Abstract
An antigen capture immunoassay to detect West Nile (WN) virus antigen in infected mosquitoes and avian tissues has been developed. With this assay purified WN virus was detected at a concentration of 32 pg/0.1 ml, and antigen in infected suckling mouse brain and laboratory-infected mosquito pools could be detected when the WN virus titer was 102.1 to 103.7 PFU/0.1 ml. In a blindly coded set of field-collected mosquito pools (n = 100), this assay detected WN virus antigen in 12 of 18 (66.7%) TaqMan-positive pools, whereas traditional reverse transcriptase PCR detected 10 of 18 (55.5%) positive pools. A sample set of 73 organ homogenates from naturally infected American crows was also examined by WN virus antigen capture immunoassay and TaqMan for the presence of WN virus. The antigen capture assay detected antigen in 30 of 34 (88.2%) TaqMan-positive tissues. Based upon a TaqMan-generated standard curve of infectious WN virus, the limit of detection in the antigen capture assay for avian tissue homogenates was approximately 103 PFU/0.1 ml. The recommended WN virus antigen capture protocol, which includes a capture assay followed by a confirmatory inhibition assay used to retest presumptive positive samples, could distinguish between the closely related WN and St. Louis encephalitis viruses in virus-infected mosquito pools and avian tissues. Therefore, this immunoassay demonstrates adequate sensitivity and specificity for surveillance of WN virus activity in mosquito vectors and avian hosts, and, in addition, it is easy to perform and relatively inexpensive compared with the TaqMan assay.
Prior to the outbreak of West Nile (WN) virus encephalitis in New York City during the late summer of 1999, St. Louis encephalitis virus (SLE virus) was the most important agent of epidemic viral encephalitis in North America and the only mosquito-borne human pathogen in the family Flaviviridae found in this continent (27). WN virus had been previously recognized as the cause of outbreaks of encephalitis in humans and/or horses in Europe during the 1990s (15). It was originally isolated from an adult female in Uganda in 1937 and has historically circulated throughout Africa, Asia, southern Europe, the Middle East, and Australia (subtype Kunjin [KUN]) (25). The virus normally circulates in natural transmission cycles involving mosquito vectors (usually Culex species) and birds, whereas humans and horses are considered incidental or dead-end hosts.
WN virus is a member of the family Flaviviridae, genus Flavivirus, belonging to the Japanese encephalitis virus (JE virus) serocomplex, which includes SLE virus, JE virus, Alfuy virus, Koutango virus, KUN virus, Murray Valley encephalitis virus, Cacipacore virus, and Yaounde virus (10). Members of the JE serocomplex are closely related antigenically but can be differentiated by plaque-reduction neutralization (PRNT) assays, by enzyme-linked immunosorbent assay (ELISA) and indirect fluorescent antibody assay with virus-specific monoclonal antibodies (MAbs), or by detecting WN virus-specific RNA sequences with reverse transcriptase PCR (RT-PCR) detection systems (17, 28).
Since there is no proven therapy for WN encephalitis in humans or animals, nor any human vaccine to prevent WN virus infection, preventative public health measures, such as the reduction of mosquito vector density, are of primary importance in the control of both WN and SLE viruses. Effective mosquito control requires rapid and sensitive surveillance of virus activity in natural transmission cycles prior to human infection (3, 18). Historically, the most effective means of predicting flavivirus activity has been serologic surveillance of sentinel or wild bird populations as well as determination of numbers of and infection rates in vector mosquitoes (3, 18). Monitoring fatal WN virus infection in birds, especially crows, seemed to be the most sensitive method for determining the geographic range of virus activity in the North American outbreak (15). Even though surveillance programs are similar for both WN and SLE viruses, the presence of both viruses in the same geographic area requires that adequate tests be available to differentiate these viruses.
An antigen (Ag) detection ELISA developed for SLE virus-infected mosquito pools compared favorably in sensitivity with the standard, traditional methods of virus isolation in cell culture and suckling mice and was a much more rapid and less costly assay (28). We have now developed a similar Ag capture ELISA to detect WN virus in mosquito pools and avian tissue homogenates that compares favorably in sensitivity to both virus isolation in cell culture and traditional RT-PCR. In combination, these two Ag detection assays can differentiate between WN and SLE viruses in both laboratory-infected and naturally infected specimens. Even though the Ag capture ELISA is generally less sensitive than the TaqMan assay for WN virus, it is a reasonable and less expensive alternative for those laboratories not equipped for quantitative RT-PCR analysis. Moreover, the use of well-characterized MAbs in these ELISAs readily permits standardization among laboratories.
MATERIALS AND METHODS
Viruses.
All virus strains were obtained from the reference collection at the Division of Vector-Borne Infectious Diseases (DVBID), Centers for Disease Control and Prevention, Ft. Collins, Colo. WN virus strain NY99 and SLE virus strain MSI-7 were grown in Vero and SW-13 cell cultures, respectively, and purified from clarified cell culture supernatants by polyethylene glycol precipitation followed by rate-zonal and isopycnic centrifugation on glycerol-tartrate gradients as previously described (26). Protein concentrations of purified virions were determined by the Bio-Rad protein assay (Bio-Rad, Hercules, Calif.). Stock viral seeds were prepared from infected suckling mouse brain (SMB) or infected C6/36 cell culture supernatant and included the following virus strains: WN (Eg 101), SLE (TBH-28), yellow fever (17D), Ilheus (original), Rio Bravo (M64), dengue 1 (Hawaii), dengue 2 (New Guinea C), dengue 3 (H87), dengue 4 (H241), eastern equine encephalitis (EEE) (NJ60) and Venezuelan equine encephalomyelitis (TC-83).
Antibodies.
