Abstract
We report improved ability to name pictures at 2 and 8 months after repetitive transcranial magnetic stimulation (rTMS) treatments to the pars triangularis portion of right Broca’s homologue in a 57 year-old woman with severe nonfluent/global aphasia (6.5 years post left basal ganglia bleed, subcortical lesion). TMS was applied at 1 Hz, 20 minutes a day, 10 days, over a two-week period. She received no speech therapy during the study. One year after her TMS treatments, she entered speech therapy with continued improvement. TMS may have modulated activity in the remaining left and right hemisphere neural network for naming.
Introduction
Global aphasia is defined as severe impairment across all language modalities (Goodglass and Kaplan, 1983) and is among the most difficult aphasia syndromes to treat (Kertesz and McCabe, 1977; Sarno and Levita, 1981; Helm-Estabrooks and Albert, 2004). It is also considered to be an aphasia syndrome with the least potential for improvement in overt propositional speech, picture naming, language comprehension, reading or writing, especially during the chronic stage (Demeurisse and Capon, 1987; Rosenbek et al., 1989).
Brain reorganization underlying language recovery in aphasia post-stroke is largely unknown. Over thirty years ago, a possible role for the right hemisphere (RH) was suggested by Kinsbourne (1971) and Czopf (1972) when injection of intracarotid amobarbital into the right (R) carotid of patients with nonfluent speech after a left hemisphere (LH) stroke produced customary speech arrest, whereas injection into the left (L) carotid produced almost no alteration on recovered speech. More recently, Basso et al., (1998) reported that patients who had partially recovered from aphasia after LH lesion showed worsening of language functions after subsequent RH lesion. Some functional imaging studies have also suggested a RH role (Cappa et al., 1997; Thulborn et al., 1999; Musso et al., 1999; Rosen et al., 2000; Naeser et al., 2004). Kim et al. 2002 have observed predominantly R lateral frontal activity during language production in patients with ischemic anterior L perisylvian lesion and intact L basal ganglia. In functional neuroimaging studies with Wernicke’s aphasia patients, increased activation in the R posterior superior temporal gyrus region (and some remaining LH language areas) has been associated with improvement (Weiller et al., 1995; Musso et al., 1999). Some studies have suggested a LH role in better aphasia recovery (Metter 1987; Heiss et al., 1997; 1999; Karbe et al., 1998; Miura et al., 1999; Warburton et al., 1999). Many studies have suggested that both hemispheres are important, depending on the type of language behavior and when it was examined (Weiller et al., 1995; Belin et al., 1996; Basso et al., 1998; Mimura et al., 1998; Cao et al., 1999; Hund-Georgiadis et al., 1999; Gold & Kertesz, 2000; Ansaldo et al., 2002; Zahn et al., 2004).
Functional imaging studies where only nonfluent aphasia patients were examined have observed unusually high activation levels in R perisylvian language homologues during various language tasks (Belin et al., 1996; Thulborn et al., 1999; Rosen et al., 2000; Naeser et al., 2004). This high activation in R perisylvian language homologues is not correlated with improved language performance (Belin et al., 1996; Rosen et al., 2000; Perani et al., 2003; Naeser et al., 2004). Rather, it may represent, in part, increased effort, a maladaptive plasticity, or the breakdown of normal inter-hemispheric control. Rosen et al., 2000 concluded that “… the anomalous R frontal response after L frontal damage may reflect the loss of active inhibition or competitive interaction from the homologous L frontal area, or an inefficient ‘dead-end’ strategy.”
When functional imaging has been obtained before and after speech therapy, better language has been associated with improved L hemisphere or L peri-lesional activation following therapy (Small et al., 1998; Musso et al., 1999; Leger et al., 2002; Cornelissen et al., 2003). The patients examined in these studies, however, did not have chronic, severe nonfluent/global aphasia.
Transcranial Magnetic Stimulation
Transcranial magnetic stimulation (TMS) is a painless, non-invasive procedure that utilizes magnetic fields to create electric currents in discrete brain areas (Walsh and Pascual-Leone, 2003). A current is discharged through a coil of copper wire that is held over the subject’s scalp. The current generates a rapidly fluctuating magnetic field that penetrates the scalp and skull unimpeded and induces a changing electrical field in the cerebral cortex below the coil. This leads to neuronal depolarization that can excite or inhibit cortex (Rothwell, 1997). Repetitive TMS (rTMS) of appropriate frequency, intensity and duration can lead to transient increases or decreases in excitability of the affected cortex that last beyond the duration of the train itself (Pascual-Leone et al., 1998).
The possibility of modulating cortical excitability with rTMS has generated clinical trials applying rTMS for treatment of various neuropsychiatric conditions. The hypothesis underlying most of these studies is that modulation of cortical excitability in cortical areas of dysfunction (as evidenced by functional neuroimaging) may result in clinical benefit for the patients (Pascual-Leone et al., 1998; George and Bellmaker, 2000). For example, slow, 1 Hz rTMS appears capable of normalizing abnormally enhanced motor cortical excitability in some patients with dystonia and has led to symptomatic improvement for hours to days (Siebner et al., 1999). In studies with normal subjects or epilepsy patients, rTMS has been observed to have an effect on language, ranging from facilitation of naming (Topper et al., 1998; Mottaghy et al., 1999) to arrest of speech (Pascual-Leone et al., 1991; Jennum et al., 1994; Epstein et al., 1996; Epstein et al., 1999), depending on the rTMS parameters and location of the coil. In stroke patients with R hemisphere lesion who have left-sided neglect, slow, 1 Hz rTMS has been applied to the posterior parietal area in the undamaged L hemisphere resulting in a significant reduction in neglect lasting for two weeks (Brighina et al., 2003). Similar improvement in neglect has been observed in other rTMS studies with this type of condition (Oliveri et al., 1999; Hilgetag et al., 2001).
TMS studies to treat aphasia
Our ongoing research with rTMS in stroke patients with nonfluent aphasia has observed a significant increase in ability to name pictures following application of slow, 1 Hz rTMS to an anterior portion of R Broca’s homologue, pars triangularis, for 10 minutes (90% motor threshold) (Naeser et al., 2002). There was a concomitant, significant decrease in reaction time to name the pictures. Application of rTMS to the posterior portion of R Broca’s homologue, pars opercularis, was associated with a significant decrease in the pictures named, and increased reaction time (Naeser et al., 2002). Application to other areas (motor cortex mouth; posterior, superior temporal gyrus, R Wernicke’s homologue) was not associated with increased picture naming. The improved naming that was observed immediately following application of rTMS to R pars triangularis was only temporary, however, lasting less than a half hour.
Therefore, in a separate study, we suppressed R pars triangularis with 1 Hz rTMS for a longer period of time (20 minutes) over more days (5 days per week for 2 weeks) in four R-handed, chronic aphasia patients (5 to 11 years poststroke) (Naeser et al., 2005). On testing at 2 months after the tenth rTMS treatment, there was significant improvement on three naming tests: 1) the first 20 items, Boston Naming Test (BNT, Kaplan et al., 2001); 2) the Animal Naming subtest, Boston Diagnostic Aphasia Exam, 3rd Edition (BDAE, Goodglass et al., 2001); and 3) Tools/Implements on the BDAE. At 8 months, all three naming scores continued to improve relative to pre-rTMS testing, but only Tools/Implements was significant. This was the first study to report lasting, improved naming at 2 months and 8 months following application of rTMS treatments in chronic aphasia patients.
The purpose of the present case report is to present in detail, the language improvements observed in a severe nonfluent/global aphasia patient who was treated with rTMS for 2 weeks in the study reviewed above (Naeser et al., 2005). At one year post-rTMS (7.5 years poststroke), her language improvement was sufficient to warrant referral for speech therapy. Her continued language improvement following speech therapy (post-rTMS) is reported.
