Abstract
Using a polyphasic approach (including cellular protein and fatty acid analysis, biochemical characterization, 16S ribosomal DNA sequencing, and DNA-DNA hybridizations), we characterized 51 bacterial isolates recovered from respiratory secretions of cystic fibrosis (CF) patients. Our analyses showed that 24 isolates belong to taxa that have so far not (or only rarely) been reported from CF patients. These taxa include Acinetobacter sp., Bordetella hinzii, Burkholderia fungorum, Comamonas testosteroni, Chryseobacterium sp., Herbaspirillum sp., Moraxella osloensis, Pandoraea genomospecies 4, Ralstonia gilardii, Ralstonia mannitolilytica, Rhizobium radiobacter, and Xanthomonas sp. In addition, one isolate most likely represents a novel Ralstonia species, whereas nine isolates belong to novel taxa within the α-Proteobacteria. Eight of these latter isolates are classified into the novel genus Inquilinus gen. nov. as Inquilinus limosus gen. nov., sp. nov., or as Inquilinus sp. The remaining 17 isolates are characterized as members of the family Enterobacteriaceae. The recovery of these species suggests that the CF lung is an ecological niche capable of supporting the growth of a wide variety of bacteria rarely seen in clinical samples. Elucidation of the factors that account for the association between these unusual species and the respiratory tract of CF patients may provide important insights into the pathophysiology of CF infection. Because accurate identification of these organisms in the clinical microbiology laboratory may be problematic, the present study highlights the utility of reference laboratories capable of identifying unusual species recovered from CF sputum.
Cystic fibrosis (CF), an autosomal-recessive hereditary disease caused by mutations in the CF transmembrane conductance regulator gene, is characterized by the production of abnormally thickened, viscous mucus and a disturbance in electrolyte transport across epithelial membranes (34). Defects in the CF transmembrane conductance regulator gene mainly affect the respiratory tract and the pancreas, and exacerbations of pulmonary infections cause significant morbidity and mortality in persons with CF (16). Typical CF pathogens include Pseudomonas aeruginosa, Staphylococcus aureus, Haemophilus influenzae, and Burkholderia cepacia complex organisms (16), but other glucose nonfermenters such as Ralstonia pickettii, Alcaligenes xylosoxidans, Stenotrophomonas maltophilia, and Burkholderia gladioli can be recovered from CF sputum culture as well (3, 16). Correct identification of CF pathogens is important, since it underlies effective infection control measures and therapuetic intervention (24, 25, 36); however, several studies have shown that the identification of CF pathogens, including B. cepacia complex organisms, is far from straightforward (3, 8, 22, 28).
Here we report the characterization of 51 bacterial isolates recovered from respiratory secretions of CF patients. The majority of these isolates were identified by referring microbiology laboratories as B. cepacia complex, Burkholderia sp., or R. pickettii or could not be identified. Our initial assessment (using growth on selective media, biochemical tests, and PCR-based assays) indicated that they were in fact not members of these species. A polyphasic taxonomic study (including cellular protein and fatty acid analysis, 16S ribosomal DNA [rDNA] sequencing, DNA-DNA hybridizations, and phenotypical tests) showed that these isolates either belonged to novel taxa or to taxa that so far have not (or only rarely) been reported for isolates from CF patients; others were atypical (and hence difficult to identify) strains from taxa previously found in CF patients.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The 51 isolates investigated were obtained from the respiratory secretions of 47 CF patients attending 16 different treatment centers in the United States; all were sent to the B. cepacia Research Laboratory and Repository (University of Michigan, Ann Arbor, Mich.) for further analysis. Identifications by the referring laboratory (based on a wide variety of biochemical test kits) were as follows: B. cepacia complex (29 isolates), Burkholderia sp. (3 isolates), R. pickettii (2 isolates), Acinetobacter sp. (2 isolates), Rhizobium radiobacter (1 isolate), Comamonas acidovorans (1 isolate), Pseudomonas mendocina (1 isolate), and Pseudomonas sp. (1 isolate). Eleven isolates were unidentified. All isolates were grown aerobically on Mueller-Hinton broth (Becton Dickinson, Cockeysville, Md.) supplemented with 1.8% (wt/vol) agar and incubated at 32°C unless otherwise indicated. Preliminary investigations employing standard techniques and several previously described genus- and species-specific PCR-based assays (targeting 16S rRNA and recA genes [26, 27]) indicated that these organisms were not members of bacterial species typically recovered from CF sputum culture.
Phenotypic tests.
For conventional biochemical testing, bacteria from fresh (24 to 48 h) Mueller-Hinton agar plates were inoculated into the test reagent. Oxidase and catalase testing of each isolate was performed with 1% tetramethyl p-phenylenediamine dihydrochloride and 3% hydrogen peroxide, respectively. Growth in 1, 6, and 10% NaCl was determined in Mueller-Hinton broth incubated at 37°C. Lysine decarboxylase, o-nitrophenyl-β-d-galactoside (ONPG), and oxidation-fermentation sugars (sucrose and lactose) were obtained from Remel (Lenexa, Kans.). These tests were incubated at 37°C and were examined daily for 7 days, except ONPG, which was examined at 24 and 48 h. The RapID NF Plus (Remel) and API20E (bioMerieux, Hazelwood, Mo.) commercial identification systems were used according to the recommendations of the manufacturers. Growth on B. cepacia selective agar (BCSA) (containing 600 U of polymyxin B, 10 μg of gentamicin, and 2.5 μg of vancomycin per ml) (18) was determined at 32°C.
