Abstract
Monohexosylceramides (CMHs, or cerebrosides) have been reported as membrane and cell wall constituents of both pathogenic and nonpathogenic fungi, presenting remarkable differences in their ceramide moiety compared to mammalian CMHs. Current evidence suggests that CMHs are involved in fungal differentiation and growth and contribute to host immune response. Here we describe a structural diversity between cerebrosides obtained from different forms of the human pathogen Fonsecaea pedrosoi. The major CMH species produced by conidial forms displayed the same structure previously demonstrated by our group for mycelia, an N-2′-hydroxyhexadecanoyl-1-β-d-glucopyranosyl-9-methyl-4,8-sphingadienine. However, the major cerebroside species purified from sclerotic cells carries an additional hydroxyl group, bound to its long-chain base. The structural difference between cerebrosides from mycelial and sclerotic cells was apparently not relevant for their antigenicity, since they were both recognized at similar levels by sera from individuals with chromoblastomycosis and a monoclonal antibody to a conserved cerebroside structure. Preincubation of fungal cells with anti-CMH monoclonal antibodies had no effect on the interaction of F. pedrosoi sclerotic cells with murine macrophages. In contrast to what has been described for other fungal species, sclerotic bodies are resistant to the antifungal action of anti-CMH antibodies. Immunofluorescence analysis showed that recognition of sclerotic cells by these antibodies only occurs at cell wall regions in which melanization is not evident. Accordingly, melanin removal with alkali results in an increased reaction of fungal cells with anti-CMH antibodies. Our results indicate that cerebroside expression in F. pedrosoi cells is associated with dimorphism and melanin assembly on the fungal cell wall.
The dematiaceous fungus Fonsecaea pedrosoi is the principal etiologic agent of chromoblastomycosis, a chronic and granulomatous mycosis usually confined to skin and subcutaneous tissues (15). Predominant in tropical and subtropical areas, this disease is normally described in arms and legs of labor workers, which are constantly in contact with soil, where F. pedrosoi grows as a saprophyte (5). Characterized by dry, crusted, warty, and violaceous lesions, chromoblastomycosis has a complicated treatment. It includes a combination of antifungal drugs and surgical excision; however, incorrect diagnosis, relapses, and therapy interruption are frequent, causing an elevated percentage of morbidity (5). Cryotherapy and laser surgery are alternative options for removing the lesions (6).
Although fungal infection occurs after traumatic inoculation of mycelium fragments and conidial forms, excised chromoblastomycosis lesions reveal mostly sclerotic bodies and a small number of mycelium fragments (5, 6, 10). The morphological changes from conidial forms to sclerotic bodies occur inside the host, associated with an intense granulomatous response (11, 27). Interestingly, sclerotic cells display a unique shape along with a muriform arrangement within the tissue, which impairs an efficient host cell attack and antifungal drug access (10).
Initially described as mammalian cell membrane building blocks (14), monohexosylceramides (CMH) have been demonstrated to be involved in relevant cellular functions (4, 14). Several studies have shown CMH and more complex glycosphingolipids (GSL) as antigens (4), mediators of cell adhesion (14), and key molecules in signal transduction upon cell-cell interaction (14). Special attention has been given to fungal CMH in the last two decades. All fungal species studied so far were able to synthesize CMH, with Saccharomyces cerevisiae being the unique exception (4). Comparing CMH from several pathogenic fungi, a very conservative structure has been observed, consisting of a ceramide moiety containing 9-methyl-4,8-sphingadienine in amidic linkage to 2-hydroxyoctadecanoic or 2-hydroxyhexadecanoic acids and glucose or galactose as the carbohydrate portion (4).
Antigenic properties have also been described for fungal CMH. Rodrigues and colleagues (24) purified human antibodies against CMH from sera of patients with cryptococcosis. These antibodies reacted with the cell wall and reduced cell budding and growth of Cryptococcus neoformans. Moreover, sera from patients with histoplasmosis, aspergillosis, and paracoccidioidomycosis reacted with C. neoformans CMH (24). Antibodies to CMH also inhibited cell differentiation of Colletotrichum gloeosporioides (9), Pseudallescheria boydii (23), and Candida albicans (24). We recently tested the activity of a monoclonal anti-CMH antibody against conidial forms of F. pedrosoi (22) and detected a direct fungicidal action. Preincubation of conidial cells with anti-CMH also increased the murine peritoneal macrophage capacity to engulf and kill the fungus. CMH were also identified as specific targets for the antifungal plant defensin RsAFP2 (30). Together, these data confirmed that these GSL are not only antigenic molecules but also targets for the action of antifungal compounds.
Here, we purified and characterized CMH from sclerotic, mycelial, and conidial forms of F. pedrosoi cultured in a defined medium. The major CMH of mycelial and conidial forms present the same structure, an N-2′-hydroxyhexadecanoyl-1-β-d-glucopyranosyl-9-methyl-4,8-sphingadienine, described previously for mycelial forms cultured in complex medium (22). However, sclerotic bodies synthesize a unique cerebroside species, containing a 9-methyl-4,8-sphingadienine carrying an extra hydroxyl group. Such difference suggests the association between F. pedrosoi CMH and its dimorphism process. Although structurally different, these molecules react against sera from patients with chromoblastomycosis and a monoclonal antibody to a conserved cerebroside in equivalent levels, as determined by enzyme-linked immunosorbent assay (ELISA). The monoclonal antibody to CMH neither killed sclerotic cells nor influenced their adhesion by murine macrophages, in contrast to a previous description for conidia (22). Finally, we observed by immunofluorescence assays that melanin expression at the cell wall of F. pedrosoi interferes with recognition of CMH, which may explain the resistance of sclerotic forms to anticerebroside antibodies.
MATERIALS AND METHODS
Microorganism and growth conditions.
F. pedrosoi strain VLP was isolated from a human case of chromoblastomycosis (1). Stock cultures were maintained on Sabouraud dextrose agar under mineral oil and kept at 4°C. Transfers were made at 6-month intervals. Mycelial and sclerotic bodies were obtained from inoculation in Butterfield's chemically defined medium (7) and cultured for 30 days at room temperature at pH 6.5 and 2.7; respectively. Conidial forms were obtained under constant agitation with a stirring bar for 5 days in the same medium, pH 5.5, and at room temperature. Conidial, mycelial, and sclerotic cells were collected by filtration and washed 3 times in 0.01 M phosphate-buffered saline (PBS), pH 7.2, before all of the experiments. For interactions with macrophages and immunofluorescence assays, sclerotic cells were strongly vortexed to disrupt aggregated cells, followed by centrifugation (200 × g) for 1 min to pellet the enduring aggregated forms, leaving the disaggregated cells in solution.