Case report
Patient history and lesion on structural MRI scan
At age 51, this college-educated, R-handed woman (homemaker) had a left intracerebral hemorrhage (basal ganglia bleed), which resulted in severe R hemiplegia, and severe nonfluent/global aphasia. Her L hemisphere lesion on the T1-weighted MRI scan obtained 6.5 years poststroke, was subcortical and she had no lesion in L Broca’s or Wernicke’s cortical areas (Figure 1). Extensive lesion was present in the two white matter areas near ventricle, compatible with severe nonfluent speech (arrows on MRI scan in Figure 1). These areas include: 1) medial subcallosal fasciculus, deep to Broca’s area, adjacent to the L frontal horn (affecting pathways from SMA and cingulate gyrus Brodmann area (BA) 24 to head of caudate; and 2) periventricular white matter, located deep to sensorimotor cortex, adjacent to the L body of lateral ventricle (affecting sensori-motor pathways deep to mouth, inter-and intra-hemispheric pathways including, in part, limbic and motor thalamo-cortical pathways) (Naeser et al., 1989). Although no lesion was present in Wernicke’s cortical area, subcortical white matter lesion was present in the anterior temporal isthmus area (superior to the temporal horn, affecting auditory pathways from medial geniculate body to Heschl’s gyrus) (Naeser and Palumbo, 1994). This overall subcortical white matter lesion pattern is compatible with a severe nonfluent/global aphasia (Naeser et al., 1989; Naeser & Palumbo, 1994). We hypothesize that her severe aphasia was likely associated with impaired connections to language cortex, associated with her extensive white matter lesion pattern as described above. It is also possible, however, that the basal ganglia portion of the lesion itself affected the severity of her aphasia (Hillis et al., 2004), and/or there was a significant neuronal loss in otherwise intact cortical areas caused by peri-ictal pressure ischemia from the original stroke. Without perfusion MRI studies, however, the potential contribution of these latter factors remains unknown.
Fig. 1.
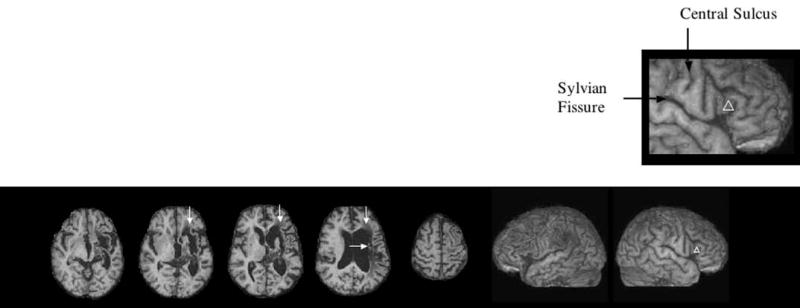
T1-weighted structural MRI scan for this severe nonfluent/global aphasia patient (57yr. F, 6.5 yr. poststroke). The lesion is primarily subcortical only, without lesion in Broca’s or Wernicke’s cortical areas. Extensive lesion was present in the two white matter areas adjacent to ventricle compatible with severe nonfluent speech: 1) the medial subcallosal fasciculus located anterolateral to the L frontal born, deep to Broca’s area (vertical arrows); and 2) the middle 1/3 periventricular white matter located deep to sensorimotor cortex mouth, adjacent to the L body of lateral ventricle (horizontal arrow). See text for pathways in these areas. The L and R lateral views are reconstructed from the 3D MPRAGE MRI scan. The white triangle on the R pars triangularis marks the area of cortex (R BA 45) where 1 Hz rTMS was applied during each 20-minute treatment session (Phase 2), utilizing the Brainsight program to maintain consistent placement of the TMS coil (Fig. 2). The enlarged box shows this targeted area (R BA 45) is located rostral to the anterior, vertical (ascending) ramus of the Sylvian fissure.
Speech therapy at 2 years poststroke onset
At 21 months poststroke onset, this patient was too severe to be tested with the BDAE or the BNT; therefore, she was tested with the Boston Assessment of Severe Aphasia test (BASA, Helm-Estabrooks et al., 1989). Her scores at that time were: Oral/Gestural raw score, 5/21; Auditory Comprehension, 13/16; and Overall BASA score, 42/61. Her speech output was limited to occasional one-word utterances and she was classified as having global aphasia.
She received one year of treatment with the Computer-assisted Visual Communication program (C-ViC). C-ViC is an icon-based alternative communication system designed for patients with severe aphasia who have limited expressive output, either oral, gestural, or written. All have some impairment of auditory comprehension, ranging from moderately impaired to severely impaired; and impaired syntactic performance (Baker et al., 1975; Gardner et al., 1976; Steele et al., 1989; Weinrich et al., 1995; Weinrich et al., 1997; Naeser et al., 1998). She was considered to have “best response” to the C-ViC treatment program and was able to use the program on a laptop computer to initiate communication and reply to questions in the home (patient #4, Naeser et al., 1998). Her overt speech output remained limited to one-word utterances, however, and she was considered too severe for other speech therapy programs. Multiple testings during C-ViC training showed that her picture naming ability remained poor, naming only 6/30 items, as tested at 11 and 41 weeks post-Entry into C-ViC (Table 1).
Table 1.
Test results for naming pictures aloud during C-ViC therapy at Entry into C-ViC (21 months poststroke), and 11, 24 and 41 weeks post-Entry. Picture naming remained stable from week 11 to week 41 during C-ViC therapy
| Entry into C-ViC
|
Time post- Entry into C-ViC
|
|||
|---|---|---|---|---|
| C-ViC Naming Task | 21 Months Poststroke | 11 Weeks | 24 Weeks | 41 Weeks |
| People (max=10) | 3 | 4 | 4 | 4 |
| Verbs (max=10) | 0 | 0 | 1 | 1 |
| Objects (max=10) | 0 | 2 | 2 | 1 |
| Total (max=30) | 3 | 6 | 7 | 6 |
TMS treatment program at 6.5 years poststroke onset
At age 57 (6.5 years poststroke) her language was re-evaluated. She was still globally aphasic and essentially unchanged from previous, post-therapy assessment. She was entered into our experimental rTMS aphasia treatment program. Signed informed consent was obtained. The rTMS treatment protocol was approved by the Institutional Review Boards at the three Boston hospitals with which the authors are affiliated, as well as by the Scientific Advisory Committee at the Harvard-Thorndike General Clinical Research Center, where the rTMS was applied.
Within a few weeks prior to the first rTMS session, a 3-dimensional magnetization prepared rapid gradient echo (3D MPRAGE) MRI scan was obtained in the Radiology Department, Beth Israel Deaconess Medical Center (TR=11.08ms; TE=4.3ms; flip angle 8°). The images were acquired in the sagittal plane (1mm slice thickness, no gap) using a Siemens Vision Symphony/Quantum 1.5T scanner. This MRI scan was later used to guide the exact position of the hand-held TMS coil on the patient’s scalp during rTMS treatment. This imaging also included the ears and the tip of the nose as landmarks to help guide the frameless stereotaxic system (Brainsight, Rogue Industries, Montreal, Quebec). An example of this system is shown in Figure 2.
Fig. 2.