SDS-PAGE of whole-cell proteins.
Strains were grown aerobically on nutrient agar (Oxoid CM3) supplemented with 0.04% (wt/vol) KH2PO4 and 0.24% (wt/vol) Na2HPO4·12H2O (pH 6.8) and incubated for 48 h at 28°C. Preparation of whole-cell proteins and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were performed as described previously (33). Densitometric analysis, normalization, and interpolation of the protein profiles and numerical analysis by using Pearson's product-moment correlation coefficient were performed with the GelCompar 4.2 software package (Applied Maths, Kortrijk, Belgium). Isolates were identified by comparison to a database containing profiles of all Burkholderia, Ralstonia, Pandoraea, Alcaligenes, and Bordetella species and various other gram-negative nonfermenters (5, 6, 7, 10, 38, 39).
FAME analysis.
Strains were grown aerobically on Trypticase soy agar (Becton Dickinson). After an incubation period of 24 to 48 h at 28°C (35°C for isolates AU0476, AU0578, AU0599, AU0771, AU1109, AU1714, AU2065, and AU1979), a loopful of well-grown cells was harvested, and fatty acid methyl esters (FAMEs) were prepared, separated, and identified by using the Microbial Identification System (MIDI, Inc., Newark, Del.) as described previously (40). Isolates were identified by comparison to commercially available databases (MIDI, Inc.).
16S rDNA sequencing.
DNA was prepared by heating one or two colonies (picked from a plate grown overnight) at 95°C for 15 min in 20 μl of lysis buffer containing 0.25% (wt/vol) SDS and 0.05 M NaOH. After lysis, 180 μl of distilled water was added, and the DNA solutions were stored at −20°C. The nearly complete sequence (corresponding to positions 9 to 1500 in the Escherichia coli numbering system) of the 16S rRNA gene was amplified by PCR with the conserved primers UFPL (5′-AGTTTGATCCTGGCTCAG-3′) and URPL (5′-GGTTACCTTGTTACGACTT-3′). The PCR product was purified by using the Promega Wizard PCR Preps kit (Promega, Madison, Wis.) according to the manufacturer's instructions. Sequence analysis was performed with an Applied Biosystems 3700 DNA sequencer and the protocols of the manufacturer (PE Applied Biosystems, Foster City, Calif.) by using the BigDye Terminator Cycle Sequencing Ready Reaction kit. The sequencing primers used were UFPL, URPL, 16SF1 (5′-GCCTTCGGGTTGTAAAGCAC-3′), 16SF2 (5′-CCTTACCTACCCTTGACA-3′), 16SB1 (5′-GCGCTCGTTGCGGGACT-3′), and 16SB2 (5′-GTATTACCGCGGCTGCTG-3′). Sequence assembly was performed by using EditSeq (DNAStar, Inc., Madison, Wis.). The sequences were compared to sequences available in the GenBank database by using FASTA3 (http://www.ebi.ac.uk/fasta33/) (31), and the results obtained with FASTA3 were used to identify isolates to the genus or species level. Phylogenetic trees based on the neighbor-joining method were constructed by using the MegAlign (DNAStar) software package.
Determination of the DNA base composition and DNA-DNA hybridizations.
DNA was prepared as described previously (7). The DNA base composition was determined by high-pressure liquid chromatography, and DNA-DNA hybridizations were performed with photobiotin-labeled probes in microplate wells as described previously (7). The hybridization temperature was 55°C.
Nucleotide accession numbers.
All 16S rDNA sequences determined in the present study were deposited in the GenBank database under the following accession numbers (strain): AY043368 (AU0783), AY043369 (AU1523), AY043370 (AU0939), AY043371 (AU2210A), AY043372 (AU2339), AY043374T (AU0476T), AY043375 (AU1979), AY043376 (AU1220), AY043377 (AU1775), AY043378 (AU0428), AY043379 (AU0255), AY043380 (AU0378), AY043381 (AU0626), AY043382 (AU2259), AY043383 (AU2502), AY043384 (AU2539), AY043385 (AU0664), AY043386 (AU0736), AY043387 (AU1209), AY043388 (AU1285), AY043389 (AU1388), AY043390 (AU14191), AY043391 (AU1496), and AY043392 (AU1713).
