Abstract
Susceptibility of six isolates of Mycobacterium tuberculosis (CB3.3, CDC1551, RJ2E, C.C.13, H37Rv, and H37Ra) and two isolates of Mycobacterium bovis (Ravenel and BCG) to reactive oxygen intermediates (ROI) and reactive nitrogen intermediates (RNI) was determined by standard in vitro survival assays. After 21 days of incubation, the survival of most strains exposed to either acidified sodium nitrite (ASN) or hydrogen peroxide (H2O2) was significantly lower than the same strains unexposed to these RNI or ROI products. However, after 50 days of incubation, these differences in susceptibility became less apparent for strains exposed to ASN but not for strains exposed to H2O2. The recovery of these strains after exposure to RNI suggests that the effect of RNI on M. tuberculosis is bacteriostatic. The in vitro concentrations of ROI and RNI used in these assays were higher than those expected in vivo. These observations suggest that, in vivo, RNI expression at physiologically achievable concentrations may keep M. tuberculosis from proliferating but that removal of RNI may allow the organisms to proliferate. Furthermore, the ability of some M. tuberculosis strains to cause rapidly progressive disease may relate to their intrinsic levels of RNI and ROI resistance.
Although current estimates suggest that one third of the world's population is infected with Mycobacterium tuberculosis, the majority of such infections remain clinically latent. Factors that contribute to the success of M. tuberculosis as a pathogen include its ability to resist the harsh environment of the host macrophage and to persist within immunocompetent hosts even after a clinical cure of the disease is achieved.
The ability of M. tuberculosis to cause disease in the human or animal host may vary according to host susceptibility and to intrinsic biologic differences among clinical strains. The latter may reflect a particular strain's specific adaptation to the antimicrobial defenses of the host macrophage. For example, numerous studies have demonstrated that strains of Mycobacterium differ in susceptibility to reactive oxygen intermediates (ROI) and reactive nitrogen intermediates (RNI) (7, 12, 16, 17, 18, 20, 23, 26).
The role of ROI and RNI in controlling acute infections with M. tuberculosis has been fairly well studied in the murine system (1, 3, 4, 8, 9, 10, 19). However, little is known about the role of these intermediates during latent infections. Studies have demonstrated that the inducible form of nitric oxide synthase (iNOS) expression is required to control mycobacterial infection in mice (1, 9, 13, 19). However, the induction of iNOS does not lead to complete elimination of M. tuberculosis in vivo (22). In addition, Flynn et al. (11) demonstrated that M. tuberculosis reactivation occurs if the production of RNI is inhibited in a murine model of latency (11). This suggests that reactivation tuberculosis may result from the removal of host defense mechanisms, such as RNI, that keep M. tuberculosis from replicating in vivo. Consequently, it is possible that ROI and RNI may inhibit replication (bacteriostatic) but do not eradicate the bacteria (bactericidal). We decided to test this concept in vitro by using RNI and ROI susceptibility assays applied to both clinical and laboratory strains of mycobacteria.
MATERIALS AND METHODS
The mycobacterial strains used in the present study are listed in Table 1. All strains of Mycobacterium were grown in Middlebrook 7H9 broth with 0.5% glycerol, 0.02% Tween 80, and 10% ADC enrichment to mid-log phase. Single-cell suspensions of each isolate were prepared according to a previously published method (15) and quantified by enumeration of their CFU on Middlebrook 7H11 agar plates. A 0.1-ml aliquot of this suspension was added to 0.9 ml of 7H9 broth (pH 5.3 for ASN assays or pH 7.0 for H2O2 assays) containing various concentrations of either sodium nitrite (NaNO2) or hydrogen peroxide (H2O2). As a control, the culture was added to 7H9 broth at the appropriate pH without NaNO2 or H2O2 (unexposed). Each suspension was incubated at 37°C for 24 h, plated onto 7H11 agar, and incubated at 37°C for 3 weeks. At day 21 (or day 26 for H37Ra), the CFU recovered from isolates exposed to either ASN or H2O2 were marked, quantified, and compared to the CFU for recovered isolates not exposed to ASN or H2O2; the data were then calculated as the percent survival ([number of CFU exposed at day 21/number of CFU unexposed at day 21] × 100). Incubation at 37°C was allowed to continue to day 50, at which time each plate was reexamined to enumerate additional CFU recovered during the interval. The total number of CFU was again compared to the total number of CFU from recovered isolates not exposed to ASN or H2O2 at 50 days of incubation, and the data were converted to percent survival ([number of CFU exposed at day 50/number of CFU unexposed at day 50] × 100). Each strain was tested similarly in triplicate, and each assay was repeated at least twice. Comparison of the mean CFU recovery of each strain was made by Student t test, and a P value of <0.05 was considered to show a significant difference.