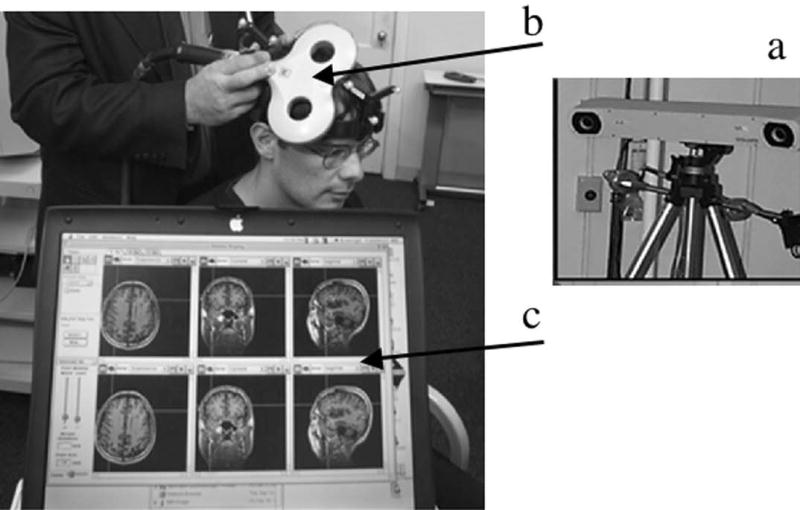
Illustration showing the TMS equipment and treatment procedure: a) An infrared camera is used to detect the position of the TMS coil; b) The figure 8-shaped TMS coil is placed on the participant’s scalp to affect brain cortex (approximately 1 cm × 1 cm) directly beneath the center of the coil; c) The 3D MPRAGE MRI scan of the participant is shown on a laptop computer to guide positioning of the TMS coil directly over the targeted cortical region of interest (Brainsight, Rogue Industries, Montreal, Quebec).
Our rTMS treatment protocol included two phases. During Phase 1, the best RH region of interest (ROI) to suppress with 1 Hz rTMS was determined. During Phase 2, that “best response” ROI was treated 10 times (20 minutes per session), over a two-week period.
Phase 1
During Phase 1, four RH perisylvian language homologues were each transiently suppressed with 1 Hz rTMS for 10 minutes; two ROIs per visit, with a half-hour break in between. Thus, in different rTMS sessions, slow, 1 Hz rTMS was applied to R pars triangularis; R pars opercularis; R posterior superior temporal gyrus (R BA 22); and R motor cortex (M1, lips, orbicularis oris). The anterior portion of Broca’s area (pars triangularis) and the posterior portion of Broca’s area (pars opercularis) were defined according to Amunts et al. 1999: The two areas are often referred to in cytoarchitectonic studies as BA 45 and 44, respectively, although cytoarchitectonic borders do not consistently coincide with sulcal contours. These two areas are anatomically separated by the anterior, vertical (ascending) ramus of the Sylvian fissure and this landmark was used to locate R BA 45 and 44 in our study.
During Phase 1, the rTMS was applied for 10 minutes (600 pulses) at 90% motor threshold using a 7cm diameter, figure 8-shaped coil (MagStim, NY). A frameless stereotaxic system (Brainsight, Rogue Industries, Montreal) guided the position of the coil on the patient’s scalp and documented its accurate targeting of a specific ROI throughout rTMS. See Fig 2. Mathematical models suggest that when applied tangentially to the scalp at perithreshold intensity, this coil affects a volume of approximately 1 cc of cortex (Roth et al., 1991; Wagner et al., 2004).
Phase 1, naming ability on Snodgrass & Vanderwart picture lists
During Phase 1, the effect of rTMS application to a specific ROI was determined by testing the patient’s ability to name 20 Snodgrass & Vanderwart pictures, immediately post-rTMS to that area. Prior to the first rTMS session, the patient’s Baseline Naming ability and response time (RT) had been determined across five different Snodgrass & Vanderwart (1980) (S&V) lists (20 items per list). The lists were controlled for complexity and familiarity, and were as similar as possible in frequency (Kucera & Francis, 1967 rating, listed in Snodgrass & Vanderwart, 1980). The internal order and list presentations were randomized; no list was presented twice in a rTMS session. Items beginning with the same phoneme, or of similar category were not presented immediately following each other in any list. Responses were tape-recorded; RTs were computed. If the patient’s ability to name pictures (within 10 minutes post-rTMS to a specific ROI), was at least 2 SD above Baseline Naming ability, then that ROI was considered the Best Response ROI for that patient.
Phase 1, results for best response ROI to treat with rTMS
Figure 3 shows location of the four RH ROIs for this patient, which were transiently suppressed for 10 minutes each, with 1 Hz rTMS during Phase 1; and the effects on naming and RT post-rTMS for each of these ROIs are presented in graph form. Only suppression of R BA 45 was associated with improved naming for her, immediately post-rTMS. Her Baseline Naming ability on the five S&V lists pre-rTMS was 4.45 pictures (SD, 1.37); with a mean RT of 3407 ms (SD, 1415 ). Following application of rTMS to R BA 45, she named 6 pictures, with a mean RT of 2685 ms. Suppression of the other RH ROIs (R motor cortex, lips and R BA 22) impaired her naming ability, where she named only 3 pictures post-rTMS to each area; and suppression of R BA 44 (posterior R Broca’s homologue) impaired her naming ability the most, where she named only 1 picture post-rTMS and the RT was especially long (4851 ms). Thus, although she did not reach a post-rTMS naming score that improved by 2 SD (which in her case would have been 7 pictures), her score of 6 pictures post-rTMS to R BA 45 was considered adequate for inclusion into Phase 2; it was clearly a cortical area which improved naming for her, post-rTMS. Therefore, within a month after the completion of Phase 1, she was entered into Phase 2, where 1 Hz rTMS would be applied to R BA 45 for 20 minutes a day, for 10 treatments over a two-week period.
Fig. 3.
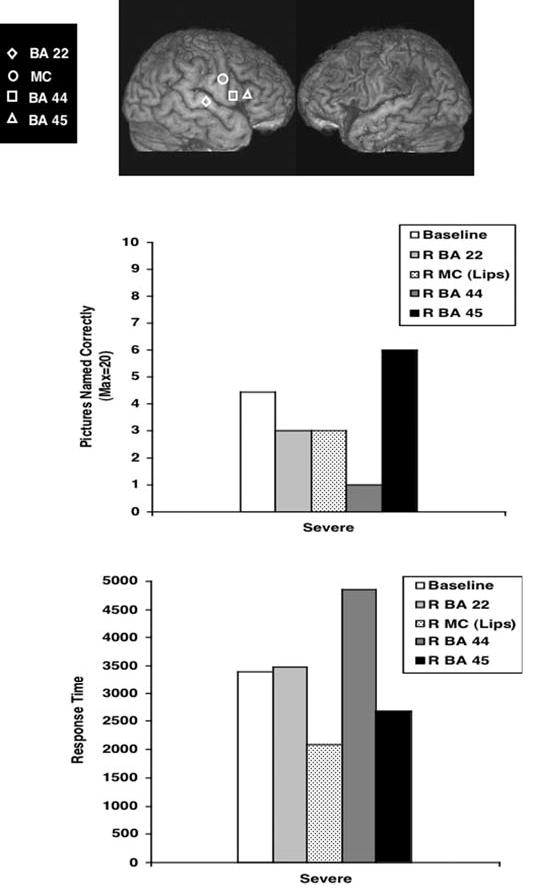
Phase 1, Top: Location on this patient’s 3D MPRAGE MRI scan, for the four RH ROIs which were transiently suppressed for 10 minutes each, with 1 Hz rTMS during Phase 1. Middle: Her Snodgrass & Vanderwart (1980) naming scores as tested immediately post-rTMS to each RH ROI. Bottom: Her mean response time (RT) to name each picture, post-rTMS to each RH ROI. Only suppression of R BA 45 was associated with improved naming (and decreased RT) immediately post-rTMS.
Phase 2
Within 2 weeks prior to the first rTMS treatment in Phase 2, her language was examined with standardized aphasia tests, and again at 2 months and 8 months following the 10th rTMS treatment. These tests included the first 20 items on the BNT (Kaplan et al., 2001) and parts of the BDAE, 3rd Edition (Goodglass et al., 2001). See Table 2.
Table 2.