The isolation and characterization of the murine MAbs 3.91D, 6B6C-1, 4A4C-4, and 4G2 have been described previously (1, 6, 23, 24). WN virus capture MAb 3.91D, an immunoglobulin G3 (IgG3) subclass antibody, is specific for the closely related WN and KUN viruses, and SLE virus capture MAb 4A4C-4, an IgG2A subclass antibody, is SLE virus type specific. The detecting MAb 6B6C-1 (subclass IgG2A) is flavivirus group reactive and was commercially conjugated to horseradish peroxidase (HRP) (Jackson Immunoresearch Laboratories, West Grove, Pa.). MAb 4G2, a flavivirus group-reactive, subclass IgG2A antibody, was used as a capture antibody for heterologous flaviviruses. High-titer antiviral antibody preparations in the form of murine hyperimmune ascitic fluids (HIAF) for WN, SLE, and EEE viruses were used in the Ag capture inhibition tests. These reagents were obtained from the DVBID.
Mosquito inoculation and processing.
To evaluate the sensitivity of the WN virus Ag capture assay for mosquito pool specimens, Aedes albopictus (Keyport strain) mosquitoes were inoculated intrathoracically with WN virus (NY99-6480) and Aedes aegypti (DQ strain) mosquitoes were inoculated intrathoracically with SLE virus (TBH-28). The infected mosquitoes were held at least 6 days at 27°C. Uninfected A. aegypti mosquitoes were used for negative controls.
WN and SLE virus laboratory-infected mosquitoes were processed either as single specimens or in pools of 50 (1 virus positive and 49 virus negatives). Field-collected mosquitoes, identified as Culex spp., Culex pipiens, or Culex restuans, were placed in pools of 3 to 51 individuals. Single mosquitoes or pools were placed in 4-ml cryogenic, polypropylene, round-bottom vials (Corning Inc., Corning, N.Y.) with 2 ml of BA-1 diluent (1× medium 199 with Hanks' balanced salt solution, 0.05 M Tris buffer [pH 7.6], 1% bovine serum albumin, 0.35 g of sodium bicarbonate per liter, 100 μg of streptomycin per ml, 100 U of penicillin G per ml, 1 μg of amphotericin B [Fungizone] per ml). Mosquito preparations were ground with a MOSAVEX device for 20 to 30 min by placing four 4.5-mm-diameter, copper-clad steel beads (BB-caliber airgun shot) into the tube with the mosquitoes and diluent (5). The mosquito homogenate and steel beads were centrifuged at 3,700 rpm for 20 min to create a pellet of solids. Aliquots of the supernatant were used for plaque assay, Ag capture ELISA, and RNA extraction for TaqMan and RT-PCR (2, 17).
Avian organ homogenates.
Twenty American crow (Corvus brachyrhynchos) carcasses, collected in New Jersey in the fall of 1999, were processed to remove samples of various organs: brain, liver, spleen, kidney, heart, and lung (21). Approximately 0.5 cm3 of each tissue was placed in 1.8 ml of BA-1 diluent, homogenized in glass Ten Broeck tissue grinders, and then clarified by centrifugation. The supernatants were used for plaque assay, Ag capture ELISA, and RNA extraction for TaqMan (2, 17).
Antigen capture ELISA.
The Ag capture immunoassay procedure is essentially that described by Tsai et al. (28). Four WN virus- or WN and KUN virus-specific MAbs were evaluated for their ability to serve as an adequate Ag capture reagent (1, 7, 8, 24) (data not shown). We identified 3.91D, a WN- and KUN-specific MAb, as a satisfactory capture antibody because it was able to capture WN viral Ag with a sensitivity similar to that shown by MAb 4A4C-4 for SLE viral Ag, and it also showed good reactivity with a wide range of WN and KUN virus isolates (1, 24, 29). One hundred microliters of an optimum dilution (1:2,000) of 3.91D ascites, determined by a boxed titration with WN virus-infected SMB, was used to coat Immulon 2 microtiter plates (Dynex Technologies, Chantilly, Va.). Plates were washed following each incubation step with phosphate-buffered saline (PBS), pH 7.2, with 0.05% Tween 20 (PBS-T) and blocked prior to sample addition with 300 μl of blocking buffer (1% nonfat dry milk, 0.1% Tween 20 in PBS). One hundred-microliter volumes of homogenized mosquito pool, virus-infected SMB, or avian tissue supernatant, including uninfected controls, were added to duplicate wells and incubated overnight at 4°C for the greatest sensitivity or, alternatively, at 37°C for 3 h. To reduce background or increase test sensitivity, mosquito pool supernatants were treated with a final concentration of 0.5% Tween 20 in PBS for 15 to 30 min at room temperature before being added to the blocked plate. Detector MAb 6B6C-1-HRP (100 μl of a 1:10,000 dilution in blocking buffer) was added, the mixture was incubated for 1 h at 37°C, and this was followed by the addition of substrate, 3,3′,5,5′-tetramethylbenzidine (TMB-ELISA; Gibco BRL, Gaithersburg, Md.). The plates were incubated for 15 min in the dark, and color development was stopped by the addition of 50 μl of 1 N H2SO4. The absorbance was measured in an ELx808 microplate reader at 450 nm (BioTek Instruments, Winooski, Vt.).
The SLE virus Ag capture test was run in a similar manner, as previously described (28), using a 1:4,000 dilution of MAb 4A4C-4 ascites as capture antibody and positive controls consisting of SLE virus-infected SMB seed virus stocks or inactivated Ag. For either capture assay, the mean absorbance was calculated for each sample tested in duplicate, as well as for the uninfected controls. Test samples were scored as presumptive positives if their mean absorbance was greater than twice the mean absorbance of the normal controls. This value can be expressed as an absorbance ratio, (mean A450 of test sample)/(mean A450 of normal control).
To examine the specificity of the WN virus Ag capture ELISA, parallel tests were run comparing the reactivity of WN- and KUN-specific MAb 3.91D and group-reactive MAb 4G2 as capture antibodies. Plates were coated with either 3.91D (1:2,000) or 4G2 (1:4,000) diluted in coating buffer, pH 9.6, and incubated overnight at 4°C. The test was run as described for the Ag capture assay with a panel of flavivirus-infected SMB or C6/36 infectious seed pools as Ags in the test. MAb 6B6C-1-HRP was used to detect captured Ag.