RESULTS
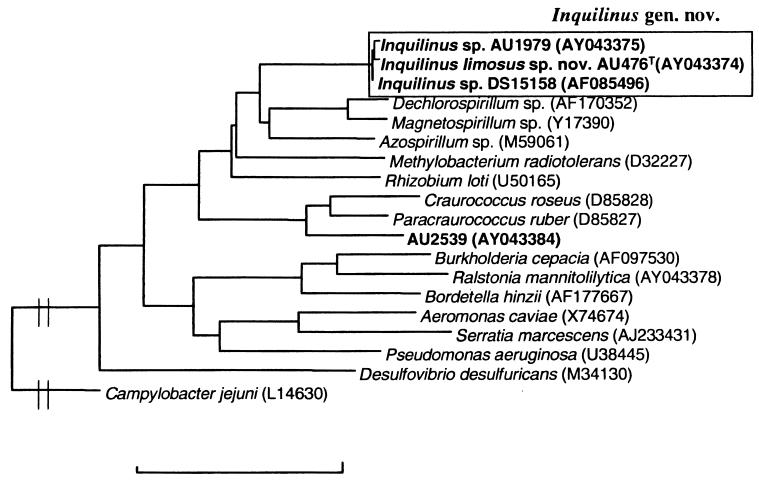
Of the 51 isolates investigated, 11 could be identified to the genus or species level by SDS-PAGE of whole-cell proteins. These were identified as Ralstonia mannitolilytica (five isolates), Ralstonia gilardii (one isolate), Ralstonia sp. (one isolate), Pandoraea genomospecies 4 (two isolates), Burkholderia fungorum (one isolate), or Bordetella hinzii (one isolate). The isolates showed variable growth on BCSA (Table 1). The remaining 40 isolates could not be identified by SDS-PAGE of whole-cell proteins and were further analyzed by means of cellular fatty acid analysis and/or 16S rDNA sequence analysis. Among these were 14 isolates identified as Acinetobacter sp. (two isolates), Comamonas testosteroni (two isolates), Chryseobacterium sp. (two isolates), Herbaspirillum sp. (two isolates), Moraxella osloensis (one isolate), Rhizobium radiobacter (four isolates), or Xanthomonas sp. (one isolate). These strains also showed variable growth on BCSA (Table 2). Seventeen additional isolates were identified as belonging to the Enterobacteriaceae by cellular fatty acid analysis; these were further characterized by using the API 20E identification system and 16S rDNA sequencing. Among these 17 isolates, whole-cell protein electrophoresis differentiated three clusters with identical protein profiles and 4 isolates with unique protein profiles (Table 3). Several isolates (AU0476, AU0578, AU0599, AU0771, AU1109, AU1714, and AU2065) could not be classified into any existing taxon. These isolates formed a homogeneous group, as determined by SDS-PAGE (designated group 29), but could not be identified by cellular fatty acid analysis. The following fatty acids were present in all group 29 isolates investigated (AU0476T, AU0599, AU1109, AU1714, and AU2065) (mean values ± the standard deviation): 16:0-2OH (5.1% ± 1.2%), 16:0-3OH (3.7% ± 0.9%), 18:1Ω7c (35.6% ± 1.2%), 17:0-2OH (5.0% ± 1.7%), 17:0-3OH (4.3% ± 1.3%), 19:0-cyclo ω8c (7.9% ± 2.5%), 18:1-2OH (15.5% ± 5.0%), and 18:0-3OH (9.0% ± 0.7%). In addition, small amounts of 10:0-3OH, SF2 (14:0-3OH, 16:1 iso I, or 12:0 ALDE), 16:0, 17:1ω7c, 17:0, and 18:0 could be detected in some but not all strains. The fatty acid composition of another isolate (AU1979) was similar to that of group 29 isolates, but its protein pattern was clearly different (data not shown). The 16S rDNA sequences of isolates AU1979 and group 29 representative AU0476 were determined and compared with each other and with available 16S rDNA sequences in the GenBank database. The sequences were similar to each other (>99.5% similarity) and to the previously determined 16S rDNA sequence of isolate DS15158 (GenBank accession no. AF85496) (99.4 to 99.9% similarity). Based on the phylogenetic analysis, these isolates clearly belong to the α-Proteobacteria, with their closest relatives being members of the genera Azospirillum (<88.6% similarity), Dechlorospirillum (<87.8% similarity), Magnetospirillum (<87.4% similarity), and Rhizobium (≤86.2% similarity) (Fig. 1). DNA was prepared from group 29 isolates AU476 and AU2065 and strain AU1979, the G+C content of the genome of the isolates was determined, and these strains were included in the DNA-DNA hybridization experiments. Isolates AU476, AU2065, and AU1979 had G+C contents of 70.3, 70.7, and 70.9%, respectively. The hybridization experiments confirmed that group 29 strains AU476 and AU2065 formed one genomic group (DNA-DNA binding value of 93%). Hybridization values of AU1979 toward AU476 and AU2065 were lower (53 and 62%, respectively). We also determined the phenotypic characteristics of all group 29 isolates and of AU1979 (Table 4). Finally, the remaining strain AU2539 also could not be identified to the species level by means of fatty acid analysis. 16S rDNA sequence analysis revealed that it also belonged to the α-Proteobacteria, with the genera Paracraurococcus and Craurococcus as closest neighbors (with similarities lower than 93.3 and 91.2%, respectively) (Fig. 1). The biochemical characteristics of AU2539 were as follows: growth at 25, 28, 32, and 37°C; production of pink pigment on Mueller-Hinton plates; no growth on BCSA; no lysine and ornithine decarboxylase, β-galactosidase, arginine dihydrolase, N-acetylglucosaminidase, α-glucosidase, β-glucosidase, pyrollidonyl aminopeptidase, γ-l-glutamyl aminopeptidase, tryptophan aminopeptidase, or N-benzyl-arginine aminopeptidase activity present; oxidase, catalase, urease, gelatinase, proline aminopeptidase, lipase, and phosphatase activity present; no acid production from lactose or sucrose; no H2S, indole, or acetoin production; no denitrification; and no utilization of glucose, mannitol, inositol, sorbitol, rhamnose, sucrose, melibiose, amygdalin, arabinose, or citrate.