Boston Naming Test (BNT) and Boston Diagnostic Aphasia Exam, 3rd Edition (BDAE) scores pre-rTMS (6.5 years poststroke) and at 2 months and 8 months after 10 rTMS treatments
| Pre-rTMS | 2 Months Post-rTMS | 8 Months Post-rTMS | |
|---|---|---|---|
| BNT, First 20 Items (Max = 20) | 4 | 7 | 12 |
| BDAE, Longest Number of Words per Phrase Length (Max = 7) | 1 | 1 | 1 |
| Articulatory Agility (Max = 7) | 3 | 3 | 3 |
| Repetition of Single Words (Max = 10) | 4 | 4 | 3 |
| Repetition of Sentences (Max = 10) | 0 | 0 | 0 |
| Comprehension of Single Words (Max = 37) | 26.5 | 27 | 30 |
| Commands (Max = 15) | 3 | 6 | 4 |
She received ten, 20-minute, 1 Hz rTMS treatments applied to the R pars triangularis (R BA 45), five days a week for two weeks. The location of this area is marked with the white triangle on the R lateral view of her MRI scan shown in Figure 1. The coil was placed on the gyrus immediately rostral to the anterior, vertical (ascending) ramus of the Sylvian fissure (Amunts et al., 1999). For review, see Devlin et al. 2003. The motor threshold for the L first dorsal interosseus muscle was determined prior to rTMS treatment each day. Each rTMS treatment was administered at 90% of motor threshold. She did not receive any speech therapy during her participation in the rTMS study or follow-up language testing at 2 months and 8 months post-rTMS.
Phase 2, Results for post-rTMS Language Testing
Table 2 and Figure 4 show that she improved in naming on the BNT and BDAE (relative to pre-rTMS testing) at 2 months and 8 months after 10 rTMS treatments. Her pre-rTMS score on the first 20 items of the BNT was 4; at 2 months post-rTMS she named 7 items; and at 8 months, 12 items. Her pre-rTMS score on the BDAE Naming Animals subtest was 0/12; at 2 months she named 1 animal; and at 8 months post-rTMS, 6 animals. On the BDAE Naming Tools/Implements subtest, she named 2/12 pre-rTMS; 3/12 at 2 months, and 5/12 at 8 months post-rTMS (Figure 4).
Fig. 4.
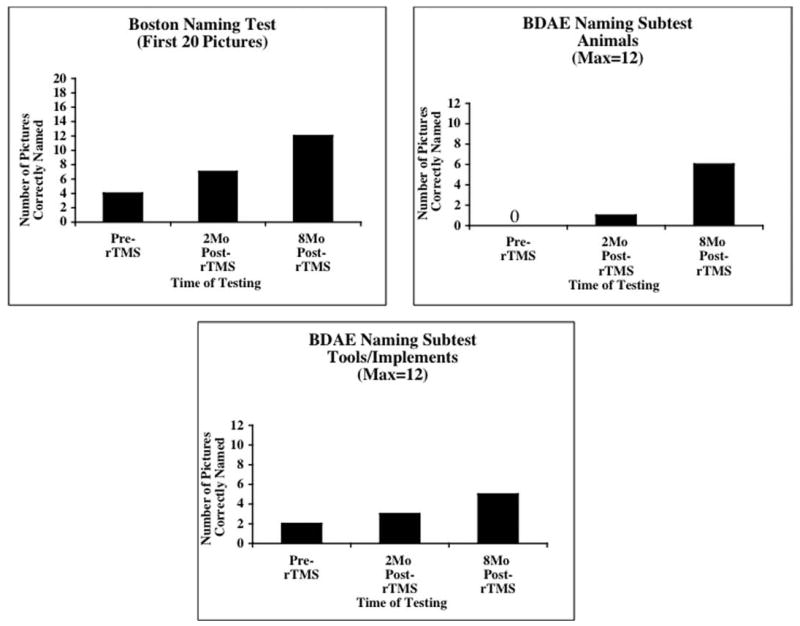
Phase 2. Graphs showing BNT and BDAE naming scores for this global aphasia patient pre-rTMS (6.5 years poststroke) and at 2 months and 8 months after 10, 20 minute, 1 Hc rTMS treatments to RBA 45.
Speech therapy at 7.5 years poststroke onset (1 year post-rTMS)
Because of the patient’s sustained improvement in naming pictures at 8 months post-rTMS, she was referred to the Aphasia Rehabilitation Research Clinic at the Harold Goodglass Aphasia Research Center (HGARC) for language therapy in an effort to further improve her language skills. When first seen at the HGARC clinic, she was 58 years old, and 7.5 years poststroke. Her language was evaluated on two separate occasions to establish stable baseline performance prior to initiation of therapy. The evaluation tasks used were the Auditory Comprehension subtests from the BDAE, 2nd Edition (Goodglass and Kaplan, 1983), and the Cognitive Linguistic Quick Test (CLQT; Helm-Estabrooks, 2001). Due to unreliable “yes” “no” responses for the BDAE Auditory Comprehension subtest of Complex Ideational Material, the overall percentile rank for Auditory Comprehension was computed using only three subtests: 1) Word Discrimination, 46/72 (40th percentile); 2) Body Part Identification, 9/20 (28th percentile); and 3) Commands, 4/15 (20th percentile). The mean of these three percentile scores placed her overall Auditory Comprehension at the 29th percentile. In addition, she produced only occasional, accurate one-word responses in conversation, and she was diagnosed as having global aphasia.
Her performance on the CLQT indicated a Moderate Impairment in Attention and Visuospatial Skills and Severe Impairment in Memory, Executive Function, and Language. It should be noted that the “verbal” aspects of the Memory tasks probably skewed the earned score in this domain. Her CLQT Total Composite Cognitive Score of 1.4 was within the range of Severe Impairment for her age.
She had been referred to the clinic for a possible course of Melodic Intonation Therapy (MIT) (Albert et al., 1973; Sparks et al., 1974; Sparks and Holland, 1976; Helm-Estabrooks et al., 1989; Helm-Estabrooks and Albert, 2004). Both her BDAE overall Auditory Comprehension score of 29th percentile and her cognitive test scores were well below those predictive of good response to MIT with functional carryover of any treatment effects. Therefore, it was determined that she should receive a course of Cognitive-Linguistic Therapy (Helm-Estabrooks and Albert, 2004) in an effort to improve her overall cognitive status and auditory comprehension skills.
She completed two phases of Cognitive-Linguistic Therapy (Helm-Estabrooks & Albert 2004) using treatment activities employing materials from the Cognitive-Linguistic Task Book (Helm-Estabrooks, 1995), the experimental Attention Training Program (Helm-Estabrooks et al., 2000) and the Problem-Solving Therapy Program (Helm-Estabrooks & Karrow, in preparation). Both phase one and two consisted of five, two-hour treatment sessions. All treatment activities were supplemented by homework assignments.
In the first phase of the Cognitive-Linguistic Therapy Program, treatment focused on the cognitive domain of attention utilizing tasks such as cancellation of nonlinguistic symbols and numbers (arranged in rows, random scatter patterns, rows with background distractions), and completion of repeated grapho-motor patterns. The other cognitive area addressed in this phase was cognitive flexibility as required for trail-making tasks that required connection of nonlinguistic symbols according to increasing size, and alphabet and numbers trails.
The second phase of the Cognitive-Linguistic Therapy Program continued to focus on attention and cognitive flexibility using tasks of increasing complexity including those requiring higher-order inferences and cognitive formulation. These included tasks such as logical groupings and pairings, semantic decision-making, estimations, and organization of items by size, weight, speed, cost and so on.
Following the 5th treatment session (representing 10 hours of direct therapy) she was re-evaluated. She showed an increase from the 29th to the 37th percentile in overall Auditory Comprehension on the BDAE (Word Discrimination, 50/72, 46th percentile; Body Part Identification, 11.5/20, 36th percentile; and Commands, 6/15, 30th percentile). On the CLQT, she showed improvement in her Total Composite Cognitive Score (earning a score of 1.8, which is within the range of Moderate Impairment). The domains with notable improvements (from Moderate to Mild Impairment) were Attention and Visuospatial Skills. Following the second phase of treatment (5 more treatment sessions, representing an additional 10 hours of direct therapy), the BDAE and the CLQT were re-administered. She tested at the 35th percentile for Auditory Comprehension on the BDAE (Word Discrimination, 51/72, 46th percentile; Body Part Identification, 10/20, 30th percentile; and Commands, 7/15, 30th percentile). Although there was slight improvement in the areas of Symbol Trails, Design Memory and Design Generation, the CLQT Total Composite Score remained in the Moderate Impairment range at 1.8.