Lipid extraction and purification of CMH.
GSL from different forms of F. pedrosoi were extracted at room temperature successively with mixtures of chloroform-methanol (2:1, 1:1, and 1:2 [vol/vol]). The extracts were pooled and dried under vacuum (crude lipid). The crude lipid extract was partitioned according to the method of Folch et al. (12). The lipids recovered from Folch's lower phase were fractionated on a silica gel column by elution with chloroform, acetone, and then methanol. The enriched glycolipid fraction was then purified by another silica gel column chromatography. This column was eluted sequentially with different mixtures of chloroform-methanol (95:5, 9:1, 8:2, and 1:1 [vol/vol]) and, finally, with methanol. Samples were analyzed on high-performance thin-layer chromatography (HPTLC) plates developed with chloroform-methanol-water (65:25:4 [vol/vol/vol]). The spots were visualized with iodine vapor and by charring with orcinol-H2SO4 (26). Quantification of GSL was made by using the Scion Image software (Scion Corporation, NIH), with glucose as the colorimetric standard.
ESI-MS analysis.
Glycolipids were analyzed by electrospray ionization-mass spectrometry (ESI-MS) using a Finnigan LCQ-Duo ion trap instrument (Thermo Electron, San Jose, CA). Samples were diluted in chloroform-methanol (1:1, [vol/vol]), containing 10 mM lithium iodide, and introduced into ESI-MS at a 5- to 10-μl/min flow rate, with the assistance of an infusion micropump (Harvard Apparatus, Cambridge, MA). Analyses were carried out in the positive (ESI+) mode. The source and capillary voltages were 4.5 kV and 3 V, respectively (ESI+). The capillary temperature was kept at 200°C. Spectra were collected at a 200- to 2,000-m/z range. Source-induced dissociation was obtained at 25 V. Ion trap collision-induced dissociation (ESI-MS/MS or ESI-MSn) experiments were carried out at 20 to 60% (1 to 3 eV) normalized relative collision energy. All spectra were processed using the Xcalibur software (Thermo Electron).
CMH permethylation.
The purified fraction containing GlcCer from sclerotic bodies of F. pedrosoi was permethylated essentially according to the method of Ciucanu and Kerek (8). Briefly, a few milligrams of NaOH powder was added to the completely dried sample placed on borosilicate tubes. Then, 30 μl of an iodomethane-dimethyl sulfoxide (1:1 [vol/vol]) mixture was added and the tube sealed. After sonication for 2 to 3 min, the samples were incubated at room temperature for 1 h with a quick agitation every 15 min. The reaction was stopped by the addition of 15% acetic acid in methanol (200 μl), and 100 μl of water and 300 μl of chloroform were added. After a 15-minute centrifugation at 2,000 × g, the aqueous phase was discarded and the organic phase washed 4 to 5 times with water. After evaporation of chloroform under a nitrogen stream, the samples were analyzed as described above.
Carbohydrate compositional analysis.
GSL were hydrolyzed with 3 M trifluoroacetic acid at 100°C for 3 h, and the resulting monosaccharides were identified by HPTLC and gas chromatography as their alditol-acetate derivatives (25) using an OV-225 fused silica capillary column (30 m by 0.25 mm, internal diameter), with the temperature programmed from 50 to 220°C at 5°C/min. Glucose and galactose (Sigma-Aldrich) were used as standards.
Reactivity of purified CMH with antibodies to cerebrosides.
Ninety-six-well microplates were washed with n-butanol and ethanol and then dried at room temperature (29). Purified CMH from mycelial and sclerotic cells were dissolved in a chloroform-methanol (2:1 [vol/vol]) mixture to form a 4 μM solution. This preparation was diluted in a mixture of ethanol-methanol (1:1, vol/vol) to a final concentration of 0.04 μM. Fifty microliters (2 nmol) of this solution was added to each well of a solvent-washed 96-well plate and evaporated to dryness at room temperature. After blocking with Tris-buffered saline (TBS) supplemented with bovine serum albumin (3 mg/ml) overnight at 4°C, the wells were washed with TBS and incubated (1 h, room temperature) with serially diluted sera from chromoblastomycosis patients. Sera from 17 individuals infected with F. pedrosoi presenting typical lesions of chromoblastomycosis were pooled and used in this study. Sera from healthy individuals with no previous laboratory exposure to F. pedrosoi were also collected and tested against the same preparation by ELISA. Alternatively, a monoclonal anti-CMH antibody (12 μg/ml, starting dilution) was used as a probe to detect CMH (9, 22). After washing in TBS, an alkaline phosphatase-labeled goat anti-mouse or anti-human immunoglobulin was added (1:5,000), followed by incubation for 1 h at room temperature. The plate was subsequently washed three times with TBS and once with assay buffer (0.1 M glycine buffer containing 1 mM MgCl2, 1 mM ZnCl2, pH 10.4). Reactions were visualized by the addition of assay buffer containing 1 mg/ml of p-phenyl phosphate, followed by determination of absorbance (405 nm) in a microplate reader spectrophotometer.
Effects of antibodies to CMH on cell growth.
F. pedrosoi sclerotic cells (2 × 102) were suspended in 100 μl of PBS supplemented with antibodies to CMH in concentrations varying to 1.5 to 100 μg/ml. In this range of concentrations, antibodies to CMH were demonstrated to be inhibitory for different fungal species (9, 21-23). Cell suspensions were then incubated in the presence of antibodies to CMH for 8 h at 28°C. Viable CFU were evaluated by plating 100 μl of the samples onto Sabouraud dextrose agar plates, followed by incubation for 10 days at room temperature. Control systems were similarly treated with an irrelevant immunoglobulin (mouse immunoglobulin G2b [IgG2b] to human laminin).
Interaction of sclerotic cells with J774.16 macrophages.