Antigen capture inhibition assay.
Mosquito pools or avian tissue homogenates that had positive reactions in the WN virus Ag capture ELISA were confirmed in an inhibition assay, performed as described by Tsai et al. (28). Twenty microliters of a 1:20 dilution of WN HIAF was mixed with 100 μl of each sample that was positive for WN viral Ag, and incubated for 1 h at 37°C. A control antibody (EEE HIAF) was also mixed with each sample in a separate reaction mixture. These mixtures were retested in the capture ELISA by adding 100 μl of each mixture to a capture antibody-coated, blocked plate and completing the assay as usual. Test samples were considered to be confirmed as positive for WN virus if the calculated inhibition was greater than 50%, i.e., if [1.00 − (A450 of sample +WN HIAF)/(A450 of sample + EEE HIAF)] × 100 was >50.
TaqMan assay and traditional RT-PCR.
The method for isolating viral RNA from virus seeds, mosquito pools, and homogenized avian tissues was essentially that described by Lanciotti et al. (17). The QIAamp viral RNA kit (QIAGEN, Valencia, Calif.) was used to extract RNA from an aliquot of the same supernatant used for Vero cell plaque assay. RNA was always eluted from the QIAGEN columns in a volume of elution buffer equal to the volume of the extracted sample and was stored at −70°C. WN virus (NY99 strain, GenBank accession number AF196835), TaqMan, and RT-PCR primers used with all laboratory-infected or naturally infected samples were those previously described: WNENV-forward, reverse, and probe primers for TaqMan, and WN233 and WN640c primers for RT-PCR (17). SLE virus TaqMan primers and probe were designed using the envelope glycoprotein sequence information from several SLE virus strains stored at DVBID (16).
The standard RT-PCR assay was performed with the TITAN One-Tube RT-PCR kit (Roche Molecular Biochemicals, Indianapolis, Ind.) using 5 μl of RNA and 50 pmol of each primer in a 50-μl reaction mixture and was analyzed on a 3% NuSieve 3:1 agarose gel (FMC Bioproducts, Rockland, Maine) (17). The TaqMan assay, with 5 μl of RNA, 50 pmol of each primer, and 10 pmol of a probe labeled with the reporter dye 6-carboxyfluorescein and the quencher dye 6-carboxytetramethylrhodamine, was performed and analyzed as previously described (17). Quantitation of WN or SLE virus in laboratory-infected mosquito pools and WN virus in avian tissue homogenates was carried out by generating a standard curve with plaque assay-titrated virus seeds with the PE 7700 Sequence Detection System Software (PE Applied Biosystems, Foster City, Calif.).
RESULTS
Sensitivity evaluations.
The sensitivity of this Ag capture assay was evaluated by determining the minimum amount of virus or viral Ag that could be detected from three sources of virus: purified virus, virus-infected SMB preparations, and laboratory-infected mosquito pools. Because the SLE virus Ag capture test required an overnight incubation of Ag and use of TMB as the HRP substrate to achieve the greatest sensitivity, both the overnight incubation step and the TMB substrate were used for the WN virus Ag capture ELISA (28).
The ranges of viral Ag detected for WN or SLE purified virus (based on amount of viral protein) and virus-infected SMB (based on infectious titer) were very comparable: 21.5 to 43 pg/0.1 ml and 102.25 to 102.9 PFU/0.1 ml for WN virus, and 62 pg/0.1 ml and 102.5 to 103.0 PFU/0.1 ml for SLE virus. Detection of Ag in laboratory-infected mosquito pools, whether the pool consisted of a single infected mosquito alone or mixed with 49 uninfected mosquitoes, was slightly more sensitive for the SLE virus Ag capture test (102.3 to 102.8 PFU/0.1 ml), regardless of Tween 20 treatment. The WN virus Ag capture test detected 103.1 to 103.95 PFU of WN virus/0.1 ml in untreated mosquito pools and 102.8 to 103.75 PFU of virus/0.1 ml in Tween 20-treated mosquito pools. The number of mosquitoes in the pool, 1 or 50, did not affect the sensitivity of WN virus Ag detection. Incubation of the virus-infected SMB or mosquito pool sample for 15 to 30 min at room temperature with a final concentration of 0.5% Tween 20 was done in an attempt to increase test sensitivity by disrupting membranes containing the envelope glycoprotein, a procedure that did increase the sensitivity of an Ag capture assay for EEE virus (4). Treatment with Tween 20 did result in some reduction in the test background, but very little or no increase in sensitivity was observed for most samples; therefore, the additional time and effort for detergent treatment did not seem warranted. The results with all WN virus laboratory-infected mosquito pools were confirmed by the WN virus Ag capture inhibition test by using WN HIAF as the positive polyclonal antibody inhibitor; SLE HIAF as a cross-reactive, positive antibody inhibitor; and EEE HIAF as a normal control antibody (noninhibitor). All 10 WN virus-infected pools showed high levels of inhibition with WN HIAF (mean of 85.9% inhibition) but lower levels of inhibition with SLE HIAF (mean of 63.6% inhibition), indicating that it is important to use a homologous HIAF inhibitor for the most sensitive test.
The ability to detect WN virus Ag in laboratory-infected mosquitoes depended upon the length of the extrinsic incubation period following virus inoculation. The WN virus Ag capture ELISA could detect viral Ag in pools composed of 1 infected plus 49 uninfected mosquitoes at 48 h postinoculation (hpi) if the homogenized pool was treated with 0.5% Tween 20 or at 72 hpi without detergent treatment (data not shown). The infectious titer present in the mosquito pools as measured by plaque assay increased from <102 PFU/0.1 ml at 24 hpi to 103.5 or 105.2 PFU/0.1 ml at 48 and 72 hpi, respectively.
Specificity determinations.