TABLE 1.
Mycobacterial strains used in this study
| Strain | Source |
|---|---|
| M. bovis | |
| Ravenel | Virulent reference laboratory strain |
| BCG | Attenuated reference laboratory strain |
| M. tuberculosis | |
| H37Rv | Virulent reference laboratory strain |
| H37Ra | Attenuated reference laboratory strain |
| CB3.3 | Clinical isolate (New York City outbreak) |
| CDC1551 | Clinical isolate (Kentucky outbreak) |
| RJ2E | Clinical isolate (Rio de Janiero, Brazil) |
| C.C.13 | Clinical isolate (Northern California) |
RESULTS
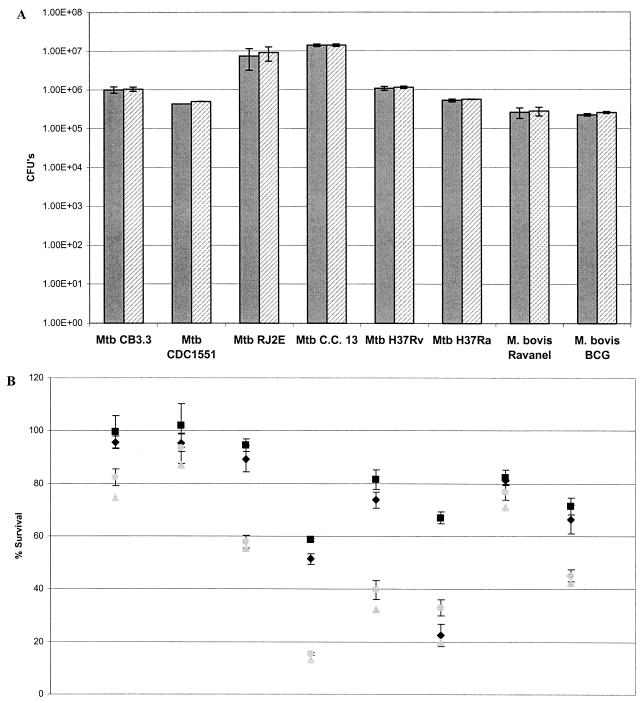
Each strain of mycobacteria demonstrated similar levels of growth between days 21 and 50 when not exposed to ASN or H2O2 (Fig. 1A and 2A). At day 21, at a lower concentration of ASN (3 mM), the percent survival of all strains of mycobacteria was ≤80% compared to the same strains not exposed to ASN. At day 50, however, the percent survival of all of the clinical strains exceeded 80% (Fig. 1A). Compared to the laboratory strain H37Ra, at 3 mM ASN all of the clinical strains of M. tuberculosis and M. bovis (Ravenel and BCG) were significantly more resistant at day 21 or 50 of incubation (P < 0.05, Student t test) (Fig. 1B and Table 2). In addition, at 3 mM ASN the percent survival at day 21 versus that at day 50 was significantly different for each strain. On the other hand, at a higher concentration of ASN (6 mM), the percent survival of the strains at days 21 and 50 showed no significant difference, except for H37Ra and M. bovis Ravenel (Fig. 1B). At 6 mM ASN all of the strains, except M. bovis BCG, remained relatively resistant compared to H37Ra (Table 2).