The murine macrophage cell line J774.16 was cultured in Dulbecco's minimal essential medium (Gibco-BRL, Gaithersburg, MD) supplemented with 2 mM l-glutamine and 10% complement-inactivated fetal bovine serum (Gibco-BRL, Gaithersburg, MD). Cells were harvested with trypsin-EDTA and washed twice with Dulbecco's minimal essential medium. Cell viability was checked by trypan blue staining. The cells were allowed to adhere onto coverslips placed in 24-well culture plates (5 × 105 per well) or into the wells of a 96-well plate (105 per well). After 30 min at 37°C in a 5% CO2 atmosphere, nonadherent cells were then removed. Adhered macrophages were washed twice with culture medium and then cultured for 24 h. Sclerotic cells of F. pedrosoi were treated with anti-CMH monoclonal antibody (10 μg/ml), PBS, or an irrelevant IgG (10 μg/ml) for 1 h before interaction with macrophages. Fungal cells were washed in PBS and allowed to interact with macrophages at fungi/macrophage ratios of 1:1 or 1:10 for 1 h at 37°C. The proportion of sclerotic cells per phagocyte was established based on the property that these forms of F. pedrosoi are much larger than other fungal cells. After removal of nonadherent fungi by washing, the coverslips were fixed with methanol and stained with Giemsa. In each system, 1,000 macrophages were counted and the results expressed as the number of adhered fungi per 100 macrophages. Control experiments with conidial cells were performed at a fungi/macrophage ratio of 1:10, and the adhesion index was at least 3 times higher than that for sclerotic cells (data not shown). These experiments were repeated 3 times with similar results.
Killing assays were performed in 96-well plates. After 1 h of incubation, the wells were washed three times with PBS and macrophages were lysed with sterile cold water. The resulting suspension was plated onto brain heart infusion agar plates. After 10 days, the number of CFU was determined. All experiments were performed in triplicate sets and statistically analyzed by using Student's t test.
Partial depletion of melanin in F. pedrosoi cells (2).
A pellet of 108 cells (conidia) or 10 mg (mycelia) was resuspended in 1 ml of 0.5 M NaOH and incubated overnight at 25°C and under constant agitation. Sclerotic cells (10 mg) that are more melanized (3) and more resistant to alkali extraction were suspended in 2 M NaOH (1 ml, final volume) and then strongly vortexed for 30 s. This suspension was slightly homogenized with a Dounce homogenizer (3 strokes) and then incubated as described for mycelia and conidia. Cell suspensions were then washed exhaustively with PBS and fixed in 4% paraformaldehyde. Melanin removal was evaluated visually or by immunofluorescence analysis with antibodies to melanin, as described below.
Immunofluorescence analysis.
The antibodies used in this study consisted of a monoclonal antibody to conserved CMH, produced and characterized in previous studies (9, 22), and a polyclonal preparation of antimelanin antibodies, obtained from sera of human individuals with chromoblastomycosis. Human antibodies to melanin were purified as previously described by our group (3) using sera described above (see “Reactivity of purified CMH with antibodies to cerebrosides”). Control or alkali-treated cells of F. pedrosoi were fixed in 4% paraformaldehyde cacodylate buffer (0.1 M, pH 7.2) for 1 h at room temperature. Fixed cells were washed twice in PBS and incubated in the same buffer containing 1% bovine serum albumin for 1 h. Cells were washed in PBS and sequentially incubated with antibodies to CMH (at 10 μg/ml) and a fluorescein isothiocyanate-labeled anti-mouse IgG (at 1:100 dilution) for 1 h at room temperature. The cells were washed again and then incubated with human antibodies to melanin at 10 μg/ml. After washing, fungal preparations were incubated with a Texas Red-labeled anti-human IgG at a 1:100 dilution. Control systems consisting of fungal cells incubated only with secondary antibodies were also prepared. Alkali-treated and untreated cells were finally washed and microscopically observed with an Axioplan 2 (Zeiss, Germany) fluorescence microscope. Images were acquired using a Color View SX digital camera and processed with the software system analySIS (Soft Image System).
RESULTS
Lipid extraction and CMH purification.
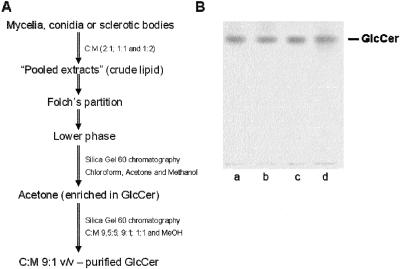
Crude lipid extraction and CMH purification were carried out as previously described (4). Identical strategies of CMH purification were used for all different forms of F. pedrosoi (Fig. 1A). After Folch's partition (12), CMH present at the lower phase was partially purified using a Silica Gel 60 chromatography column. According to HPTLC analysis, the fraction eluted with acetone was enriched in CMH (data not shown). This fraction was dried, resuspended in CHCl3, and then reapplied to another silica gel column. In all cases, the fraction containing purified CMH was eluted with chloroform-methanol (9:1 [vol/vol]) (data not shown). The HPTLC analysis of purified CMH from all different forms of F. pedrosoi revealed a retention factor (Rf) (Fig. 1B) identical to that obtained for a previously characterized CMH purified from F. pedrosoi mycelia cultivated in Kauffman's medium (22).
FIG. 1.
Purification of CMH from different F. pedrosoi forms. A summary of the purification strategy is shown in panel A. C, chloroform; M, methanol. Purified samples from mycelia (a), conidia (b), sclerotic cells (c), and a CMH standard (d) were analyzed by HPTLC and are shown in panel B.
Structural analysis of CMH from F. pedrosoi.
