. J. Clin. Microbiol. 38:152-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niemann, S., E. Richter, and S. Rusch Gerdes. 2002. Biochemical and genetic evidence for the transfer of Mycobacterium tuberculosis subsp. caprae Aranaz et al. 1999 to the species Mycobacterium bovis Karlson and Lessel 1970 (Approved Lists 1980) as Mycobacterium bovis subsp. caprae comb. nov. Int. J. Syst. Evol. Microbiol. 52:433-436. [DOI] [PubMed] [Google Scholar]
- 13.Njanpop Lafourcade, B., J. Inwald, A. Ostyn, B. Durand, S. Hughes, M. Thorel, G. Hewinson, and N. Haddad. 2001. Molecular typing of Mycobacterium bovis isolates from Cameroon. J. Clin. Microbiol. 39:222-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Reilly, L. M., and C. J. Daborn. 1995. The epidemiology of Mycobacterium bovis infections in animals and humans: a review. Tuber. Lung Dis. 76(Suppl.):11-46. [DOI] [PubMed] [Google Scholar]
- 15.Pavlic, M., F. Allerberger, M. P. Dierich, and W. M. Prodinger. 1999. Simultaneous infection with two drug-susceptible Mycobacterium tuberculosis strains in an immunocompetent host. J. Clin. Microbiol. 37:4156-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlerka, G., and P. Fehr. 1995. Über eine Infektion eines Rinderbestandes mit Mycobacterium bovis. Wien. Tierärztl. Mschr. 82:48-53.2382445 [Google Scholar]
- 17.Scorpio, A., and Y. Zhang. 1996. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat. Med. 2:662-667. [DOI] [PubMed] [Google Scholar]
- 18.Serraino, A., G. Marchetti, V. Sanguinetti, M. Rossi, R. Zanoni, L. Catozzi, A. Bandera, W. Dini, W. Mignone, F. Franzetti, and A. Gori. 1999. Monitoring of transmission of tuberculosis between wild boars and cattle: genotypical analysis of strains by molecular epidemiology techniques. J. Clin. Microbiol. 37:2766-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sreevatsan, S., P. Escalante, X. Pan, D. A. Gillies, S. Siddiqui, C. N. Khalaf, B. N. Kreiswirth, P. J. Bifani, L. G. Adams, T. Ficht, V. S. Perumaalla, M. D. Cave, J. D. van Embden, and J. M. Musser. 1996. Identification of a polymorphic nucleotide in oxyR specific for Mycobacterium bovis. J. Clin. Microbiol. 34:2007-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tweddle, N. E., and P. Livingstone. 1994. Bovine tuberculosis control and eradication programs in Australia and New Zealand. Vet. Microbiol. 40:23-39. [DOI] [PubMed] [Google Scholar]
- 21.van der Zanden, A. G., A. H. Hoentjen, F. G. Heilmann, E. F. Weltevreden, L. M. Schouls, and J. D. van Embden. 1998. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis complex in paraffin wax embedded tissues and in stained microscopic preparations. Mol. Pathol. 51:209-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Embden, J. D., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, T. M. Shinnick, and P. M. Small. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Soolingen, D., P. E. de Haas, J. Haagsma, T. Eger, P. W. Hermans, V. Ritacco, A. Alito, and J. D. van Embden. 1994. Use of various genetic markers in differentiation of Mycobacterium bovis strains from animals and humans and for studying epidemiology of bovine tuberculosis. J. Clin. Microbiol. 32:2425-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zumarraga, M. J., C. Martin, S. Samper, A. Alito, O. Latini, F. Bigi, E. Roxo, M. E. Cicuta, F. Errico, M. C. Ramos, A. Cataldi, D. van Soolingen, and M. I. Romano. 1999. Usefulness of spoligotyping in molecular epidemiology of Mycobacterium bovis-related infections in South America. J. Clin. Microbiol. 37:296-303. [DOI] [PMC free article] [PubMed] [Google Scholar]