TABLE 1.
Isolates identified by SDS-PAGE of whole-cell proteins
| Strain(s) | Identification | Growth on BCSAa |
|---|---|---|
| AU2290, AU2300, AU0428, AU0255, and AU0378 | Ralstonia mannitolilytica | + |
| AU1589 | Ralstonia gilardii | + |
| AU0626 | Ralstonia sp. | − |
| AU2911 | Burkholderia fungorum | − |
| AU1775 and AU2478 | Pandoraea genomospecies 4 | + |
| AU1784 | Bordetella hinzii | − |
+, Growth; −, no growth.
TABLE 2.
Isolates identified by cellular fatty acid analysis and/or 16S rDNA sequencing
| Strain | Identification by FAME (score) | Phylogenetic affiliation based on 16S rDNA sequencing | Growth on BCSAd |
|---|---|---|---|
| AU0783 | Acinetobacter baumanii (0.335) | Genus Acinetobacter | − |
| AU1523 | Acinetobacter baumanii (0.616) | Genus Acinetobacter | + |
| AU1747a | Comamonas testosteroni (0.194) | − | |
| AU1748a | Comamonas testosteroni (0.737) | − | |
| AU0939b | Chryseobacterium meningosepticum (0.544) | Flavobacterium-Cytophaga-Bacteroides group | + |
| AU0999b | Chryseobacterium meningosepticum (0.341) | + | |
| AU2210A | Chromobacterium violaceum (0.606) | Genus Herbaspirillum | + |
| AU2339 | Pseudomonas huttiensis (0.558) | Genus Herbaspirillum | + |
| AU1220 | Moraxella catarrhalis (0.323) | Moraxella osloensis | − |
| AU2259c | Rhizobium radiobacter (0.432) | Genus Rhizobium | − |
| AU1577c | Rhizobium radiobacter (0.444) | − | |
| AU1253c | No match | − | |
| AU1254c | Rhizobium radiobacter (0.795) | − | |
| AU2502 | Xanthomonas hyacinthi (0.314) | Xanthomonas campestris group | + |
AU1747 and AU1748 have identical whole-cell protein profiles and therefore may represent a single clone.
AU0939 and AU0999 have similar whole-cell protein profiles and therefore may represent a single species.
AU2259, AU1577, AU1253, and AU1254 have similar whole-cell protein profiles and therefore may represent a single species.
−, No growth; +, growth.
TABLE 3.
Enterobacterial isolates identified by cellular fatty acid analysis, 16S rDNA sequencing, and/or API 20E
| Straina | Identification by FAME (score) | Identification by API 20E | Phylogenetic affiliation based on 16S rDNA sequence |
|---|---|---|---|
| AU0664a | Not done | Good identification as Serratia marcescens | Serratia marcescens |
| AU0736a | Cedecea davisae (0.453) | Very good identification as Serratia marcescens | |
| AU0791a | Cedecea davisae (0.548) | Good identification as Serratia marcescens | |
| AU1209a | Salmonella typhimurium (0.467) | Good identification as Vibrio vulnificus | Serratia marcescens |
| AU1285a | Kluyvera ascorbata (0.492) | Good identification as Aeromonas hydrophila | Serratia marcescens |
| AU2544a | Cedecea davisae (0.491) | Good identification as Aeromonas hydrophila | |
| AU0774b | Cedecea davisae (0.217) | Doubtfull identification as Vibrio vulnificus | |
| AU1676b | Cedecea davisae (0.467) | Very good identification as Serratia marcescens | |
| AU1677b | Cedecea davisae (0.608) | Very good identification as Serratia marcescens | |
| AU1274c | Cedecea davisae (0.331) | Unidentified (unacceptable profile) | |
| AU1392c | Salmonella typhimurium (0.300) | Unidentified (unacceptable profile) | |
| AU1398c | Cedecea davisae (0.237) | Unidentified (unacceptable profile) | |
| AU1388 | Yersinia pseudotuberculosis (0.020) | Good identification as Aeromonas hydrophila | Serratia marcescens |
| AU1491 | Morganella morganii (0.573) | Excellent identification as Morganella morganii | Genus Morganella |
| AU1496 | Klebsiella pneumoniae (0.392) | Acceptable identification as Escherichia hermanii | Klebsiella pneumoniae |
| AU1713 | Enterobacter cancerogenus (0.242) | Very good identification as Enterobacter cloacae | Escherichia coli |
Isolates indicated by the same superscript letter belong to different protein electrophoretic subgroups (a, b, and c, respectively) of the same species; based on 16S rDNA sequencing and/or very good identification as Serratia marcescens by the API 20E system, all of these isolates were identified as Serratia marcescens.