After the course of Cognitive-Linguistic Therapy, she began MIT, even though her BDAE overall Auditory Comprehension score was ten percentile points below the 45th percentile (BDAE 2nd Edition) recommended for MIT candidacy. The MIT program is hierarchically structured into three linguistic levels, beginning with maximal clinician support that gradually tapers off, thereby increasing the need for greater patient initiation (Helm-Estabrooks & Albert, 2004). Pictures that illustrate target responses accompany phrase and sentence stimuli.
She was fully cooperative in the MIT program and was seen for two, one-hour sessions twice a week. After two weeks, she had met the criterion (90% over five consecutive sessions) for the Elementary Level of MIT and progressed to the Intermediate Level that involves less rigorous cueing. After 8 sessions at the Intermediate Level, she failed to meet scoring criterion for advancement and showed no changes in functional verbal communication and MIT was discontinued. After four months of behavioral therapy (23 sessions) partially supplemented with home assignments, she was re-tested with the BDAE Auditory Comprehension subtests. Her overall Auditory Comprehension had improved to the 40th percentile (Word Discrimination, 51.5/72, 47th percentile; Body Part Identification, 12/20, 38th percentile; and Commands, 7/15, 35th percentile).
She is now enrolled in a course of Voluntary Control of Involuntary Utterances (VCIU) (Helm-Estabrooks and Barresi, 1980; Helm-Estabrooks and Albert, 2004) that has been used successfully with other stroke patients with left subcortical lesion who have severe nonfluent speech. Her response to the first five sessions of VCIU has been encouraging. She can now name 28/40 of her group of VCIU pictured items without prompting.
Her husband has commented that she is more involved with activities in the home. For example, she now initiates setting the table before a meal. Also, following a party, family and friends commented to her husband that she was initiating communication more often and effectively communicating more information. She and her husband recently completed a trip to Russia, and in an Aphasia Rounds interview at the HGARC she was able to communicate that she ate “borscht,” and that they visited the ballet, and so on.
Discussion
This case study is the first to report sustained improved naming following application of a series of rTMS treatments in a chronic, global aphasia patient long after the initial insult and despite stable deficits for many years. In this study, the application of 1 Hz rTMS to R pars triangularis (R BA 45) resulted in improved picture naming on the BNT, and the Animals and Tools/Implements subtests on the BDAE at 2 months and 8 months following 10 rTMS treatments, relative to pre-rTMS testing.
The improvements post-rTMS in this severe aphasia patient are particularly striking, because global aphasia patients are expected to have the least potential for change (Demeurisse and Capon, 1987; Rosenbek et al., 1989). Multiple testings during the C-ViC therapy program, begun at 21 months poststroke in her case, had showed picture naming ability to be poor and stable at that time (only 6/30 pictures named correctly at 11 and 41 weeks post- Entry into the C-ViC program). Thus, although her improved naming at 2 and 8 months post-rTMS could be due to a number of factors, that is, repeated tests or the increased attention given to the patient during the rTMS treatment series, this may not be the case for her, because repeated testing and almost one year of the C-ViC therapy program (initiated at 2 years poststroke) had not resulted in improved naming at that time. She had entered the rTMS study at 6.5 years post-stroke and showed consistent improvement in naming in three different naming tasks at 2 and 8 months post-rTMS (Figure 4). This consistent increase was in obvious contrast to the lack of improvement during the earlier nonverbal communication treatment program which had been provided at 2 years poststroke. At one year post-rTMS she had improved enough to be referred for further speech therapy (7.5 years poststroke), where she continued to show improvement in language skills (especially in auditory comprehension, and in the voluntary use of words and phrases appropriate to her environment). While her level of improvement remains modest, a patient with this level of severity (global aphasia) has a type of aphasia which is among the most difficult of aphasia syndromes to treat (Kertesz and McCabe, 1977; Sarno and Levita, 1981; Helm-Estabrooks and Albert, 2004). Thus, improvement in a global aphasia patient with rTMS treatments, after 6.5 years poststroke is encouraging, and further studies are warranted.
Our results should be considered preliminary, however, as this was an open-protocol, single case report without sham rTMS control. Nevertheless, this patient was studied well beyond the spontaneous recovery period of 3 to 6 months poststroke (Demeurisse and Capon, 1987), and was considered to be in the chronic, stable phase of global aphasia.
Possible mechanisms for rTMS effect
The mechanism of action underlying improved naming following the application of 1 Hz rTMS to suppress the pars triangularis portion of R Broca’s homologue is unknown. Possibilities may rest on understanding better the role of L Broca’s area. The participation of Broca’s area (pars triangularis and pars opercularis portion of L inferior prefrontal cortex, L IPC) along with posterior temporal lobe structures has been observed in semantic tasks during functional imaging with normals (Gold and Buckner, 2002), as well as aphasia patients (Price et al., 2001). Some fMRI studies with normals have underscored the importance of L IPC for selection of competing semantic knowledge (Gabrieli et al., 1998; Gold and Buckner, 2002). Indeed, in studying aphasia patients, Thompson-Schill et al. 1998 have observed a direct correlation between extent of lesion within L BA 44, and selection-related errors on a task requiring the patient to generate a verb for a written noun. In word-stem completion tasks, patients with L IFG lesions have been observed to primarily shift activation to the R hemisphere (R IFG, R fusiform and R lateral occipital cortex) with improved modulation (decreased response) in R-sided structures as performance improved with practice (Blasi et al., 2002).
We hypothesize that in the present study, application of 1 Hz rTMS to R pars triangularis likely affected the structures included in the semantic/phonological processing suggested by Gold and Buckner (2002) to include connections from L inferior prefrontal cortex (L IPC) to L BA 21 (semantic processing); and connections from L IPC to an area near precentral L BA 6 and inferior parietal (L BA 40) (phonological processing). The application of 1 Hz rTMS to R pars triangularis may have suppressed “over-activation” in this region, and permitted better modulation of the remaining temporo-parietal structures in the bi-hemispheric neural network for naming. Thus, the application of rTMS to R pars triangularis may have, in part, facilitated better selection among competing semantic choices for naming.
This patient improved in general naming as tested on the first 20 items of the BNT, at 2 months and 8 months post-stroke. The neural network involved with naming these BNT items may generally be associated with the neural networks for semantic and phonological processing as mentioned above (Price et al., 2001; Gold and Buckner, 2002). However, her scores also improved on specific naming category subtests of the BDAE—for example, animals and tools/implements. The neural networks involved with naming in these specific categories may vary, from those involved with more general picture naming on the BNT (Damasio et al., 1996; Murtha et al., 1999; Damasio et al., 2004). For example, Damasio et al. 2004 have reviewed converging evidence from lesion studies and functional neuroimaging studies with normals, and suggest that naming animals involves mesial occipital cortices and middle infero-temporal areas, whereas naming tools involves posterior infero-temporal areas and anterior regions of the supramarginal gyrus (a somatosensory region possibly related to manipulability). Thus, in our patient, emergence of a new ability to name animals and tools/implements suggests that rTMS application to R BA 45 may have had a more widespread, modulating effect possibly on mesial occipital cortices and infero-temporal cortex, which were not part of the LH lesion, and were remote from the site of rTMS stimulation itself in R BA 45. New modulation of these remote areas may also have helped her to respond to the speech therapy treatment sessions which were initiated at 7.5 years poststroke (one year after the Phase 2 rTMS treatments). Whether modulated involvement of these remote areas, especially during naming of animals and tools/implements, occurred post-rTMS in her case is unknown, however, without fMRI studies.