FIG. 1.
Mycobacterial ASN susceptibility assays. Mycobacterial isolates were incubated for 16 h in 7H9 medium (pH 5.3) and then plated onto 7H11 agar. CFU were determined at 21 and 50 days of incubation. (A) Isolates were incubated at 37°C in 7H9 broth at pH 5.3 without NaNO2, and CFU were quantified at day 21 (shaded bars) and day 50 (hatched bars). The results are expressed as the CFU ± the standard error of the mean (SEM). (B) Isolates were exposed to 3 mM (day 21 [black diamonds] and day 50 [solid squares]) and 6 mM NaNO2 (day 21 [gray triangles] and day 50 [gray circles]). The results are expressed as the percent survival based on the CFU recovered from ASN-exposed strains relative to the CFU of unexposed strains ± the SEM. Both graphs are representative of triplicate experiments and show mean values of triplicate cultures for each strain.
FIG. 2.
Mycobacterial H2O2 susceptibility assays. Mycobacterial isolates were incubated for 16 h in 7H9 medium (pH 7.0) and then plated onto 7H11 agar. CFU were determined at 21 and 50 days of incubation. (A) Isolates were incubated at 37°C in 7H9 broth at pH 7.0 without H2O2, and CFU were quantified at day 21 (shaded bars) and day 50 (hatched bars). The results are expressed as CFU ± the SEM. (B) Isolates were exposed to 2 mM (day 21 [black diamonds] and day 50 [black squares]) and 5 mM H2O2 (day 21 [gray triangles] and day 50 [gray circles]). The results are expressed as the percent survival based on CFU recovered from strains exposed to H2O2 relative to the CFU of unexposed strains ± the SEM. Both graphs are representative of triplicate experiments and show mean values of triplicate cultures for each strain.
TABLE 2.
Comparison to laboratory strain H37Ra of resistance levels of each strain to ASN and H2O2
| Strain |
Pa
|
|||||||
|---|---|---|---|---|---|---|---|---|
| ASN at:
|
H2O2 at:
|
|||||||
| 3 mM
|
6 mM
|
2 mM
|
5 mM
|
|||||
| Day 21 | Day 50 | Day 21 | Day 50 | Day 21 | Day 50 | Day 21 | Day 50 | |
| CB3.3 | S | S | S | S | S | S | S | S |
| CDC1551 | S | S | S | S | S | S | S | S |
| RJ2E | S | S | S | S | S | NS | S | NS |
| C.C.13 | S | S | S | S | S | NS | S | S |
| H37Rv | S | NS | S | S | S | NS | S | NS |
| Ravenel | S | NS | S | S | S | NS | S | S |
| BCG | S | S | NS | NS | NS | NS | NS | NS |
P values were determined by using the Student t test for comparison of the mean CFU recovery of each strain to that observed for the laboratory strain H37Ra. S, significant (P < 0.05); NS, not significant (P > 0.05).
At 2 mM H2O2, H37Ra and one clinical isolate (C.C.13) were most susceptible compared to the other strains tested, but no significant difference was detected between the two time points of incubation for each strain, except with H37Ra (Fig. 2B). At 5 mM H2O2 all of the strains except H37Rv and a clinical strain (C.C.13) remained relatively resistant compared to the laboratory strain H37Ra (Table 2). At 5 mM H2O2, no significant differences in the percent survival were observed for each individual strain at day 21 versus day 50 except with H37Ra (Fig. 2B). Two of the clinical isolates (CB3.3 and CDC1551) consistently exhibited the highest resistance to killing by 5 mM H2O2 or 6 mM ASN compared to all of the other strains tested in the present study (Fig. 1B and 2B).