FIG. 1.
Phylogenetic tree showing the position of the genus Inquilinus and isolate AU2539 within the Proteobacteria. The scalebar represents 10% sequence dissimilarity.
TABLE 4.
Phenotypic characteristics of I. limosus strains and Inquilinus sp. strain AU1979a
| Characteristic |
I. limosus
|
Inquilinus sp. strain AU1979 | ||||||
|---|---|---|---|---|---|---|---|---|
| AU0476T | AU0578 | AU0599 | AU0771 | AU1109 | AU1714 | AU2065 | ||
| Presence of: | ||||||||
| Oxidase | + | + | + | + | + | + | − | − |
| Urease | − | − | − | − | − | − | + | − |
| β-Galactosidase | + | + | + | + | − | + | − | + |
| α-Glucosidase | − | + | + | − | + | − | + | + |
| γ-l-Glutamyl aminopeptidase | + | + | − | + | + | + | + | − |
| Gelatinase | − | − | − | − | + | + | + | − |
| Growth in 6% NaCl | − | + | − | − | + | − | + | − |
| Growth on BCSA | + | − | − | − | − | − | − | − |
| Production of acid from: | ||||||||
| Sucrose | − | + | + | − | − | − | − | − |
| Lactose | − | + | + | − | + | − | − | − |
All strains tested were positive for the following characteristics: presence of catalase, β-glucosidase, phosphatase, lipase, N-acetyl glucosaminidase, proline aminopeptidase, pyrollidonyl aminopeptidase, tryptophan aminopeptidase, and N-benzyl-arginine aminopeptidase activity; growth in 1% NaCl; growth at 25, 28, 32, 37, and 42°C; and acetoin production. All strains investigated were negative for the following characteristics: presence of lysine decarboxylase or arginine dihydrolase activity; denitrification; growth in 10% NaCl; H2S or indole production; and utilization of glucose, mannitol, inositol, sorbitol, rhamnose, sucrose, melibiose, amygdalin, arabinose, and citrate.
DISCUSSION
In the present study we used a polyphasic approach (including cellular fatty acid and protein analysis, 16S rDNA sequencing, DNA-DNA hybridizations, and biochemical characterization) to characterize 51 isolates recovered from the respiratory tracts of CF patients. Our initial analyses showed that these isolates did not belong to bacterial species typically recovered from CF patients. The more extensive analyses described here revealed that these isolates (i) belong to species previously not (or only rarely) recovered from CF patients, (ii) are atypical strains from taxa previously found in CF patients, or (iii) belong to novel taxa.
Unusual species in CF sputum.
Isolates of B. cepacia complex, B. gladioli, R. pickettii, and several Pandoraea species are regularly recovered from CF patients (3, 5, 16). In the present study we identified several other species belonging to these genera in isolates obtained from CF cultures. These included several R. mannitolilytica isolates. Isolates from this recently described species have been recovered from blood, sputum, and other clinical samples; a number of hospital outbreaks involving this species have also been described (10). R. mannitolilytica may be difficult to differentiate from B. cepacia complex organisms, R. pickettii, and Pseudomonas species (10). In the present study all R. mannitolilytica isolates had been misidentified by the referring laboratories as R. pickettii or Burkholderia sp. R. gilardii has been isolated from the environment and various human clinical samples (6). Strain AU1589 represents the first reported R. gilardii isolate recovered from the respiratory tract of a CF patient. The referring microbiology laboratory identified this isolate as belonging to the B. cepacia complex. In addition to R. mannitolilytica and R. gilardii, another Ralstonia isolate, AU0626, was found. Although this strain could not be identified to the species level, SDS-PAGE of whole-cell proteins and the 16S rDNA sequence data clearly indicated that it belonged to the genus Ralstonia. Further taxonomic work is required to clarify whether this isolate represents a new Ralstonia species or is an atypical isolate from existing Ralstonia species.
The genus Pandoraea contains five named species and four unnamed genomospecies. Pandoraea strains have mainly been isolated from respiratory secretions of CF patients (5, 9). Isolates AU1775 and AU2478 were isolated from the same CF patient 10 months apart. Based on 16S rDNA sequence analysis, these isolates clearly belong to the genus Pandoraea (98.9 to 99.9% similarity to other Pandoraea species), and comparison of the one-dimensional protein profiles showed that they belonged to Pandoraea genomospecies 4 (data not shown). The only previously known isolate belonging to Pandoraea genomospecies 4 was also isolated from the sputum of a CF patient (9). Both AU1775 and AU2478 were identified by the referring laboratory as belonging to the B. cepacia complex. The fact that they were obtained from the same patient 10 months apart indicates the capacity for chronic colonization by this species.