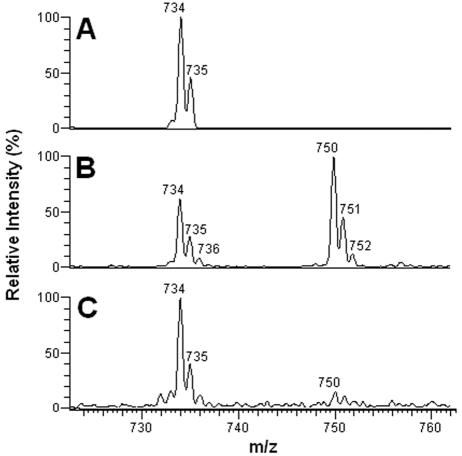
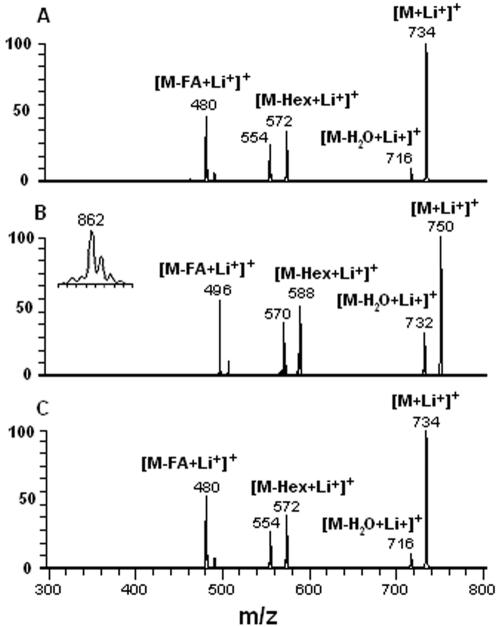
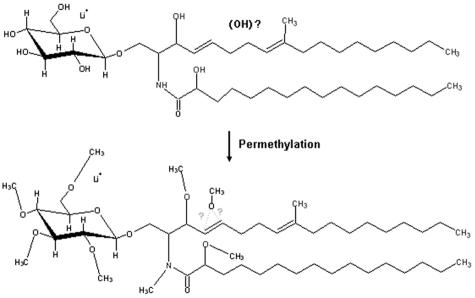
Purified CMH from different stages of F. pedrosoi were analyzed by positive ion mode ESI-MS. Scanning from 700 to 800 m/z of purified CMH samples revealed a profile similar to what has been described for other fungal cerebrosides (4). Abundant monolithiated peaks between m/z 730 and 760 were observed (Fig. 2). CMH purified from mycelia presented a major species at m/z 734 [M + Li+]+ (Fig. 2A), while two major ion species were observed at m/z 734 [M + Li+]+ and 750 [M + Li+]+ for sclerotic cell CMH (Fig. 2B). Conidial forms also presented both species; however, the intensity of the peak at m/z 750 was relatively low (Fig. 2C). The molecular species corresponding to these major peaks were submitted to ESI-MS/MS analysis (Fig. 3). The fragmentation profile was identical for the parental ions with m/z values corresponding to 734, which represented the major peak obtained for purified molecules from mycelia and conidia (Fig. 2A and C). The loss of 162 units, common to all CMH analyzed and diagnostic of a monosaccharide unit, gave rise to daughter ions at m/z 572 and 588 [M − Hexose + Li+]+ corresponding to the ceramide monolithiated ion from the parental ions at m/z 734 and 750, respectively. The daughter ions at m/z 480, observed in CMH of all three stages of F. pedrosoi, and m/z 496 observed in conidial and sclerotic cell CMH are consistent with a loss of an OH C16 fatty acid. The difference of 16 units observed among the conidial and sclerotic bodies' CMH parental ions (Fig. 2A and B) remained detectable after loss of either monosaccharide or fatty acid, suggesting that the long chain base could possibly present an extra hydroxyl group. This hypothesis was strongly supported after the analysis of the permethylated CMH derivative showing a molecular and monolithiated ion at m/z 862, consistent with an addition of 8 methyl group units (Fig. 3, inset). Fragmentation of permethylated CMH from a sclerotic body suggests that the additional hydroxyl group is linked to C-4 or -5 of its long chain base (data not shown). In all CMH studied in this work, the sugar portion was defined as glucose after HPTLC and gas chromatography analysis (data not shown). According to these data, the CMH purified from mycelia of F. pedrosoi cultivated in defined medium is identical to the one synthesized by mycelia grown in Kauffman's medium (22), consisting of 9-methyl-4,8-sphingadienine in amidic linkage to 2-hydroxyhexadecanoic acid. The same structure was assigned as the principal CMH from conidial cells. On the other hand, the major CMH synthesized by sclerotic cells bears an extra hydroxyl group in its long chain base (trihydroxyl-sphingadienine), which is also produced in minor amounts in conidial forms (Fig. 4).
FIG. 2.
ESI-MS spectra of the intact CMH purified from mycelial (A), sclerotic (B), and conidial (C) forms of F. pedrosoi.
FIG. 3.
ESI-MS/MS spectra showing the analysis of lithiated fragments derived from CMH molecular ions from F. pedrosoi mycelia (A), sclerotic bodies (B), and conidia (C). CMH from sclerotic cells were permethylated, and the corresponding molecular ion, derived from the peak at m/z 750, is shown in the inset in panel B.
FIG. 4.
Representation of native (top) and permethylated (bottom) CMH (m/z 750) from sclerotic cells. The binding site of the additional hydroxyl group, undefined in underivatized samples, involves C-4 or -5 in the fatty acid moiety of CMH.
F. pedrosoi CMH are similarly recognized by anticerebroside antibodies.
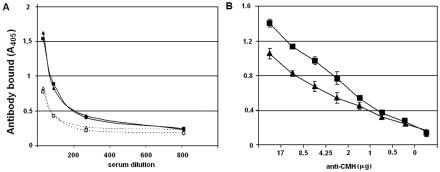
Anti-CMH antibodies have been detected in patients with different mycoses, such as aspergillosis, cryptococcosis, histoplasmosis, and paracoccidioidomycosis (24). Based on this information, we evaluated the production of antibodies to cerebrosides during the human infection by F. pedrosoi (Fig. 5A). Our data reveal that anti-CMH antibodies are in fact produced during chromoblastomycosis, and the serological reactivity is similar when cerebrosides from mycelial or sclerotic forms are used. Similar results were obtained when a monoclonal antibody to conserved fungal CMH was used. According to the results shown in Fig. 5B, the monoclonal antibody reacts well with both CMH, although the affinity level for the cerebroside extracted from sclerotic cells seems to be less than that observed for the mycelial glycolipid.
FIG. 5.
Reactivity of F. pedrosoi cerebrosides with sera from human individuals (A) or with a monoclonal antibody to conserved CMH (B). (A) Sera from patients with chromoblastomycosis (closed symbols, continuous lines) reacted similarly with CMH from sclerotic cells (triangles) or mycelia (squares). Sera from healthy individuals (open symbols, dotted lines) reacted with cerebrosides with significantly lower intensities. (B) A similar pattern of antibody reactivity was observed when cerebrosides from sclerotic cells (triangles) and mycelia (squares) were tested by ELISA using a monoclonal antibody to conserved CMH. A higher level of affinity was observed when the mycelial cerebroside was used.
Anti-CMH antibodies have no direct effect on sclerotic cells.
Considering that anti-CMH monoclonal antibodies are able to kill conidia of F. pedrosoi (22) and yeast cells of C. neoformans (24) and inhibit fungal differentiation of C. albicans, C. gloesporioides, and P. boydii (9, 23, 24), we decided to test the direct effect of monoclonal anti-CMH antibodies on sclerotic cell viability and cell differentiation. We concluded that, even after incubation of higher concentrations of anti-CMH antibodies (100 μg/ml) with sclerotic cells, there was no difference in the ability to grow or differentiate between control and antibody-treated cells (data not shown). Therefore, although sclerotic cells produce antigenic CMH (Fig. 5), they seem to be especially resistant to the direct effect of anti-CMH antibodies.