Functional MRI studies would also be necessary, pre-rTMS and post-rTMS, to determine whether, in fact, our Phase 2 rTMS treatment protocol suppressed R BA 45 in this patient, or not. It is likely the area was suppressed, however, given the known effect of 1 Hz rTMS on motor cortex (Chen et al., 1997; Maeda et al., 2000; Romero et al., 2002). In separate studies, we have been able to obtain overt naming fMRI on some of our aphasia patients pre- and post-rTMS. We have observed suppression of activation in R frontal lobe areas (including, in part, R BA 45) post-rTMS, during overt naming fMRI, with improved naming scores post-rTMS (personal observation).
Suppression of R hemisphere language areas may be compatible with improvement in nonfluent aphasia. For example, the single published functional neuroimaging study with melodic intonation therapy in nonfluent aphasia patients observed a decrease in R hemisphere activation on PET scans, and an increase in L frontal activation, during improved speech with melodic intonation therapy. Belin et al. (1996, p. 1508) wrote that, “Introducing MIT in the repetition condition resulted in a relative CBF decrease in seven of the nine right hemisphere regions of interest, and for the group of subjects this was significant for the right homologue of Wernicke’s area (p<0.02). In the left hemisphere, there was a significant relative CBF increase in Broca’s area, and in the adjacent prefrontal cortex (p<0.04).” Thus, suppression of a R language homologue with rTMS in nonfluent aphasia and improved naming in our study could be compatible with decreased R hemisphere activation on PET scans during MIT, and improved bisyllabic word repetition in the Belin et al. 1996 study.
In summary, at this time, it is unknown whether application of our 1 Hz rTMS protocol to R BA 45 in this severe nonfluent/global aphasia patient suppressed this area. We hypothesize that this occurred, but we have no pre-rTMS and post-rTMS fMRI comparisons for her. It is unlikely that 1 Hz rTMS promoted activation in R BA 45, although this is possible. It seems more likely that the 1 Hz rTMS promoted suppression in R BA 45, and this in turn promoted a better modulation throughout the bi-hemispheric neural network for naming. In future studies, pre- and post-rTMS overt fMRI studies are critical to better understand the effects of rTMS on activation levels in specific ROIs, both local and remote from the site of rTMS.
While it may seem paradoxical to expect improved naming following the suppression of R pars triangularis, there are cases where additional, new lesions in a chronic stroke patient improved behavior. For example, the disappearance of a L-sided neglect was observed in a stroke patient with R parietal lesion, following a new L frontal lobe lesion (Vuilleumier et al., 1996). Kapur (1996) has labeled this effect as “paradoxical functional facilitation.” Future overt naming fMRI studies with aphasia patients, before and after a series of rTMS treatments, are needed to examine whether rTMS can, indeed, suppress R pars triangularis and modulate activation in a specific manner, in the bi-hemispheric network for naming. This would provide insight into mechanisms underlying language improvement post-rTMS in aphasia patients.
TMS may provide a novel treatment approach for aphasia, including severe nonfluent/global aphasia. A better language outcome for aphasia patients may be observed where rTMS is combined with speech therapy administered immediately after each rTMS treatment, as well as for a few months following the last rTMS treatment.
Footnotes
Research supported by NIH grant RO1 DC05672 from the National Institute on Deafness and Other Communication Disorders, Bethesda, MD and a grant from the Medical Research Service, Department of Veterans Affairs, Washington, D.C. (to M.A.N.); a K24 NIH award (RRO18875, to A.P.-L) and the Harvard-Thorndike General Clinical Research Center (NCRR MO1 RR01032); and a P30 DC05207 grant to the Harold Goodglass BU Aphasia Research Center from the National Institute on Deafness and Other Communication Disorders.
References
- Albert ML, Sparks RW, Helm NA. Melodic intonation therapy for aphasia. Arch Neurol. 1973;29:130–131. doi: 10.1001/archneur.1973.00490260074018. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HBM, Zilles K. Broca’s region revisited: cytoarchitecture and intersubject variability. Journal of Comparative Neurology. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Ansaldo AI, Arguin M, Lecours AR. The contribution of the right cerebral hemisphere to the recovery from aphasia: a single longitudinal case study. Brain Lang. 2002;82:206–222. doi: 10.1016/s0093-934x(02)00017-2. [DOI] [PubMed] [Google Scholar]
- Baker E, Berry T, Gardner H, Zurif E, Davis L, Verof A. Can linguistic competence be dissociated from natural language functions? Nature. 1975;254:609–619. [Google Scholar]
- Basso G, Romero S, Pietrini P, Beeson PM, Rapczack S, Grafman J. Neurofrontal correlates of language reorganization after massive hemisphere stroke. Poster presented at the 4th International Conference on Functional Mapping of the Human Brain, Montreal, Quebec, Canada, June 7–12, 1998. Neuroimage. 1998;7(4):S472. [Google Scholar]
- Belin P, Van Eeckhout Ph, Zilbovicious M, Remy Ph, Francois C, Gallium FS, et al. Recovery from nonfluent aphasia after melodic intonation therapy: A PET study. Neurology. 1996;47:1504–1511. doi: 10.1212/wnl.47.6.1504. [DOI] [PubMed] [Google Scholar]
- Blasi V, Young AC, Tansy AP, Petersen SE, Snyder AZ, et al. Word retrieval learning modulates right frontal cortex in patients with left frontal damage. Neuron. 2002;35:159–170. doi: 10.1016/s0896-6273(02)00936-4. [DOI] [PubMed] [Google Scholar]
- Brighina F, Bisiach E, Oliveri M, Piazza A, LaBua V, Daniele O, Fierro B. 1 Hz repetitive transcranial magnetic stimulation of the unaffected hemisphere ameliorates contralesional visuospatial neglect in humans. Neuroscience Letters. 2003;336:131–133. doi: 10.1016/s0304-3940(02)01283-1. [DOI] [PubMed] [Google Scholar]
- Cao Y, Vikingstad EM, George KP, Johnson AF, Welch KM. Cortical language activation in stroke patients recovering from aphasia with functional MRI. Stroke. 1999;30(11):2331–2340. doi: 10.1161/01.str.30.11.2331. [DOI] [PubMed] [Google Scholar]
- Cappa SF, Perani D, Grassi F, Bressi S, Alberoni M, Franceschi M, et al. A PET follow-up study of recovery after stroke in acute aphasics. Brain Lang. 1997;56:55–67. doi: 10.1006/brln.1997.1737. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Cornelissen K, Laine M, Tarkiainen A, Jarvensivu T, Marin N, Salmelin R. Adult brain plasticity elicited by anomia treatment. J Cogn Neurosci. 2003;15(3):444–461. doi: 10.1162/089892903321593153. [DOI] [PubMed] [Google Scholar]
- Czopf J. Role of the non-dominant hemisphere in the restitution of speech in aphasia. Arch Psychiatr Nervenkr. 1972;216(2):162–171. doi: 10.1007/BF00346417. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR. A neural basis for lexical retrieval. Nature. 1996;380(6574):499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski R, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92:179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- Demeurisse G, Capon A. Language recovery in aphasic stroke patients: clinical, CT and CBF studies. Aphasiology. 1987;1(4):301–315. [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MFS. Semantic processing in the left inferior prefrontal cortex: A combined functional magnetic resonance imaging and transcranial magnetic stimulation study. Journal of Cognitive Neuroscience. 2003;15(1):71–84. doi: 10.1162/089892903321107837. [DOI] [PubMed] [Google Scholar]
- Epstein CM, Lah JJ, Meador K, Weissman JD, Gaitan LE, Dihenia B. Optimum stimulus parameters for lateralized suppression of speech with magnetic brain stimulation. Neurology. 1996;47(6):1590–1593. doi: 10.1212/wnl.47.6.1590. [DOI] [PubMed] [Google Scholar]
- Epstein CM, Meador KJ, Loring DW, Wright RJ, Weissman JD, et al. Localization and characterization of speech arrest during transcranial magnetic stimulation. Clin Neurophysiol. 1999;110:1073–79. doi: 10.1016/s1388-2457(99)00047-4. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, Poldrake RA, Desmond JE. The role of left prefrontal cortex in language and memory. Proc Natl Acad Sci USA. 1998;95:906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner H, Zurif E, Berry T, Baker E. Visual communication in aphasia. Neuropsychologia. 1976;14:275–292. doi: 10.1016/0028-3932(76)90023-3. [DOI] [PubMed] [Google Scholar]
- George MS, Bellmaker RH. Transcranial magnetic stimulation in neuropsychiatry. Washington D.C: American Psychiatric Press, 2000.