B. fungorum is a recently described Burkholderia species that has been isolated from the environment, animals, and human clinical samples (7). Isolate AU2911 was identified as B. fungorum by SDS-PAGE of whole-cell proteins. Recently, several B. fungorum strains were isolated from CF patients (7). Several xenobiotic-compound-degrading strains with potential biotechnological applications as biodegradation agents (including Burkholderia sp. strain LB400) are closely related to B. fungorum (7, 13), and the finding that B. fungorum can be isolated from clinical samples (including respiratory secretions of CF patients) highlights the need for a general consensus on the potential large-scale use of members of the genus Burkholderia as biocontrol or biodegradation agents.
Bordetella hinzii strains have mainly been isolated from poultry with respiratory disease, although isolated cases of human infection have been reported (15, 20). Funke et al. (15) reported two strains obtained from a CF patient over a 3-year period; the clinical picture and laboratory data were clearly consistent with episodes of infectious pulmonary exacerbations and, despite aggressive antimicrobial therapy, the organism could not be eradicated from the patient. Isolate AU1784 was identified as Bordetella hinzii by SDS-PAGE of whole-cell proteins but could not be identified with several commercial biochemical systems and was misidentified as Alcaligenes piechaudii by fatty acid analysis (data not shown). This confirms previous data that this organism is difficult to identify (20).
Based on fatty acid composition and 16S rDNA sequence, isolates AU0783 and AU1523 were identified as belonging to the genus Acinetobacter. Acinetobacter spp. are widely distributed in nature and in the hospital environment and may also be recovered from the skin of healthy individuals. Misidentification of acinetobacters is relatively common due to the taxonomic complexity of the genus (which includes 9 named species and 14 unnamed genomic groups), the lack of discriminatory power of identification schemes based on phenotypic characteristics alone, and the frequent occurrence of isolates that do not seem to belong to any of the described genomic groups (29, 30). The 16S rDNA sequence of isolate AU0783 was most similar to sequences of Acinetobacter calcoaceticus-Acinetobacter baumanii complex organisms. The 16S rDNA sequence of AU1523 is most similar to sequences of representatives of the recently described species Acinetobacter ursingii, a taxon considered to be of medical importance (29, 30). More data are required to establish whether these isolates belong to any of the described genomic groups within the genus Acinetobacter or whether these isolates truely represent novel taxa.
Isolates AU1747 and AU1748 were recovered from two siblings with CF and were both identified as Comamonas testosteroni by fatty acid analysis. Both isolates were obtained at the same time, and their whole-cell protein profiles are identical, suggesting patient-to-patient spread. Comamonas species are mainly environmental in origin and are generally not associated with human disease (41).
Isolates AU0939 and AU0999 were identified as belonging to the Flavobacterium-Cytophaga-Bacteroides phylum. The closest relatives were Chryseobacterium species (93.8 to 94.4% 16S rDNA sequence similarity). Chryseobacterium isolates are mainly isolated from the natural environment, but Chryseobacterium meningosepticum occurs in human clinical samples (including cases of meningitis, sepsis, pneumonia, and eye infections) (41). Although the relatively low similarity between the 16S rDNA sequence of AU0939 and representatives of Chryseobacterium species suggests that AU0939 and AU0999 constitute a novel taxon within this genus, a thorough taxonomic study would be required to clarify their taxonomic status.
16S rDNA sequence analysis of isolates AU2210A and AU2339 showed that they were most similar to Pseudomonas huttiensis (99.5%) and Herbaspirillum frisingense (99.6%), respectively. Recently, P. huttiensis has been shown to be misclassified, being more properly placed in the genus Herbaspirillum (1). Herbaspirillum spp. are mainly environmental nitrogen-fixing organisms and are frequently isolated from the roots and stems of various plants (21), although they also have been cultured from various clinical samples, including wounds and the eye (2).
Strain AU1220 was identified as Moraxella osloensis based on 16S rDNA sequence analysis (>99.3% similarity to other M. osloensis isolates; <93.3% similarity to other Moraxella species). This species has been associated with human infections, including meningitis, arthritis, and endocarditis. M. osloensis strains have also been isolated from hospital environments, as well as from the respiratory tracts of healthy adults (41).
Isolates AU2259, AU1577, AU1253, and AU1254 were identified as Rhizobium (Agrobacterium) radiobacter. Rhizobium radiobacter infections have been associated with long-term indwelling catheters, and several cases also have been reported in immunocompromised individuals (12, 19). Our data suggest that Rhizobium radiobacter may have the potential to spread from patient to patient (AU1253 and AU1254 were simultaneously isolated from two siblings with CF).
Genuine Xanthomonas species (excluding Stenotrophomonas maltophilia) are notorious plant pathogens and have, to our knowledge, not been recovered from humans. Based on 16S rDNA sequence analysis and fatty acid analysis, isolate AU2502 clearly belongs to the genus Xanthomonas (the Xanthomonas campestris cluster) (17).
Atypical Enterobacteriaceae.