We tested the ability of 3.91D to capture heterologous virus from high-titer virus-infected SMB preparations. Although these high-titer, heterologous Ags were captured by 3.91D, absorbance ratios were 6.2- to 62.5-fold lower than those obtained with the homologous WN virus (Table 1). Conversely, all flaviviruses tested (except dengue 1 virus) had similar absorbance ratios when the flavivirus group-reactive MAb 4G2 was used as the capture antibody, indicating that the inability of 3.91D to capture heterologous flaviviruses was a result of its specificity and not due to insufficient Ag. The reactivity of all dengue 1 virus preparations examined was low with 4G2, most likely reflecting the low titer of the preparations. Not unexpectedly, SLE virus had the highest level of cross-reactivity with the 3.91D capture antibody; however, the absorbance ratio on 3.91D for SLE virus was significantly lower (6.2-fold) than the absorbance ratio for WN virus.
TABLE 1.
Specificity of the WN virus Ag capture ELISA with other flaviviruses
| Virusb | Absorbance ratioa of viral Ags on capture MAb
|
|
|---|---|---|
| WN virus-specific MAb 3.91D | Flavivirus group-reactive MAb 4G2 | |
| WN | 81.3 | 69.4 |
| SLE | 13.1 | 71.5 |
| Yellow fever | 1.3 | 65.9 |
| Ilheus | 6.6 | 62.3 |
| Rio Bravo | 10.9 | 65.6 |
| Dengue 1 | 1.8 | 30.0 |
| Dengue 2 | 6.0 | 71.6 |
| Dengue 3 | 1.4 | 52.8 |
| Dengue 4 | 6.0 | 57.4 |
| VEEc | 1.0 | 0.95 |
Absorbance ratio, A450 of test sample/A450 of normal control sample.
Virus seed preparation in SMB or tissue culture fluid.
VEE, Venezuelan equine encephalomyelitis virus, an alphavirus control.
Results of testing 10 WN virus and 10 SLE virus laboratory-infected mosquito pools in both the WN and SLE virus Ag capture assays revealed excellent specificity (data not shown). Five WN virus laboratory-infected mosquito pools composed of a single infected mosquito (P1) or five pools of 1 infected mosquito plus 49 uninfected mosquitoes (P50) had average WN virus Ag capture ELISA endpoints within twofold of one another (corresponding to infectious titers of 102.8 to 103.1 PFU/0.1 ml), but none of these WN virus-infected pools were detected in the SLE virus Ag capture assay. The same specificity was shown for the SLE virus laboratory-infected mosquito pools, since none of the pools cross-reacted in the WN virus Ag capture ELISA. The average SLE virus Ag capture ELISA endpoints for the P1 and P50 mosquito pools were within fourfold of one another (corresponding to infectious titers of 102.2 to 102.8 PFU/0.1 ml).
Validation testing with field-collected specimens.
A blindly coded set of 100 naturally infected mosquito pools (collected in New Jersey during 2000) were tested in the WN virus Ag capture ELISA; presumptive positive pools were retested in the inhibition ELISA. The pools found to be positive for WN virus contained from 3 to 51 mosquitoes identified as Culex spp., C. pipiens, or C. restuans. The complete set of mosquito pools was first evaluated by virus isolation in a plaque assay and by TaqMan for the presence of viral RNA. In addition, all isolation-positive samples were also tested by traditional RT-PCR. Of the 100 pools, 20 were positive for virus isolation, but only 18 isolates could be confirmed as WN virus. The comparative results for the 18 confirmed WN virus-positive pools are shown in Fig. 1. The WN virus Ag capture ELISA detected Ag in 12 of the 18 positive pools (66.7%). The Ag capture test was not as sensitive as TaqMan, which detected 100% of the positive pools, but it showed good specificity in that all pools found to be negative by plaque assay and TaqMan were also negative by WN virus Ag capture; i.e., no false positives were found. For this set of samples, the Ag capture assay showed slightly greater sensitivity than traditional RT-PCR, which detected 10 of 18 TaqMan-positive pools (55.5%), whereas 2 of 18 pools (11.1%) gave equivocal results. All but one of the RT-PCR-positive pools were also positive by Ag capture ELISA. The WN virus Ag capture inhibition test was performed on the 12 mosquito pools scored as presumptive positives in the Ag capture assay; all 12 pools were confirmed as positive, with WN HIAF inhibition values ranging from 78.9 to 96.6% (data not shown).
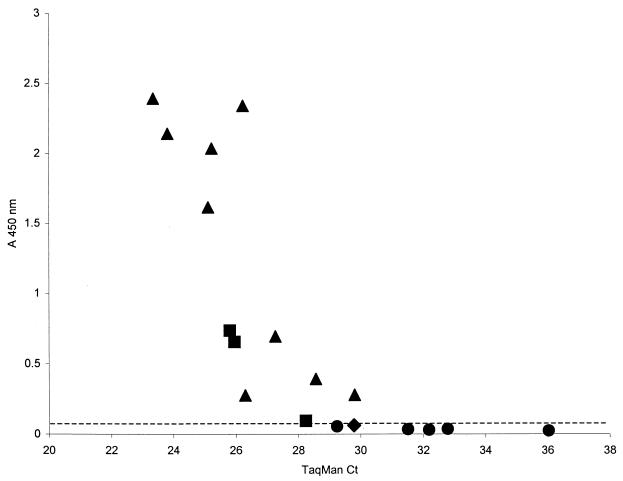
FIG. 1.
Comparison of Ag capture ELISA, TaqMan, and traditional RT-PCR used to test pools of naturally infected mosquitoes. TaqMan Ct values represent the threshold cycle number at which fluorescence increases above a fixed threshold value. Symbols: ▴, pools positive by TaqMan, Ag capture ELISA, and RT-PCR; ▪, pools positive by TaqMan and Ag capture ELISA but equivocal or negative by RT-PCR; ♦, pool positive by TaqMan and RT-PCR, but negative by Ag capture ELISA; •, pools positive by TaqMan only. ELISA plates were read at 450 nm; all symbols below the dotted line represent negative results by Ag capture ELISA.