Association of sclerotic cells with murine macrophages in the presence of anti-CMH.
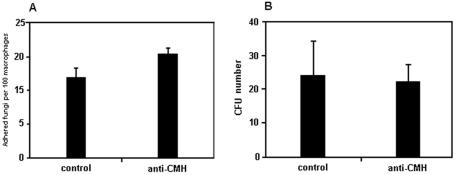
Antibodies to CMH are opsonic in a model of phagocytosis involving F. pedrosoi conidia and mouse peritoneal macrophages (22). We therefore investigated the influence of the monoclonal antibody to CMH on the adhesion and killing of sclerotic cells by the murine macrophage cell line J774.16. For this purpose, we incubated sclerotic bodies, previously treated with irrelevant IgG or anti-CMH antibodies (10 μg/ml), for 1 h with a monolayer of murine macrophages in ratios of 1 fungus per macrophage or 10 phagocytes per fungal cell. The results shown in Fig. 6A suggest that preincubation of sclerotic bodies with the antibody did not enhance fungal association to macrophages in a significant way (n = 1,000 macrophages, P > 0.05), although a small tendency of higher adhesion of antibody-treated sclerotic cells was observed. Results are shown for experiments using the 10:1 fungus/phagocyte ratio, but similar profiles were obtained when fungal cells and phagocytes were incubated in equivalent amounts (data not shown).
FIG. 6.
Influence of a monoclonal antibody to CMH on the association of F. pedrosoi with macrophages (A) and on phagocyte antimicrobial activity (B). The antibody to CMH did not influence the interaction between fungi and macrophages, since no significant differences in association or killing (P > 0.05) were observed when sclerotic cells were preincubated with irrelevant antibodies (control) or antibodies to cerebrosides (anti-CMH). Student's t test was used for statistical analysis. As a positive control of antibody efficacy, sclerotic cells were replaced with conidia. Using these F. pedrosoi forms, phagocytosis and microbial killing were enhanced after treatment with antibodies to CMH (data not shown).
Pretreatment of sclerotic cells with anti-CMH antibodies did not influence the killing capacity of macrophages (Fig. 6B). Such results demonstrated that although antibodies to CMH do recognize cerebrosides from sclerotic cells, this is not sufficient to enhance fungal attachment or macrophage killing functions. We have recently demonstrated that the anti-CMH antibody currently used interferes with ingestion and killing of F. pedrosoi conidia by mouse macrophages (22). Therefore, a positive control of antibody efficacy was achieved by replacing sclerotic cells with conidia. Using these F. pedrosoi forms, phagocytosis and microbial killing were enhanced after treatment with antibodies to CMH (data not shown).
Analysis of CMH cellular distribution by immunofluorescence.
The fact that antibodies to CMH were unable to kill sclerotic bodies or to influence macrophage function was somewhat surprising, since they recognize CMH from these cells and produce such effects in several fungal models (4, 9, 21-24). We therefore evaluated the expression of surface CMH in sclerotic cells as a function of melanin expression, since we have previously demonstrated that F. pedrosoi aggregates containing pigmented sclerotic forms react very poorly with anticerebroside antibodies (22). After a slight centrifugation step, we were able to separate aggregated from disaggregated cells and proceeded with indirect immunofluorescence. Focusing our analysis on highly melanized cells, we found that the edges of sclerotic bodies which enclose higher amounts of pigment are not recognized by the antibody, while portions with less melanin are more reactive (Fig. 7). Confirming prior data, the surface of growing filaments is recognized in all of its extension, with some points where the reactivity is increased. The relationship between melanin expression and CMH recognition was then investigated based on the property that, while the F. pedrosoi melanin is alkali soluble (2), cerebrosides and most of the cell wall glycans are not. Therefore, cell shape and CMH expression are maintained in alkali-treated F. pedrosoi.
FIG. 7.
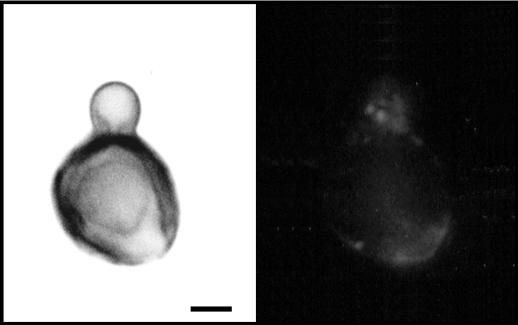
Binding of antibodies to CMH to the cell surface of sclerotic cells is inhibited by pigmentation. Immunofluorescence analysis using antibodies to CMH demonstrated that antibody reactivity is only observed in cell wall regions in which melanin is poorly expressed. The left panel shows microbial cells observed under differential interferential microscopy. The right panel shows the same cells observed under fluorescence. Bar, 1.2 μm.
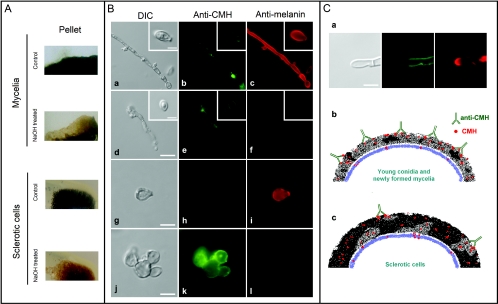
After treatment of fungal cells with NaOH, pigmentation was decreased, as determined visually in control or alkali-treated cell pellets (Fig. 8A). Fungal preparations that were not exposed to alkali treatment reacted clearly with antibodies to melanin, but not with anti-CMH antibodies (Fig. 8B). Melanin removal from different forms of F. pedrosoi was confirmed by the decreased reaction of alkali-treated fungi with the purified preparation of antimelanin antibodies. These melanin-depleted cells were strongly recognized by the monoclonal antibody to CMH, confirming that the pigment masks recognition of cerebrosides. Based on these results, we propose, in the legend to Fig. 8C, an explanation for the resistance of sclerotic cells to the antimicrobial action of antibodies to CMH.
FIG. 8.