Enteric bacilli are occasionally recovered from the respiratory secretions of CF patients, but they are usually transient colonizers and are in general not associated with severe disease (4, 16). Enterobacterial species recovered from CF respiratory tract cultures include E. coli, Klebsiella pneumoniae, and Serratia marcescens (3, 4). Identification of enterobacterial species is typically performed by using commercially available biochemical test kits, but there are several problems associated with their use (14). Slow-growing or fastidious strains require extended incubation for biochemical reactivity, and “pleiotropic” mutants isolated from patients who are taking antimicrobial agents often react atypically (14, 23). Occasionally, strains are isolated that grow rapidly but do not have biochemical reactions that fit the described taxa; these represent atypical strains or strains belonging to new taxa (14). In the present study we examined 17 isolates that were initially identified as enterobacteria by cellular fatty acid analysis. The majority had been identified as Burkholderia sp. by the referring laboratories. Identification of these isolates to the species level by cellular fatty acid analysis was only partially successful, confirming previous results that enterobacterial species cannot reliably be separated by using fatty acid analysis (M. Vancanneyt and P. Vandamme, unpublished data). Use of 16S rDNA sequence analysis in the identification of enterobacterial species is also problematic, since not all members of the Enterobacteriaceae have been subjected to extensive 16S rDNA sequence analysis (11, 37). Nevertheless, the combination of all available data strongly suggests that several of these isolates were Serratia marcescens, while one each was identified as Morganella morganii, E. coli, and K. pneumoniae.
Inquilinus gen. nov.
The seven isolates that constituted protein electrophoretic group 29 and isolate AU1979 could not be identified by cellular protein or fatty acid analysis. Sequence analysis of the 16S rDNA gene from strains AU0476 and AU1979 revealed that these strains were closely related to the unidentified α-proteobacterium DS15158, which was previously isolated from a CF patient (32). The similarity values toward other taxa were much lower (Fig. 1). Our data clearly indicate that these eight isolates should be classified into a novel genus, for which we propose the name Inquilinus gen. nov. below. Based on data derived from SDS-PAGE of whole-cell proteins and DNA-DNA hybridizations, isolates AU0476, AU0578, AU0599, AU0771, AU1109, AU1714, and AU2065 clearly belong to a single species, for which we propose the name I. limosus gen. nov., sp. nov. below. Isolate AU1979 clearly belongs to a different species within the genus Inquilinus; however, we do not here propose a formal name for this isolate, pending the study of additional similar isolates and the availability of discriminating phenotypic characteristics.
Isolate AU2539.
This isolate was identified with a low probability score as Methylobacterium mesophilicum by cellular fatty acid analysis, suggesting that it belonged to the α-Proteobacteria. Analyis of the 16S rDNA sequence of this isolate confirmed this and showed that its closest neighbors are members of the genera Paracraurococcus and Craurococcus. These two genera were recently described for bacteriochlorophyll a-containing strains isolated from soil in Japan (35). From our data it is obvious that isolate AU2539 belongs to a novel genus within the α-Proteobacteria. However, we do not propose a formal name for this organism here, pending the availability of additional isolates.
Conclusions.
The recovery of these unusual species from the respiratory secretions of CF patients clearly shows that the bacterial biodiversity in the airways of CF patients still is underestimated. This has several important implications. Although the clinical role of these and other unusual bacteria in CF lung disease is unclear, at least some of these organisms seem capable of prolonged colonization or spread among CF patients. An equally important issue is the challenge that these organisms pose to clinical microbiology laboratories: none of these organisms was initially identified correctly, with the majority either not identified or erroneously identified as B. cepacia complex or R. pickettii. The misidentification of isolates as B. cepacia complex can have a significant medical, social, and psychological impact (22, 24, 25, 28). Accurate species level identification of CF isolates is also important in order to identify outbreaks or to assess the clinical impact of unusual species. Since most of the taxa identified here have only been described recently or have not been found frequently in clinical samples, they are not included in most commercial databases; it is therefore not surprising that most were initially misidentified. Our observations suggest the need for increased awareness of this problem and highlight the usefulness of reference laboratories capable of providing polyphasic analyses of unusual isolates recovered from CF sputum. The data presented also suggest that the CF lung seems to be an ecological niche suitable for the growth of a wide variety of unusual bacteria not commonly associated with human disease. Factors that account for the association between typically nonpathogenic species and the respiratory tracts of CF patients are unknown; elucidation of these factors may provide important insights into the pathophysiology of CF infection.
Inquilinus gen. nov.
Inquilinus (In.qui′li.nus. L. masc. n. inquilinus, an inhabitant of a place that is not its own). Cells are gram-negative, nonsporulating rods. Catalase activity is present. Growth is observed at 25, 28, 32, 37, and 42°C and in 1% NaCl. No growth occurs in 10% NaCl. There is no denitrification. There is no indole production. There is no lysine decarboxylase, ornithine decarboxylase, or arginine dihydrolase activity. There is no pigment production. Lipase, phosphatase, N-acetylglucosaminidase, β-glucosidase, proline aminopeptidase, pyrollidonyl aminopeptidase, tryptophan aminopeptidase, and N-benzyl-arginine aminopeptidase activity are present. There is no utilization of glucose, mannitol, inositol, sorbitol, rhamnose, sucrose, melibiose, amygdalin, arabinose, or citrate. Additional characteristics are shown in Table 4. The G+C content of the genome is between 70.3 and 70.9%. It is isolated from the respiratory secretions of CF patients. The type species is Inquilinus limosus.