The plaque assay data for the WN virus-positive pools that were detected by Ag capture revealed that the number of PFU per 0.1 ml of suspension varied from 1 to 46 countable plaques up to an undeterminable number of plaques that covered the entire cell sheet (data not shown). Therefore, for some pools, a very low infectious virus titer was detectable by Ag capture. On the other hand, the average number of PFU per 0.1 ml for isolation-positive, Ag-capture-negative pools was 15 (range, 3 to 32 PFU/0.1 ml). Comparison of the TaqMan and WN virus Ag capture data for WN virus-positive pools showed that samples with a TaqMan threshold cycle (Ct) number of >29 but <30 may or may not be detected in the Ag capture test. However, all samples with TaqMan Ct values of <29 were positive by Ag capture, while all samples with Ct values of >30 were negative in the Ag capture ELISA (Fig. 1). TaqMan Ct values are inversely related to the amount of viral RNA in the sample, i.e., samples with high amounts of viral RNA are detected after fewer cycles and have a relatively lower Ct value.
The principal use of the SLE virus Ag capture assay is as a surveillance tool to monitor virus activity in the mosquito vector. However, because of the importance of the dead-bird monitoring system as a sensitive surveillance tool in the North American outbreak of WN virus, evaluation of the WN virus Ag capture assay for virus detection in avian tissue was undertaken (15). A coded set of 73 organ homogenates from 20 naturally infected American crows (C. brachyrhynchos), collected during the fall of 1999 as part of the dead-bird survey in New Jersey, was tested by plaque assay, TaqMan, and WN virus Ag capture ELISA and inhibition ELISA. In this sample set fewer tissues were positive by plaque assay (26 of 73 [35.6%]) than by TaqMan (34 of 73 [46.6%]) for WN virus. This result is not surprising because the tissues were obtained from carcasses, and recovery of infectious virus could have been dependent upon the amount of decomposition that had occurred as well as on the ability to isolate virus from different organs. The eight specimens that tested positive for virus by TaqMan and negative for virus by isolation came from five different birds, and other tissues (such as brain) from four of these five birds yielded positive plaque assay results (data not shown).
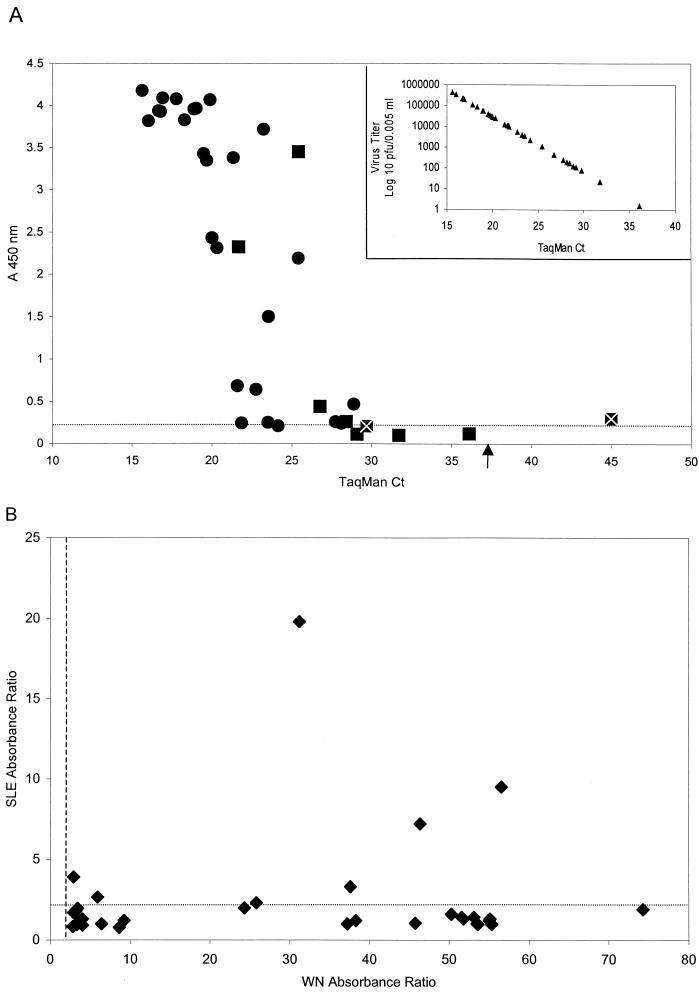
Thirty-one of 34 TaqMan-positive avian samples were determined to be presumptive positives by the WN virus Ag capture ELISA (Fig. 2A). The three samples that were virus negative by ELISA are located below the horizontal dotted line in Fig. 2A. The one sample that was virus negative by TaqMan and a presumptive positive by Ag capture could not be confirmed as positive by the WN virus Ag capture inhibition test. One other sample that was virus positive by TaqMan and a presumptive positive by Ag capture was also not confirmed by the inhibition test. All other samples identified as virus positive by the WN virus Ag capture assay were confirmed by the inhibition test, with values ranging from 67 to 96.7% inhibition (data not shown). Thus, for this sample set there was very good agreement between the number of WN virus-positive samples detected by WN virus Ag capture ELISA (41.1% positive) and the TaqMan assay (46.6% positive).
FIG. 2.
Sensitivity and specificity of the Ag capture ELISA used to test organ homogenates of naturally infected American crows. (A) Analysis of samples positive (•) and negative (▪) for virus isolation by Ag capture ELISA and TaqMan. A ▪ with a superimposed white × represents a sample which could not be confirmed as positive by the Ag capture inhibition test. A total of 35 WN virus-positive samples from 73 homogenates tested are shown. Symbols below the horizontal dotted line indicate samples negative by Ag-Cap ELISA; symbols to the right of the arrow (TaqMan Ct = 37) indicate samples below the threshold Ct for TaqMan (Ct is the threshold cycle number at which fluorescence increases above a fixed threshold value). The insert shows the linear relationship between virus titer (log10 PFU/0.005 ml) and Ct value for a set of RNA standards from a previously titrated WN virus seed included in the TaqMan assay. (B) Cross-reactivity of avian organ homogenates tested in Ag capture assays for both WN and SLE viral Ags. The absorbance ratio is calculated as A450 of test sample/A450 of normal control sample; ratios of ≥2.0 are considered significant. The dotted and dashed lines show the 2.0 absorbance ratio thresholds for the SLE and WN virus Ag capture ELISAs, respectively.