Influence of melanin shield on CMH recognition by a monoclonal antibody. (A) F. pedrosoi was treated with alkali (NaOH) or PBS (control), followed by visual analysis of pigmentation. Alkali treatment resulted in fewer pigmented mycelia and sclerotic cells. (B) Untreated mycelia (a) and conidia (a, inset) or sclerotic cells (g) reacted with the antibody to conserved cerebroside (anti-CMH) in limited regions of the cell surface, as demonstrated in panels b and h, respectively. In contrast, these cells were efficiently recognized by the antibodies to melanin (antimelanin, panels c and i). After alkali treatment, the cell shape of F. pedrosoi was maintained (d and j). In these cells, reactivity of fungal forms with the antibody to CMH was greatly increased (e and k), which was accompanied by a clear decrease in the recognition of F. pedrosoi by antibodies to melanin (f and l). Based on these results, we propose in panel C a hypothetical relationship between melanin expression and anti-CMH reactivity in F. pedrosoi. Based on the clear inverse relationship between melanin (red fluorescence) and CMH (green fluorescence) detection on the cell wall of F. pedrosoi (a), we hypothesize that young conidia and growing mycelia that express small quantities of melanin react efficiently with antibodies to CMH, resulting in microbial killing through enhanced phagocytosis or direct antimicrobial action (b). A high amount of melanin, commonly observed in sclerotic cells, would block sites for antibody binding, making fungi more resistant to the antimicrobial action of macrophages and antibodies to CMH (c). Bars, 3.5 μm (B; a, d, g and j), 1.5 μm (insets), 1 μm (C).
DISCUSSION
Dimorphism is a crucial process used by some fungal species to adapt to new environments and, in the case of pathogens, to initiate the colonization process (19). Dimorphism includes morphological transitions, and this differentiation step is usually associated with an intensive cell surface modification, involving a finely regulated control of biosynthetic pathways. In this context, F. pedrosoi and other chromoblastomycosis agents are considered unique models for the study of fungal dimorphism, since all of them are able to grow in at least 4 different morphological stages: (i) conidial, (ii) mycelial, (iii) pseudo-hyphal, and (iv) sclerotic cells.
Recent studies have suggested the importance of CMH in fungal development. For instance, Levery and coworkers demonstrated that the inhibition of the enzyme ceramide glycosyltransferase, which catalyzes the final step in CMH synthesis, affects spore germination, cell cycle, and hyphal growth (18) of Aspergillus species. A series of studies from our group corroborate with these data and strongly suggest that blocking fungal CMH with anti-CMH antibodies can be an alternative for treatment of mycoses (4, 9, 21-24), although the mechanism by which it happens remains unclear. In our models, C. albicans, C. neoformans, C. gloesporioides, and P. boydii had their growth rate at least partially reduced by direct incubation with anti-CMH antibodies (9, 23, 24). In F. pedrosoi, the incubation of conidia with a monoclonal antibody to CMH promoted a significant reduction in cell growth (22). However, immunofluorescence data suggested that only newly formed conidia and growing mycelia were reactive. Sclerotic cells, the prominent forms in infected tissues, had presented a very weak pattern of surface reactivity with these antibodies.
Structural modifications during F. pedrosoi differentiation have been studied. Kneipp and coworkers described a higher phosphatase activity in sclerotic cells than in conidial and mycelial forms (16), which was associated with parasitism. However, it also suggests that these enzymes could be involved in F. pedrosoi dimorphism, although the details by which it happens remain to be elucidated. In addition, melanin expression is more intensive in sclerotic cells than in conidia and mycelia of F. pedrosoi (20). CMH structural diversity has been considered to be linked with fungal dimorphism. For instance, the yeast-mycelium transition in Histoplasma capsulatum and Sporothrix schenckii was accompanied by fatty acid and sugar changes, respectively (31, 32). Here we demonstrate a relationship between dimorphism and CMH structural changes in F. pedrosoi.
According to our results, sclerotic cells express a CMH never reported before in the literature, consisting of a glucosyl 9-methyl-4,8 hydroxy-sphingadienine carrying an extra hydroxyl group positioned at C-4 or -5 of its long chain base. Sclerotic cells also produce conserved cerebrosides, but in lesser levels. In this regard, several attempts were done to separate the two CMH species based on their polar properties; however, none of them have succeeded (data not shown). The lithiated molecular ion at m/z 750, considered in this work as a marker of structural diversity, could be related to a previously reported mammalian CMH (17), but data derived from HPTLC and permethylation analyses confirmed the structure proposed in Fig. 4. Also, the small amounts of this CMH species at the surface of conidia support the postulate that sclerotic cells found inside the host could arise directly from F. pedrosoi spores (28).
Modifications in CMH ceramide structure can induce lipid reorientation at the membrane level, resulting in variation at side-by-side interactions (34). However, fungal CMH are deposited onto the cell wall (21, 23), and at this level, there is no information about how ceramide hydroxylation could influence the molecular wall architecture. Fatty acid length and hydroxylation have been demonstrated to be relevant for GSL recognition by antibodies (29, 34). Moreover, hydroxylation at long chain base level can also modulate antibody binding (33). Based on these findings, we evaluated the reactivity of cerebrosides from mycelial or sclerotic bodies with sera from chromoblastomycosis patients and a monoclonal antibody produced against conserved CMH (9). Interestingly, we observed that the cerebroside with an addition of an extra hydroxyl group to its long chain base presented reactivity similar to that of sera from infected individuals. A possible explanation for this finding could be the fact that, in a chronic infection by F. pedrosoi, patients are usually exposed to the different fungal forms of this pathogen. Therefore, it is reasonable to expect that antibodies to the different cerebrosides synthesized by F. pedrosoi are produced in chromoblastomycosis, which supports the result that polyclonal preparations similarly recognize different CMH. Another hypothesis is that, due to structural similarities, antibodies to CMH could cross-react with different cerebrosides from both mycelial and sclerotic cells. This hypothesis was supported by the fact that a monoclonal antibody produced against a conserved CMH (9) reacts similarly with the mycelial CMH and the new cerebroside described in this work, although a higher antibody affinity for the former was observed. In this context, the additional hydroxyl group detected in the cerebroside from sclerotic cells could represent a physical barrier in the interaction between the antibody and putative epitopes in CMH.