DISCUSSION
Our results demonstrate that, at a low concentration of ASN, the tested Mycobacterium strains display a greater level of variation in percent survival at 21 days of incubation compared to that observed at 50 days of incubation. However, at high concentrations of ASN, these differences in the percent survival at both day 21 and day 50 disappeared for each strain. Significant differences in the percent survival of the clinical isolates at the two time points were not observed when they were exposed to either concentration of H2O2. These data suggest that the RNI products, but not H2O2, exert a bacteriostatic effect at low concentrations and a bactericidal effect at high concentrations.
Considering that most mycobacterial strains were able to recover from exposure to lower concentrations of ASN when allowed to incubate beyond 3 weeks, it appears that the time of incubation after which CFU data are analyzed is important when similar assays are used to determine mycobacterial susceptibility to any type of stress. Although all of the strains in the present study exhibited some level of recovery to low concentrations of H2O2, the differences in the percent survival between day 21 and day 50 within each strain were not statistically significant, with the exception of one strain (H37Ra). One explanation for this observation is that the concentrations of H2O2 we used are bactericidal to Mycobacterium. Another possibility may be that most strains of mycobacteria, except H37Ra, are more resistant to H2O2 at 2 mM, and thus they are able to grow within 3 weeks. Hence, the concentrations of H2O2 used in the present study may have been either too low (2 mM) or too high (5 mM) to see significant differences among all of the strains when we compared the percent survival values at days 21 and 50. These hypotheses are in accord with other studies that have demonstrated either that H2O2 is bactericidal in vitro or that M. tuberculosis is relatively resistant to the effects of H2O2 in a cell-free system (2, 16, 21). Since H37Ra has a much slower growth rate than the other strains used, the significant level of recovery seen at all concentrations of ASN and H2O2 may be a consequence of not being able to visualize the colonies until much later. We tried to minimize this effect by determining the initial CFU of H37Ra at day 26 instead of at day 21, thus allowing the organism sufficient time to grow.
Collectively, our data indicate that under the in vitro conditions used here exposure to low concentrations of ASN resulted in the stasis of most clinical isolates of M. tuberculosis. In contrast to these data, a study by Chan et al. (2) found that chemically generated RNI at concentrations of between 1.0 and 10 mM are bactericidal to M. tuberculosis in vitro (2), although it should be noted that only one strain (Erdman) was analyzed and that the incubation period after which CFU were enumerated was not clearly defined. Our results are consistent with those of Rhoades and Orme (24), who demonstrated in vitro that the antimycobacterial activity of interferon-primed macrophages was bacteriostatic rather than bactericidal (24). In addition, Rhoades and Orme found that a high concentration (10 mM) of NaNO2 was mycobactericidal in a cell-free system and that lower concentrations (0.1 to 5.0 mM) showed a range of tolerance by clinical mycobacterial isolates (24). It should be noted that the cell-free assay used in the Rhoads and Orme study, although similar, was not identical to ours. Our study exposed mycobacterial strains for 16 h as opposed to a period of 10 days, and the CFU in the Rhoades and Orme study were determined at between 3 and 4 weeks.
Despite the observed bacteriostatic effect of RNI, our results also demonstrate that some clinical M. tuberculosis isolates (CDC1551 and CB3.3) are able to resist even high concentrations of ASN or H2O2. These clinical isolates were previously shown to be associated with large outbreaks of tuberculosis and were found to exhibit high levels of resistance to RNI and H2O2 compared to other clinical isolates or laboratory strains of M. tuberculosis (6, 12, 25). These observations are consistent with numerous studies that have demonstrated strain-related differences regarding susceptibility to RNI and ROI (7, 12, 16, 17, 18, 20, 23, 24, 26), and suggest that certain strains of M. tuberculosis have evolved an enhanced level of resistance to the antibacterial mechanisms elicited by the host macrophage.