As was observed for the mosquito pool sample set, a correlation was found between TaqMan Ct values and results of the WN virus Ag capture ELISA for the avian samples. In this case, samples with Ct values of <29 tested positive by Ag capture, whereas those with Ct values of >29 tested negative by Ag capture (Fig. 2A). The inset in Fig. 2A shows the linear relationship between Ct values and infectious virus titer, based upon a set of RNA standards from a WN virus seed with an established titer. It can be determined from this inset graph that a Ct value of 29 corresponds to about 102 PFU/5 μl (volume used for TaqMan) or 103.3 PFU/0.1 ml (volume used for Ag capture). Therefore, the latter value would be the calculated limit of detection, based on infectious virus, for the WN virus Ag capture ELISA. The actual numbers of PFU per 0.1 ml observed by plaque assay for the Ag capture-positive avian homogenates ranged from zero to too numerous to count (data not shown).
Although it was previously established that an overnight incubation with the test sample resulted in the most sensitive Ag detection for mosquito pool samples, results of a 37°C, 3-h incubation and an overnight incubation were compared for 22 WN virus Ag-positive avian organ homogenates. Twenty-one of the 22 samples were scored as positive in the short-incubation test, but 19 of these 22 samples had lower A450 readings than the same samples evaluated after overnight incubation. However, these results indicate that a short-incubation test might be adequate, especially for samples expected to have high concentrations of viral Ag, which is the case for a number of avian species infected with WN virus.
All 30 avian organ homogenates confirmed as positive by the WN virus Ag capture and inhibition ELISAs were also tested in the SLE virus Ag capture assay. Although there is no recognized surveillance value in testing wild bird carcasses for SLE virus, this heterologous assay was performed strictly to assess the degree of cross-reactivity that could occur with field specimens containing high concentrations of virus Ag. Sample absorbance ratios calculated from the WN and SLE virus Ag capture tests were compared in a scatter plot (Fig. 2B). Seven of the 30 samples confirmed as WN virus positive were cross-reactive in the SLE virus Ag capture test, since they had absorbance ratios above the threshold value of 2.0 (absorbance ratio range, 2.3 to 19.8). However, none of the seven samples were confirmed as positive by the SLE virus Ag capture inhibition test.
DISCUSSION
The focus of surveillance for epidemic vector-borne diseases is to measure viral activity in the vector and vertebrate host populations in order to assess the disease risk for humans and animals and to assist in targeting and evaluating vector control efforts (13, 29). For SLE virus, it is generally accepted that there are threshold levels of infection rates in vector mosquitoes and birds that must be exceeded before there is a risk of transmission to humans. The SLE virus threshold infection rate in mosquitoes for such an epidemic threat is based on virus isolation and varies from 5 to 10 per 1,000 Culex tarsalis mosquitoes (28). This general principle undoubtedly applies to WN virus, although the relationship between infectious virus titers in mosquitoes and the ability to routinely transmit virus has not been experimentally established, nor has any specific infection rate been related to epidemic transmission.
For approximately 2 decades, there has been wide acceptance of enzyme immunoassays which have proven to be both sensitive and specific as tools for rapid virus diagnosis (20, 30). These characteristics as well as the capability to rapidly process large numbers of samples are valuable in an assay used for surveillance purposes. Such assays should enhance surveillance effectiveness both by increasing the number of laboratories capable of performing tests and by lengthening the period in which vector control can be undertaken (4, 12, 28, 29, 31). Within the last 10 years, rapid, sensitive and specific nucleic acid-based assays employing RT-PCR or TaqMan have been developed for several RNA viruses, including WN virus (17, 22). In connection with developing an ELISA-based Ag detection assay for WN virus, we have also compared the performance of this test with TaqMan and traditional RT-PCR using two blindly coded sets of field-collected specimens.
The WN virus Ag capture ELISA offers the advantage of a simple, rapid in vitro procedure that is less expensive to run than TaqMan, is less sensitive to contamination problems than nucleic acid-based assays, has an internal confirmatory test, and does not require the extensive support facilities needed for conventional methods of virus isolation (cell culture and animal facilities). Another important aspect of this assay is the stability of the viral protein, which does not require a cold chain to maintain reactivity; moreover, detection of Ag may be improved after procedures such as sonication or freeze-thaw cycles (29). The capture assay is adaptable to a dipstick or wicking assay that may be easier for use in the field or for testing a small number of samples. The WN virus Ag capture test was designed as a MAb-based assay for a number of reasons: (i) to make it compatible with the previously developed SLE virus Ag capture test so that these tests could be run in tandem; (ii) to provide a theoretically infinite antibody supply; (iii) to utilize capture and detector antibodies with defined, stable antigenic specificities; and (iv) to reduce the need for routine test restandardization. Because the SLE and WN virus Ag capture tests are based on a similar protocol, the flavivirus group-reactive detector, MAb 6B6C-1-HRP, can be used in both tests, thus limiting the number of enzyme conjugates required.
Initial experiments that used purified virus, virus-infected SMB, and laboratory-infected mosquito pools revealed that the WN virus Ag capture test was as sensitive as the corresponding test for SLE virus. A MAb-based Ag capture assay for EEE virus was similar in sensitivity to the WN virus assay for virus-infected SMB but detected a minimum of only approximately 105 PFU/0.1 ml from virus-infected mosquito pools (4). The limit of detection for yellow fever virus by Ag capture ranged from 103.3 to 103.8 PFU/0.1 ml (19). Sensitivity comparisons to previously published Ag capture ELISAs for other arboviruses are difficult to make, because these studies used 50% tissue culture infective dose units as endpoints to assess infectivity levels rather than plaque assays (9, 11, 13, 14). The detection of WN virus in naturally infected mosquitoes was essentially equivalent by plaque assay and TaqMan; the Ag capture test detected 66.7% of the pools that were positive by both TaqMan and isolation. This is similar to the results of Tsai et al. (28) for SLE virus-infected mosquitoes. Those investigators stated that this limited level of sensitivity may not prevent the capture assay from detecting mosquitoes that are capable of transmitting virus, because such mosquitoes generally have a disseminated, high-titer virus infection. If one of the main objectives of surveillance efforts is identifying vectors capable of transmitting virus, then the WN virus Ag capture test would be an effective primary or confirmatory assay. Ag capture and/or traditional RT-PCR would be viable alternatives for laboratories without TaqMan or tissue culture capabilities.