The fact that the monoclonal antibody to CMH recognizes the different cerebrosides studied here was apparently in conflict with immunofluorescence assays, since antibody binding to intact sclerotic cells was limited to specific cell wall regions. During their growth in vitro, sclerotic bodies of F. pedrosoi naturally form large cellular aggregates, which diminish the access of antibodies to internally located cells (3). We therefore investigated the profile of reactivity of disaggregated cells against anti-CMH antibodies. In contrast to what has been demonstrated by our group using aggregates (22), the antibody to CMH can interact with the cell surface of disaggregated sclerotic bodies. However, our results clearly demonstrate that the antibody recognition of CMH at the cell surface only occurs in specific regions of the cell wall in which melanin, a dark cell wall pigment produced by F. pedrosoi, is poorly expressed. The fact that anti-CMH binding is inversely proportional to melanin expression suggests that melanin could block the access of external ligands to cell wall components, which may have a relationship with the well-documented observation that melanized fungi are much more resistant to antifungal agents than nonpigmented cells (13). In fact, sclerotic bodies are susceptible to the antifungal action of antimelanin antibodies (3) but not to that of antibodies to CMH.
To confirm the hypothesis that melanin masks cerebroside recognition, we partially depleted F. pedrosoi forms of melanin after overnight treatment with NaOH. Alkali-treated cells lost their reactivity to antibodies to melanin but became efficiently recognized by the anti-CMH antibody. The observation that anti-CMH antibodies do not recognize sclerotic cells with the same intensity as that observed to conidia and mycelia could therefore be explained by the cell wall distribution of cerebrosides and melanin in F. pedrosoi, in which the pigment would represent a thick cell wall layer protecting fungal cells against different external agents. Also, the observation that melanin impairs cerebroside detection in F. pedrosoi could also explain the resistance of sclerotic bodies to the antimicrobial effects of anti-CMH antibodies. We summarized this hypothesis in the legend to Fig. 8C.
Our observations are in agreement with previous studies showing that melanized fungi are more resistant to antifungal drugs than nonpigmented cells (13). The use of small molecules able to penetrate the melanin shield could be therefore a relevant strategy for chromoblastomycosis defeat. In this regard, it has been recently demonstrated that plant defensins specifically recognize fungal CMH, resulting in microbial killing (30). Also, single-chain antibody fragments derived from anti-CMH antibodies could also represent a feasible alternative to be used in the near future. Combined therapies could also be an alternative, since drugs inhibiting melanin biosynthesis could make sclerotic cells more susceptible to anti-CMH antibodies.
Acknowledgments
The present work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Universitária José Bonifácio (FUJB), Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ), Fundação de Amparo à Pesquisa de Estado de São Paulo (FAPESP) (grant no. 98/10495-5 to I.C.A.), and BBRC/Biology (NIH grant no. 5G12RR008124 to I.C.A.). I.C.A. was the recipient of a research fellowship from CNPq.
We thank Geralda R. Almeida for technical assistance and Vanila F. Palmeira and Daniela S. Alviano for preparation of sclerotic cells. We are also indebted to Luiz R. Travassos and Carlos P. Taborda for helpful suggestions.
Editor: T. R. Kozel
REFERENCES
- 1.Alviano, C. S., S. R. Farbiarz, L. R. Travassos, J. Angluster, and W. de Souza. 1992. Effect of environmental factors on Fonsecaea pedrosoi morphogenesis with emphasis on sclerotic cells induced by propranolol. Mycopathologia 119:17-23. [DOI] [PubMed] [Google Scholar]
- 2.Alviano, C. S., S. R. Farbiarz, W. De Souza, J. Angluster, and L. R. Travassos. 1991. Characterization of Fonsecaea pedrosoi melanin. Gen. Microbiol. 137:837-844. [DOI] [PubMed] [Google Scholar]
- 3.Alviano, D. S., A. J. Franzen, L. R. Travassos, C. Holandino, S. Rozental, R. Ejzemberg, C. S. Alviano, and M. L. Rodrigues. 2004. Melanin from Fonsecaea pedrosoi induces production of human antifungal antibodies and enhances the antimicrobial efficacy of phagocytes. Infect. Immun. 72:229-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barreto-Bergter, E., M. R. Pinto, and M. L. Rodrigues. 2004. Structure and biological functions of fungal cerebrosides. An. Acad. Bras. Cienc. 76:76-84. [DOI] [PubMed] [Google Scholar]
- 5.Bonifaz, A., V. Paredes-Solis, and A. Saul. 2004. Treating chromoblastomycosis with systemic antifungals. Expert Opin. Pharmacother. 5:247-254. [DOI] [PubMed] [Google Scholar]
- 6.Brandt, M. E., and D. W. Warnock. 2003. Epidemiology, clinical manifestations, and therapy of infections caused by dematiaceous fungi. J. Chemother. 15:36-47. [DOI] [PubMed] [Google Scholar]
- 7.Butterfield, W., and S. C. Jong. 1976. Effect of carbon source on conidiogenesis in Fonsecaea dermatitidis, agent of chromomycosis. Mycopathologia 58:59-62. [DOI] [PubMed] [Google Scholar]
- 8.Ciucanu, I., and F. Kerek. 1984. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 131:209-217. [Google Scholar]
- 9.da Silva, A. F. C., M. L. Rodrigues, S. E. Farias, I. C. Almeida, M. R. Pinto, and E. Barreto-Bergter. 2004. Glucosylceramides in Colletotrichum gloeosporioides are involved in the differentiation of conidia into mycelial cells. FEBS Lett. 561:137-143. [DOI] [PubMed] [Google Scholar]
- 10.da Silva, J. P., D. S. Alviano, C. S. Alviano, W. De Souza, L. R. Travassos, J. A. P. Diniz, and S. Rozental. 2002. Comparison of Fonsecaea pedrosoi sclerotic cells obtained in vivo and in vitro: ultrastructure and antigenicity. FEMS Immunol. Med. Microbiol. 33:63-69. [DOI] [PubMed] [Google Scholar]