The physiologic concentrations of ROI and RNI within human macrophages in vivo are not well established, although in vitro studies have determined that ca. 3.2 nmol of NO2−/105 cells and 287 nmol of H2O2/mg of protein/h are generated from resident murine macrophages stimulated with IFN-γ (5). In addition, other studies have demonstrated that between 34 and 241 nmol of nitrite/106 cells in culture supernatants of human peripheral blood monocytes infected with M. tuberculosis (17) and between 10 and 80 pmol of H2O2/μg of DNA from cells stimulated with polystyrene particles (14) are generated. Hence, the amount of ROI and RNI added in the cell-free system used in the present study is higher than that expected to be generated in vivo. Therefore, relatively resistant M. tuberculosis strains, such as CB3.3 and CDC1551, are less likely to be kept in check within the host. Consequently, one could postulate that such strains are more capable of causing active disease more readily after an initial infection than strains that are relatively susceptible to RNI and ROI. In contrast, the proliferation of RNI- and ROI-susceptible strains may be controlled as long as the host is able to maintain RNI and/or ROI expression. In the absence of these stresses, such M. tuberculosis strains may resume proliferation and cause the reactivation of disease.
While there may be multiple other host factors that contribute to bacteriostasis of M. tuberculosis in vivo, the observation of the bacteriostatic effect of RNI made in vitro, as well as the wide range of RNI and/or ROI susceptibilities of the clinical isolates, may provide new clues about the different clinical outcomes after M. tuberculosis infection.
Acknowledgments
This study was supported by NIH grants HL51967 and F31 DA05874.
We thank Ed Desmond, Jennifer Flood, and Lisa Pascopella at the California State Health Department and Sally Cantrell for providing C.C.13 and Thomas Shinnick for kindly providing CDC1551. We also thank Lisa Morici for technical assistance and for critical review of the manuscript.
REFERENCES
- 1.Chan, J., K. Tanaka, D. Carroll, J. Flynn, and B. R. Bloom. 1995. Effects of nitric oxide inhibitors on murine Infection with Mycobacterium tuberculosis. Infect. Immun. 63:736-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan, J., Y. Xing, R. S., Magliozzo, and B. R. Bloom. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon-γ gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed]
- 4.Dalton, D. K., S. Pitts-Meek, S. Keshav, I. S. Figari, A. Bradley, and T. A. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science 259:1739-1742. [DOI] [PubMed] [Google Scholar]
- 5.Ding, A. H., C. F. Nathan, and D. J. Stuehr. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. J. Immunol. 141:2407-2412. [PubMed] [Google Scholar]
- 6.Firmani, M. A., and L. W. Riley. 2002.. Mycobacterium tuberculosis CDC1551 is resistant to reactive nitrogen and oxygen intermediates in vitro. Infect. Immun. 70:3965-3968. [DOI] [PMC free article] [PubMed]
- 7.Flesch, I., and S. H. E. Kaufmann. 1987. Mycobacterial growth inhibition by interferon-γ-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J. Immunol. 138:4408-4413. [PubMed] [Google Scholar]
- 8.Flesch, I. E., and S. H. E. Kaufmann. 1991. Mechanisms involved in mycobacterial growth inhibition by gamma interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect. Immun. 59:3213-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for IFN-γ in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn, J. L., M. M. Goldstein, J. Chan, et al. 1995. Tumor necrosis factor is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561-572. [DOI] [PubMed] [Google Scholar]
- 11.Flynn, J. L., C. A. Scanga, K. E. Tanaka, and J. Chan. 1998. Effects of aminoguanidine on latent murine tuberculosis. J. Immunol. 160:1796-1803. [PubMed] [Google Scholar]