A high proportion (30 of 34) of the avian homogenates that were positive by TaqMan were also positive for WN virus by Ag capture ELISA. The high correlation between these two assays was most likely due to the high viral titers (or high concentrations of Ag) in many of these samples, since 25 of 34 samples positive by TaqMan had titers in excess of 104 PFU/0.1 ml (which corresponds to titers of at least 102.7 PFU/5 μl and Ct values of 25.4 or less [Fig. 2A]). However, 8 of 30 Ag capture-positive samples had very low or no PFU counts in Vero cell culture (0 to 4.5 PFU/0.1 ml), yet these samples were still detected by ELISA and had TaqMan-based titers in excess of 103 PFU/0.1 ml. These results are most likely due to detection of noninfectious nucleic acid by TaqMan and noninfectious Ag by the ELISA. It is not unlikely to expect a discrepancy between the amount of infectious virus compared with the amount of Ag due to conditions of collection, storage, transport, and number of freeze-thaw cycles to which the specimens may be subjected. Moreover, as discussed by Lanciotti et al. (17), the WN virus TaqMan assay amplifies small DNA fragments (typically <100 bp) and it is possible to generate the fluorescent signal without synthesis of full-length DNA products, both of which are factors that lead to increased assay sensitivity.
Despite the lack of absolute specificity (Table 1) (28), the WN and SLE virus Ag capture tests, in conjunction with their corresponding inhibition assays, showed sufficient specificity with the field-collected specimens tested, since none of the 30 avian tissues confirmed as WN virus positive by Ag capture had confirmed heterologous reactions in the SLE virus Ag capture assay. In addition, the two assays were able to differentiate WN virus laboratory-infected and SLE virus laboratory-infected mosquito pools. The appearance of WN virus in the United States has resulted in an important change in arbovirus disease ecology and epidemiology, both locally and, ultimately, throughout the Western Hemisphere. The great majority of states in the United States have had documented human cases of SLE within the past 4 decades, although more recent cases have been associated with the southern states (23a). Probable expansion of WN virus into areas of more-recent SLE virus activity could lead to cocirculation of these flaviviruses, which could complicate laboratory virus identification tests. Considering the continuing spread of WN virus, it is notable that the specificity of these MAb-based, Ag capture assays appears to be adequate to meet the ever-increasing surveillance needs.
REFERENCES
- 1.Adams, S. C., A. K. Broom, L. M. Sammels, A. C. Hartnett, M. J. Howard, R. J. Coelen, J. S. Mackenzie, and R. A. Hall. 1995. Glycosylation and antigenic variation among Kunjin virus isolates. Virology 206:49-56. [DOI] [PubMed] [Google Scholar]
- 2.Beaty, B. J., C. H. Calisher, and R. S. Shope. 1989. Arboviruses, p. 797-856. In N. J. Schmidt and R. W. Emmons (ed.), Diagnostic procedures for viral, rickettsial and chlamydia infections. American Public Health Association, Washington, D.C.
- 3.Bowen, G. S., and D. B. Francy. 1980. Surveillance, p. 473-499. In T. P. Monath (ed.), St. Louis encephalitis. American Public Health Association, Washington, D.C.
- 4.Brown, T. M., C. J. Mitchell, R. S. Nasci, G. C. Smith, and J. T. Roehrig. 2001. Detection of eastern equine encephalitis virus in infected mosquitoes using a monoclonal antibody based antigen-capture enzyme-linked immunosorbent assay. Am. J. Trop. Med. Hyg. 65:208-213. [DOI] [PubMed] [Google Scholar]
- 5.Doggett, S. L., I. Koevski, J. Haniotis, and R. C. Russell. 1997. MOSAVEX: a mechanical device to grind mosquitoes for arbovirus detection. Arbovirus Res. Aust. 7:75-78. [Google Scholar]
- 6.Gentry, M. K., E. A. Henchal, J. M. McCown, W. E. Brandt, and J. M. Dalrymple. 1982. Identification of distinct antigenic determinants on dengue 2 virus using monoclonal antibodies. Am. J. Trop. Med. Hyg. 31:548-555. [DOI] [PubMed] [Google Scholar]
- 7.Gould, E. A., A. Buckley, S. Higgs, and S. Gaidamovich. 1990. Antigenicity of flaviviruses. Arch. Virol. Suppl. 1:137-152. [Google Scholar]
- 8.Hall, R. A., G. W. Burgess, B. H. Kay, and P. Clancy. 1991. Monoclonal antibodies to Kunjin and Kokobera viruses. Immunol. Cell Biol. 69:47-49. [DOI] [PubMed] [Google Scholar]
- 9.Hall, R. A., B. H. Kay, and G. W. Burgess. 1987. An enzyme immunoassay to detect Australian flaviviruses and identify the encephalitic subgroup using monoclonal antibodies. Immunol. Cell Biol. 65:103-110. [DOI] [PubMed] [Google Scholar]
- 10.Heinz, F. X., M. S. Collett, R. H. Purcell, E. A. Gould, C. R. Howard, M. Houghton, R. J. Moorman, C. M. Rice, and H.-J. Thiel. 1999. Family: Flaviviridae, p. 859-866. In M. H. V. Van Regenmortel, C. M. Fauget, D. H. L. Bishop, E. B. Carstens, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wicker (ed.), Virus taxonomy: classification and nomenclature of viruses. Seventh report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, Calif.