- Gold BT, Kertesz A. Right hemisphere semantic processing of visual words in an aphasic patient: An fMRI study. Brain Lang. 2000;73:456–465. doi: 10.1006/brln.2000.2317. [DOI] [PubMed] [Google Scholar]
- Gold BT, Buckner RL. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron. 2002;35:803–812. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. The assessment of aphasia and other neurological disorders. Baltimore, MD: Williams and Wilkins, 1983.
- Goodglass H, Kaplan E, Barresi B. The assessment of aphasia and related disorders, Third Edition. Philadelphia: Lippincott, Williams & Wilkins, 2001.
- Heiss WD, Karbe H, Weber-Luxenburger G. Speech-induced cerebral metabolic activation reflects recovery from aphasia. J Neurol Sci. 1997;145:213–217. doi: 10.1016/s0022-510x(96)00252-3. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Kessler J, Thiel A. Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Ann Neurol. 1999;45:430–438. doi: 10.1002/1531-8249(199904)45:4<430::aid-ana3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Helm-Estabrooks N. Cognitive linguistic task book. Sandwich, MA: Cape Cod Institute for Communication Disorders, 1995.
- Helm-Estabrooks N. CLQT: Cognitive linguistic quick test. San Antonio, TX: The Psychological Corporation, 2001.
- Helm-Estabrooks N, Albert ML. Voluntary control of involuntary utterances. Chapter 14 in Manual of aphasia and aphasia therapy, 2nd ed. Austin, TX: Pro-Ed, 2004; 193–200.
- Helm-Estabrooks N, Albert ML. Melodic intonation therapy. Chapter 16 in Manual of aphasia and aphasia therapy, 2nd ed. Austin, TX: Pro-Ed, 2004: 221–233.
- Helm-Estabrooks N, Albert ML. Cognitive approach to improving auditory comprehension. Chapter 23 in Manual of aphasia and aphasia therapy, 2nd ed. Austin, TX: Pro-Ed, 2004: 335–362.
- Helm-Estabrooks N, Barresi B. Voluntary control of involuntary utterances: A treatment approach for severe aphasia. In: Brookshire R, editor. Clinical Aphasiology Conference Proceedings. Minneapolis, MN: BRK, 1980.
- Helm-Estabrooks N, Connor LT, Albert ML. Training attention to improve auditory comprehension in aphasia. Brain Lang. 2000;74:469–472. [Google Scholar]
- Helm-Estabrooks N, Nicholas M, Morgan A. Melodic intonation therapy program. Austin, TX: Pro-Ed, 1989.
- Helm-Estabrooks N, Ramsberger G, Morgan AR, Nicholas M. BASA: Boston Assessment of Severe Aphasia. San Antonio, TX: Special Press, Inc., 1989.
- Hilgetag CC, Theoret H, Pascual-Leone A. Enhanced visual spatial attention ipsilateral to rTMS-induced ‘virtual lesions’ of human parietal cortex. Nature Neuroscience. 2001;4:953–957. doi: 10.1038/nn0901-953. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Barker PB, Wityk RJ, Aldrich EM, Restrepo L, et al. Variability in sub-cortical aphasia is due to variable sites of cortical hypoperfusion. Brain Lang. 2004;89(3):524–30. doi: 10.1016/j.bandl.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Hund-Georgiadis M, Lex U, von Cramon DY. Activation patterns of speech function in chronic aphasia. Poster presented at the Fifth International Conference on Functional Mapping of the Human Brain, Germany, 1999.
- Jennum P, Friberg L, Fuglsand-Frederiksen A, Dam M. Speech localization using repetitive transcranial magnetic stimulation. Neurology. 1994;44(2):269–73. doi: 10.1212/wnl.44.2.269. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lippincott, Williams & Wilkins, 2001.
- Kapur N. Paradoxical functional facilitation in brain-behavior research—a critical review. Brain. 1996;119:1775–1790. doi: 10.1093/brain/119.5.1775. [DOI] [PubMed] [Google Scholar]
- Karbe H, Thiel A, Weber-Luxenburger, Kessler J, Herholz K, Heiss WD. Reorganization of the cerebral cortex in post-stroke aphasia studied with positron emission tomography. Neurology. 1998;50:A321. [Google Scholar]
- Kertesz A, McCabe P. Recovery patterns and prognosis in aphasia. Brain. 1977;100:1–18. doi: 10.1093/brain/100.1.1. [DOI] [PubMed] [Google Scholar]
- Kim YH, Ko MH, Parrish TB, Kim HG. Reorganization of cortical language areas in patients with aphasia: A functional MRI study. Yonsei Medical Journal. 2002;43(4):441–445. doi: 10.3349/ymj.2002.43.4.441. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. The minor cerebral hemisphere as a source of aphasic speech. Arch Neurol. 1971;25(4):302–306. doi: 10.1001/archneur.1971.00490040028003. [DOI] [PubMed] [Google Scholar]
- Kucera H, Franci W. Computational analysis of present-day American English. Providence, R.I.: Brown University Press, 1967.
- Leger A, D emonet J-F, Ruff S, Aithamon B, Touyeras B, Puel M, Boulanouar K, Cardebat D. Neural substrates of spoken language rehabilitation in an aphasic patient: An fMRI study. Neuroimage. 2002;17:174–183. doi: 10.1006/nimg.2002.1238. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111:800–805. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- Metter EJ. Neuroanatomy and physiology of aphasia: Evidence from positron emission tomography. Aphasiology. 1987;1:3–33. [Google Scholar]
- Mimura M, Kato M, Kato M, Sano Y, Kojima T, Naeser M, Kashima H. Prospective and retrospective studies of recovery in aphasia: Changes in cerebral blood flow and language functions. Brain. 1998;121:2083–2094. doi: 10.1093/brain/121.11.2083. [DOI] [PubMed] [Google Scholar]
- Miura K, Nakamura Y, Miura F, Yamada I, Takahashi M, Yoshikawa A, Mizobata T. Functional magnetic resonance imaging to word generation task in a patient with Broca’s aphasia. J Neurol. 1999;246(10):939–942. doi: 10.1007/s004150050486. [DOI] [PubMed] [Google Scholar]
- Mottaghy FM, Hungs M, Brugmann M, Sparing R, Boroojerdi B, et al. Facilitation of picture naming after repetitive transcranial magnetic stimulation. Neurology. 1999;53(8):1806–1812. doi: 10.1212/wnl.53.8.1806. [DOI] [PubMed] [Google Scholar]
- Murtha S, Chertkow H, Beauregard M, Evans A. The neural substrate of picture naming. J Cogn Neurosi. 1999;11(4):399–423. doi: 10.1162/089892999563508. [DOI] [PubMed] [Google Scholar]
- Musso M, Weiller C, Kiebel S, Muller SP, Bulau P, Rijntjes M. Training-induced brain plasticity in aphasia. Brain. 1999;122:1781–1790. doi: 10.1093/brain/122.9.1781. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Baker EH, Palumbo CL, Nicholas M, Alexander MP, Samaraweera R, Prete MN, Hodge SM, Weissman T. Lesion patterns in severe aphasia and outcome following treatment with a nonverbal, computer-assisted treatment program. Arch Neurol. 1998;55(11):1438–1448. doi: 10.1001/archneur.55.11.1438. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Baker EH, Hodge SM, Sczerzenie SE, Nicholas M, Palumbo CL, Goodglass H, Wingfield A, Samaraweera R, Harris G, Baird A, Renshaw, Yurgelun-Todd D. Overt propositional speech in chronic nonfluent aphasia studied with the dynamic susceptibility contrast fMRI method. Neuroimage. 2004;22:29–41. doi: 10.1016/j.neuroimage.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, Theoret H, Fregni F, Maria-Tormos J, Kurland J, Doron KW, Pascual-Leone A. Improved picture naming in chronic aphasia after TMS to part of right broca’s area, an open-protocol study. Brain and Language. 2005;93(1):95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Palumbo CL. Neuroimaging and language recovery in stroke. J Clin Neurophysiol. 1994;11(2):150–174. doi: 10.1097/00004691-199403000-00002. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Palumbo CL, Helm-Estabrooks N, Stiassny-Eder D, Albert ML. Severe non-fluency in aphasia: Role of the medial subcallosal fasciculus plus other white matter pathways in recovery of spontaneous speech. Brain. 1989;112:1–38. doi: 10.1093/brain/112.1.1. [DOI] [PubMed] [Google Scholar]
- Naeser M, Theoret H, Kobayashi M, Martin P, Nicholas M et al. Modulation of cortical areas with repetitive transcranial magnetic stimulation to improve naming in nonfluent aphasia [Abstract #133]. 8th International Conference on Functional Mapping of the Human Brain, June 2–6, 2002, Sendai, Japan. Available on CD-ROM in Neuroimage 2002, 16(2).