- 12.Friedman, C. R., G. C. Quinn, B. N. Kreiswirth, D. C. Perlman, N. Salomon, N. Schluger, M. Lutfey, J. Berger, N. Poltoratskaia, and L. W. Riley. 1997. Widespread dissemination of a drug-susceptible strain of Mycobacterium tuberculosis. J. Infect. Dis. 176:478-484. [DOI] [PubMed] [Google Scholar]
- 13.Garcia, I., R. Guler, D. Vesin, M. L. Olleros, P. Vassalli, Y. Chvatchko, M. Jacobs, and B. Ryffel. 2000. Lethal Mycobacterium bovis bacillus Calmette-Guérin infection in nitric oxide synthase 2-deficient mice: cell-mediated immunity requires nitric oxide synthase 2. Lab. Investig. 80:1385-1397. [DOI] [PubMed] [Google Scholar]
- 14.Gretzer, C., and P. Thomsen. 2000. Secretion of IL-1 and H2O2 by human mononuclear cells in vitro. Biomaterials 21:1047-1055. [DOI] [PubMed] [Google Scholar]
- 15.Grover, A. A., H. K. Kim, E. H. Wiegeshaus, and D. W. Smith. 1967. Host-parasite relationship in experimental airborne tuberculosis. II. Reproducible infection by means of an innoculum preserved at −70°C. J. Bacteriol. 94:832-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackett, P. S., V. R. Aber, and D. B. Lowrie. 1978. Virulence and resistance to superoxide, low pH, and hydrogen peroxide among strains of Mycobacterium tuberculosis. J. Gen. Microbiol. 104:37-45. [DOI] [PubMed] [Google Scholar]
- 17.Jagannath, C., J. K. Actor, and R. L. Hunter, Jr. 1998. Induction of nitric oxide in human monocytes and monocyte cell lines by Mycobacterium tuberculosis. Nitric Oxide Biol. Chem. 2:174-176. [DOI] [PubMed] [Google Scholar]
- 18.Laochumroonvorapong, P., S. Paul, C. Manca, V. H. Freedman, and G. Kaplan. 1997. Mycobacterial growth and sensitivity to H2O2 killing in human monocytes in vitro. Infect. Immun. 65:5850-5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacMacking, J. D., R. J. North, R. LaCourse, J. S. Mudgett, S. K. Shah, and C. F. Nathan. 1997. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. USA 94:5243-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manca, C., S. Paul, C. E. Barry III, V. H. Freedman, and G. Kaplan. 1999. Mycobacterium tuberculosis catalase and peroxidase activities and resistance to oxidative killing in human monocytes in vitro. Infect. Immun. 67:74-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchison, D. A., J. B. Selkon, and J. Floyd. 1963. Virulence in the guinea pig, susceptibility to hydrogen peroxide, and catalase activity of isoniazid-senstivie tubercle bacilli from South Indian and British patients. J. Pathol. Bacteriol. 86:377-386. [DOI] [PubMed] [Google Scholar]
- 22.Moreira, A. L., L. Tsenova, P. J. Murray, S. Freeman, A. Bergtold, L. Chiriboga, and G. Kaplan. 2000. Aerosol infection of mice with recombinant BCG-secreting murine IFN-γ partially reconstitutes local protective immunity. Microb. Pathol. 29:175-185. [DOI] [PubMed] [Google Scholar]
- 23.O'Brien, L., J. Carmichael, D. B. Lowrie, and P. W. Andrew. 1994. Strains of Mycobacterium tuberculosis differ in susceptibility to reactive nitrogen intermediates in vitro. Infect. Immun. 62:5187-5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhoades, E. E., and I. M. Orme. 1997. Susceptibility of a panel of virulent strains of Mycobacterium tuberculosis to reactive nitrogen intermediates. Infect. Immun. 65:1189-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valway, S. E., M. P. C. Sanchez, T. F. Shinnick, I. Orme, T. Agerton, D. Hoy, J. S. Jones, H. Westmoreland, and I. M. Onorato. 1998. An outbreak involving extensive transmission of a virulent strain of Mycobacterium tuberculosis. N. Engl. J. Med. 338:633-639. [DOI] [PubMed] [Google Scholar]
- 26.Yu, K., C. Mitchell, Y. Xing, R. S. Magliozzo, B. R. Bloom, and J. Chan. 1999. Toxicity of nitrogen oxides and related oxidants on mycobacteria: M. tuberculosis is resistant to peroxynitrite anion. Tubercle Lung Dis. 79:191-198. [DOI] [PubMed] [Google Scholar]