- 11.Hildreth, S. W., and B. J. Beaty. 1984. Detection of eastern equine encephalomyelitis virus and Highlands J virus antigens within mosquito pools by enzyme immunoassay (EIA). I. A laboratory study. Am. J. Trop. Med. Hyg. 33:965-972. [DOI] [PubMed] [Google Scholar]
- 12.Hildreth, S. W., and B. J. Beaty. 1987. Economic comparison of enzyme immunoassay and virus isolation procedures for surveillance of arboviruses in mosquito populations. J. Clin. Microbiol. 25:976-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hildreth, S. W., B. J. Beaty, H. K. Maxfield, R. F. Gilfillan, and B. J. Rosenau. 1984. Detection of eastern equine encephalomyelitis virus and Highlands J virus antigens within mosquito pools by enzyme immunoassay (EIA). II. Retrospective field test of the EIA. Am. J. Trop. Med. Hyg. 33:973-980. [DOI] [PubMed] [Google Scholar]
- 14.Hildreth, S. W., B. J. Beaty, J. M. Meegan, C. L. Frazier, and R. E. Shope. 1982. Detection of La Crosse arbovirus antigen in mosquito pools; application of chromogenic and fluorogenic enzyme immunoassay systems. J. Clin. Microbiol. 15:879-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komar, N. 2000. West Nile viral encephalitis. Rev. Sci. Off. Int. Epizoot. 19:166-176. [DOI] [PubMed] [Google Scholar]
- 16.Lanciotti, R. S., and A. J. Kerst. 2001. Nucleic acid sequence-based amplification assays for rapid detection of West Nile and St. Louis encephalitis viruses. J. Clin. Microbiol. 39:4506-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanciotti, R. S., A. J. Kerst, R. S. Nasci, M. S. Godsey, C. J. Mitchell, H. M. Savage, N. Komar, N. A. Panella, B. C. Allen, K. E. Volpe, B. S. Davis, and J. T. Roehrig. 2000. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J. Clin. Microbiol. 38:4066-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monath, T. P. 1984. Ecology and control of mosquito-borne arbovirus diseases, p. 115-134. In E. Kurstak and R. Marusyk (ed.), Control of virus diseases. Marcel Dekker, Inc., New York, N.Y.
- 19.Monath, T. P., and R. R. Nystrom. 1984. Detection of yellow fever virus in serum by enzyme immunoassay. Am. J. Trop. Med. Hyg. 33:151-157. [DOI] [PubMed] [Google Scholar]
- 20.O'Beirne, A. J., and H. R. Copper. 1979. Heterogenous enzyme immunoassay. J. Histochem. Cytochem. 27:1148-1162. [DOI] [PubMed] [Google Scholar]
- 21.Panella, N. A., A. J. Kerst, R. S. Lanciotti, P. Bryant, B. Wolf, and N. Komar. 2001. Comparative West Nile virus detection in organs of naturally infected American crows (Corvus brachyrhynchos). Emerg. Infect. Dis. 7:754-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porter, K. R., P. L. Summers, D. Dubois, B. Puri, W. Nelson, E. Henchal, J. J. Oprandy, and C. G. Hayes. 1993. Detection of West Nile virus by the polymerase chain reaction and analysis of nucleotide sequence variation. Am. J. Trop. Med. Hyg. 48:440-446. [DOI] [PubMed] [Google Scholar]
- 23.Roehrig, J. T., J. H. Mathews, and D. W. Trent. 1983. Identification of epitopes in the E glycoprotein of St. Louis encephalitis virus using monoclonal antibodies. Virology 128:118-126. [DOI] [PubMed] [Google Scholar]
- 23a.Roehrig, J. T., M. Layton, P. Smith, G. L. Campbell, R. Nasci, and R. S. Lanciotti. 2001. The emergence of West Nile virus in North America: ecology, epidemiology and surveillance. Curr. Top. Microbiol. Immunol. 267:223-240. [DOI] [PubMed] [Google Scholar]
- 24.Scherret, J. H., M. Poidinger, J. S. Mackenzie, A. K. Broom, V. Deubel, W. I. Lipkin, T. Briese, E. A. Gould, and R. A. Hall. 2001. The relationships between West Nile and Kunjin viruses. Emerg. Infect. Dis. 7:697-705. [DOI] [PMC free article] [PubMed]
- 25.Smithburn, K. C., T. P. Hughes, A. W. Burke, and J. H. Paul. 1940. A neurotropic virus isolated from the blood of a native of Uganda. Am. J. Trop. Med. 20:471-492. [Google Scholar]
- 26.Trent, D. W., and C. W. Naeve. 1980. Biochemistry and replication, p. 159-199. In T. P. Monath (ed.), St. Louis encephalitis. American Public Health Association, Washington, D.C.
- 27.Tsai, T. F., and T. P. Monath. 1987. Viral diseases in North America transmitted by arthropods or from vertebrate reservoirs, p. 1417-1456. In R. D. Feigin and J. D. Cherry (ed.), Textbook of pediatric infectious diseases. The W. B. Saunders Co., Philadelphia, Pa.
- 28.Tsai, T. F., R. A. Bolin, M. Montoya, D. B. Francy, and J. T. Roehrig. 1987. Detection of St. Louis encephalitis virus antigen in mosquitoes by capture enzyme immunoassay. J. Clin. Microbiol. 25:370-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai, T. F., C. M. Happ, R. A. Bolin, M. Montoya, E. Campos, D. B. Francy, R. A. Hawkes, and J. T. Roehrig. 1988. Stability of St. Louis encephalitis viral antigen detected by enzyme immunoassay in infected mosquitoes. J. Clin. Microbiol. 26:2620-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yolken, R. H. 1980. Enzyme-linked immunosorbent assay (ELISA): a practical tool for rapid diagnosis of viruses and other agents. Yale J. Biol. Med. 53:85-92. [PMC free article] [PubMed] [Google Scholar]
- 31.Yolken, R. H. 1982. Enzyme immunoassays for detection of infectious antigens in body fluids: current limitations and future prospects. Rev. Infect. Dis. 4:35-68. [DOI] [PubMed] [Google Scholar]