- 11.Farbiarz, S. R., T. U. De Carvalho, C. S. Alviano, and W. de Souza. 1992. Inhibitory effect of melanin on the interaction of Fonsecaea pedrosoi with mammalian cells in vitro. J. Med. Vet. Mycol. 30:265-273. [PubMed] [Google Scholar]
- 12.Folch, J., M. Less, and T. R. Tacker. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226:467-509. [PubMed] [Google Scholar]
- 13.Gómez, B. L., and J. D. Nosanchuk. 2003. Melanin and fungi. Curr. Opin. Infect. Dis. 16:91-96. [DOI] [PubMed] [Google Scholar]
- 14.Hakomori, S. 1993. Structure and function of sphingoglycolipids in transmembrane signalling and cell-cell interactions. Biochem. Soc. Trans. 21:583-595. [DOI] [PubMed] [Google Scholar]
- 15.Hamza, S. H., P. J. Mercado, H. G. Skelton, and K. J. Smith. 2003. An unusual dematiaceous fungal infection of the skin caused by Fonsecaea pedrosoi: a case report and review of the literature. J. Cutan. Pathol. 30:340-343. [DOI] [PubMed] [Google Scholar]
- 16.Kneipp, L. F., V. F. Palmeira, A. A. Pinheiro, C. S. Alviano, S. Rozental, L. R. Travassos, and J. R. Meyer-Fernandes. 2003. Phosphatase activity on the cell wall of Fonsecaea pedrosoi. Med. Mycol. 41:469-477. [DOI] [PubMed] [Google Scholar]
- 17.Levery, S. B., M. S. Toledo, R. L. Doong, A. H. Straus, and H. K. Takahashi. 2000. Comparative analysis of ceramide structural modification found in fungal cerebrosides by electrospray tandem mass spectrometry with low energy collision-induced dissociation of Li+ adduct ions. Rapid Commun. Mass Spectrom. 14:551-563. [DOI] [PubMed] [Google Scholar]
- 18.Levery, S. B., M. Momany, R. Lindsey, M. S. Toledo, J. Shayman, M. Fuller, K. Brooks, R. L. Doong, A. H. Straus, and H. K. Takahashi. 2002. Disruption of the glucosylceramide biosynthetic pathway in Aspergillus nidulans and Aspergillus fumigatus by inhibitors of UDP-Glc:ceramide glucosyltransferase strongly affects spore germination, cell and hyphal growth. FEBS Lett. 525:59-64. [DOI] [PubMed] [Google Scholar]
- 19.Madhani, H. D., and G. R. Fink. 1998. The control of filamentous differentiation and virulence in fungi. Trends Cell Biol. 8:348-353. [DOI] [PubMed] [Google Scholar]
- 20.McGinnis, M. R., and A. E. Hilger. 1987. Infections caused by black fungi. Arch. Dermatol. 123:1300-1302. [PubMed] [Google Scholar]
- 21.Nimrichter, L., M. L. Rodrigues, E. G. Rodrigues, and L. R. Travassos. 2005. The multitude of targets for the immune system and drug therapy in the fungal cell wall. Microbes Infect. 7:789-798. [DOI] [PubMed] [Google Scholar]
- 22.Nimrichter, L., E. Barreto-Bergter, R. R. Mendonca-Filho, L. F. Kneipp, M. T. Mazzi, P. Salve, S. E. Farias, R. Wait, C. S. Alviano, and M. L. Rodrigues. 2004. A monoclonal antibody to glucosylceramide inhibits the growth of Fonsecaea pedrosoi and enhances the antifungal action of mouse macrophages. Microbes Infect. 6:657-665. [DOI] [PubMed] [Google Scholar]
- 23.Pinto, M. R., M. L. Rodrigues, L. R. Travassos, R. M. Haido, R. Wait, and E. Barreto-Bergter. 2002. Characterization of glucosylceramides in Pseudallescheria boydii and their involvement in fungal differentiation. Glycobiology 12:251-260. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues, M. L., L. Travassos, K. R. Miranda, A. J. Franzen, S. Rozental, W. De Souza, C. S. Alviano, and E. Barreto-Bergter. 2000. Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infect. Immun. 68:7049-7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawardeker, J. S., J. H. Sloneker, and A. Jeanes. 1965. Quantitative determination of monosaccharides as their alditol acetates by gas chromatography. Anal. Chem. 37:1602-1604. [Google Scholar]
- 26.Schnaar, R. L., and L. K. Needham. 1994. Thin-layer chromatography of glycosphingolipids. Methods Enzymol. 230:371-389. [DOI] [PubMed] [Google Scholar]
- 27.Silva, C. L., and R. A. Fazioli. 1985. Role of the fungal cell wall in the granulomatous response of mice to the agents of chromomycosis. J. Med. Microbiol. 20:299-305. [DOI] [PubMed] [Google Scholar]
- 28.Silva, M. B., J. P. Silva, J. A. P. Diniz, P. F. Costa, S. S. M. Pereira, U. I. Salgado, and C. G. Salgado. 2004. Meios de cultura naturais para a indução de células escleróticas de Fonsecaea pedrosoi, p. 132. In Annals of the 4th Brazilian Meeting on Mycology. Brazilian Society for Medical Mycology, Ouro Preto, Brazil.
- 29.Tagawa, Y., W. Laroy, L. Nimrichter, S. E. Fromholt, A. B. Moser, H. W. Moser, and R. L. Schnaar. Anti-ganglioside antibodies bind with enhanced affinity to gangliosides containing very long chain fatty acids. Neurochem. Res. 27:847-855. [DOI] [PubMed]
- 30.Thevissen, K., D. C. Warnecke, I. E. J. A. François, M. Leipelt, E. Heinz, C. Ott, U. Zähringer, B. P. H. J. Thomma, K. K. A. Ferket, and B. P. A. Cammue. 2004. Defensins from insects and plants interact with fungal glucosylceramides. J. Biol. Chem. 279:3900-3905. [DOI] [PubMed] [Google Scholar]
- 31.Toledo, M. S., S. B. Levery, A. H. Straus, and H. K. Takahashi. 2000. Dimorphic expression of cerebrosides in the mycopathogen Sporothrix schenckii. J. Lipid Res. 41:797-806. [PubMed] [Google Scholar]
- 32.Toledo, M. S., S. B. Levery, A. H. Straus, E. Suzuki, M. Momany, J. Glushka, J. M. Moulton, and H. K. Takahashi. 1999. Characterization of sphingolipids from mycopathogens: factors correlating with expression of 2-hydroxy fatty acyl (E)-delta 3-unsaturation in cerebrosides of Paracoccidioides brasiliensis and Aspergillus fumigatus. Biochemistry 38:7294-7306. [DOI] [PubMed] [Google Scholar]
- 33.Vielhaber, G., L. Brade, B. Lindner, S. Pfeiffer, R. Wepf, U. Hintze, K. P. Wittern, and H. Brade. 2001. Mouse anti-ceramide antiserum: a specific tool for the detection of endogenous ceramide. Glycobiology 11:451-457. [DOI] [PubMed] [Google Scholar]
- 34.Villas-Boas, M. H., R. Wait, R. B. Silva, M. L. Rodrigues, and E. Barreto-Bergter. 2005. Ceramide glycosylation and fatty acid hydroxylation influence serological reactivity in Trypanosoma cruzi glycosphingolipids. FEMS Microbiol. Lett. 244:47-52. [DOI] [PubMed] [Google Scholar]