- Oliveri M, Rossini PM, Traversa R, Cicinelli P, Filippi MM, Pasqualetti P, Tomaiuolo F, Caltagirone C. Left frontal transcranial magnetic stimulation reduces contralesional extinction in patients with unilateral right brain damage. Brain. 1999;122:1731–1739. doi: 10.1093/brain/122.9.1731. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Gates JR, Dhuna A. Induction of speech arrest and counting errors with rapid-rate transcranial magnetic stimulation. Neurology. 1991;41(5):697–702. doi: 10.1212/wnl.41.5.697. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15:333–343. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- Perani D, Cappa SF, Tettamanti M, Rose M, Scifo P, et al. An fMRI study of word retrieval in aphasia. Brain Lang. 2003;85:357–368. doi: 10.1016/s0093-934x(02)00561-8. [DOI] [PubMed] [Google Scholar]
- Price CJ, Warburton EA, Moore CJ, Frackowiak RSJ, Friston KJ. Dynamic diaschisis: Anatomically remote and context-sensitive human brain lesions. J Cogn Neurosci. 2001;13(4):419–429. doi: 10.1162/08989290152001853. [DOI] [PubMed] [Google Scholar]
- Romero R, Anshel D, Sparing R, Gangitano M, Pascual-Leone A. Sub-threshold low frequency repetitive transcranial magnetic stimulation selectively decreases facilitation in the motor cortex. Clin Neurophysiol. 2002;113(1):101–107. doi: 10.1016/s1388-2457(01)00693-9. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Ojemann JG, Ollinger JM. Comparison of brain activation during word retrieval done silently and aloud using fMRI. Brain Cogn. 2000;42:201–217. doi: 10.1006/brcg.1999.1100. [DOI] [PubMed] [Google Scholar]
- Rosenbek JC, LaPointe LL, Wertz RT. Aphasia: A clinical approach. Austin, TX: Pro-Ed, 1989.
- Roth BJ, Saypol M, Hallett M, Cohen LG. A theoretical calculation of the electric field induced in the cortex during magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:47–56. doi: 10.1016/0168-5597(91)90103-5. [DOI] [PubMed] [Google Scholar]
- Rothwell JC. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J Neurosci Methods. 1997;74:113–22. doi: 10.1016/s0165-0270(97)02242-5. [DOI] [PubMed] [Google Scholar]
- Sarno MT, Levita E. Some observations on the nature of recovery in global aphasia after stroke. Brain Lang. 1981;13:1–12. doi: 10.1016/0093-934x(81)90124-3. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Tormos JM, Ceballos-Baumann AO, Auer C, Catala MD, et al. Low-frequency repetitive transcranial magnetic stimulation of the motor cortex in writer’s cramp. Neurology. 1999;52:529–37. doi: 10.1212/wnl.52.3.529. [DOI] [PubMed] [Google Scholar]
- Small SL, Flores DK, Noll DC. Different neural circuits subserve reading before and after therapy for acquired dyslexia. Brain Lang. 1998;62:298–308. doi: 10.1006/brln.1998.1951. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Sparks R, Helm N, Albert M. Aphasia rehabilitation resulting from melodic intonation therapy. Cortex. 1974;10:303–316. doi: 10.1016/s0010-9452(74)80024-9. [DOI] [PubMed] [Google Scholar]
- Sparks R, Holland A. Method: melodic intonation therapy for aphasia. J Speech Hear Disord. 1976;41:287–297. doi: 10.1044/jshd.4103.287. [DOI] [PubMed] [Google Scholar]
- Steele RD, Weinrich M, Wertz RT, Kleczewska MK, Carlson GS. Computer- based visual communication in aphasia. Neuropsychologia. 1989;27:409–426. doi: 10.1016/0028-3932(89)90048-1. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, Farah MJ, D’Esposito M, Kan IP, et al. Verb generation in patients with focal frontal lesions: A neuropsychological test of neuroimaging findings. Proc Natl Acad Sci USA. 1998;95:15855–15860. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulborn KR, Carpenter PA, Just MA. Plasticity of language-related brain function during recovery from stroke. Stroke. 1999;30(4):749–754. doi: 10.1161/01.str.30.4.749. [DOI] [PubMed] [Google Scholar]
- Topper R, Mottaghy FM, Brugmann M, Noth J, Huber W. Facilitation of picture naming by focal transcranial magnetic stimulation of Wernicke’s area. Exp Brain Res. 1998;121(4):371–378. doi: 10.1007/s002210050471. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Hester D, Assal G, Regli F. Unilateral spatial neglect recovery after sequential strokes. Neurology. 1996;46(1):184–189. doi: 10.1212/wnl.46.1.184. [DOI] [PubMed] [Google Scholar]
- Wagner TA, Zahn M, Grodzinsky AJ, Pascual-Leone AP. Three-dimensional head model simulation of transcranial magnetic stimulation. IEEE Transactions on Biomedical Engineering. 2004;51(9):1586–1598. doi: 10.1109/TBME.2004.827925. [DOI] [PubMed] [Google Scholar]
- Walsh V, Pascual-Leone A. Neurochronometrics of mind: TMS in cognitive science. Cambridge, MA (USA): MIT Press, 2003.
- Warburton E, Price C, Swinburn K. Mechanisms of recovery from aphasia: evidence from positron emission tomography studies. J Neurol Neurosurg Psychiatry. 1999;66:155–161. doi: 10.1136/jnnp.66.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiller C, Isensee C, Rijntnes M, Huber W, Muller S, Bier D, et al. Recovery from Wernicke’s aphasia: A positron emission tomographic study. Ann Neurol. 1995;37:723–732. doi: 10.1002/ana.410370605. [DOI] [PubMed] [Google Scholar]
- Weinrich M, McCall D, Weber C, Thomas K, Thornburg L. Training on an iconic communication system for severe aphasia can improve natural language production. Aphasiology. 1995;9:343–364. [Google Scholar]
- Weinrich M, Shelton JR, Cox DM, McCall D. Remediating production of tense morphology improves verb retrieval in chronic aphasia. Brain Lang. 1997;58:23–45. doi: 10.1006/brln.1997.1757. [DOI] [PubMed] [Google Scholar]
- Zahn R, Drews E, Kemeny S, Specht K, Willmes K. Recovery of semantic word processing in global aphasia: A functional MRI study. Brain Lang. 2002;83:73–75. doi: 10.1016/j.cogbrainres.2003.10.021. [DOI] [PubMed] [Google Scholar]