Description of Inquilinus limosus gen. nov., sp. nov.
Inquilinus limosus (li.mo′sus. L. masc. adj. limosus, full of slime, slimy). The description of I. limosus is the same as for the genus. The type strain, AU0476T, was isolated from the respiratory secretions of a CF patient in the United States in 1998. The description of the type strain is the same as for the species. In addition, the type strain has the following characteristics: oxidase, β-galactosidase, and γ-l-glutamyl aminopeptidase activity are present; no urease, gelatinase, or α-glucosidase activity is present; there is no acid production from sucrose or lactose; there is no growth in 6% NaCl; there is growth on BCSA at 32°C. The G+C content of the type strain is 70.3%, and the GenBank accession number for its 16S rDNA sequence is AY043374. I. limosus AU0476T was deposited in the BCCM/LMG Bacteria Collection (University of Ghent, Ghent, Belgium) as LMG 20952T and in the CCUG Culture Collection (University of Goteborg, Goteborg, Sweden) as CCUG 45653T. In addition, Inquilinus sp. strain AU1979 was deposited in the BCCM/LMG Bacteria Collection as LMG 20953.
Acknowledgments
This work was supported by a grant from the Cystic Fibrosis Foundation (United States) (to J.J.L.). T.C. is supported by the Caroll Haas Research Fund in Cystic Fibrosis.
We thank A. Balcaen, L. Lebbe, and C. Snauwaert for excellent technical assistance.
REFERENCES
- 1.Anzai, Y., H. Kim, J.-Y. Park, H. Wakabayashi, and H. Oyaizu. 2000. Phylogenetic affiliation of the pseudomonads based on 16S rRNA sequence. Int. J. Syst. E vol. Microbiol. 50:1563-1589. [DOI] [PubMed] [Google Scholar]
- 2.Baldani, J. I., B. Pot, E. Falsen, V. L. Baldani, F. L. Olivares, B. Hoste, K. Kersters, A. Hartmann, M. Gillis, and J. Dobereiner. 1996. Emended description of Herbaspirillum; inclusion of [Pseudomonas] rubrisubalbicans, a milk plant pathogen, as Herbaspirillum rubrisubalbicans comb. nov.; and classification of a group of clinical isolates (EF group 1) as Herbaspirillum species 3. Int. J. Syst. Bacteriol. 46:802-810. [DOI] [PubMed] [Google Scholar]
- 3.Burns, J. L., J. Emerson, J. R. Stapp, D. L. Yim, J. Krzewinski, L. Louden, B. W. Ramsey, and C. R. Clausen. 1998. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin. Infect. Dis. 27:158-163. [DOI] [PubMed] [Google Scholar]
- 4.Canton, R., M. I. Morosini, S. Ballestero, M. E. Alvarez, H. Escobar, L. Maiz, and F. Baquero. 1997. Lung colonization with Enterobacteriaceae producing extended-spectrum β-lactamases in cystic fibrosis patients. Pediatr. Pulm. 24:213-217. [DOI] [PubMed] [Google Scholar]
- 5.Coenye, T., E. Falsen, B. Hoste, M. Ohlén, J. Goris, J. R. W. Govan, M. Gillis, and, P. Vandamme. 2000. Description of Pandoraea gen. nov. with Pandoraea apista sp. nov., Pandoraea pulmonicola sp. nov., Pandoraea pnomenusa sp. nov., Pandoraea sputorum sp. nov., and Pandoraea norimbergensis comb. nov. Int. J. Syst. E vol. Microbiol. 50:887-899. [DOI] [PubMed] [Google Scholar]
- 6.Coenye, T., E. Falsen, M. Vancanneyt, B. Hoste, J. R. W. Govan, K. Kersters, and P. Vandamme. 1999. Classification of some Alcaligenes faecalis-like isolates from the environment and human clinical samples as Ralstonia gilardii sp. nov. Int. J. Syst. Bacteriol. 49:405-413. [DOI] [PubMed] [Google Scholar]
- 7.Coenye, T., S. Laevens, A. Willems, M. Ohlen, W. Hannant, J. R. W. Govan, M. Gillis, E. Falsen, and P. Vandamme. 2001. Burkholderia fungorum sp. nov. and Burkholderia caledonica sp. nov., two new species isolated from the environment, animals and human clinical samples. Int. J. Syst. E vol. Microbiol. 51:1099-1107. [DOI] [PubMed] [Google Scholar]
- 8.Coenye, T., P. Vandamme, J. R. W. Govan, and J. J. LiPuma. 2001. Taxonomy and identification of the Burkholderia cepacia complex. J. Clin. Microbiol. 39:3427-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daneshvar, M. I., D. G. Hollis, A. G. Steigerwalt, A. M. Whitney, L. Spangler, M. P. Douglas, J. G. Jordan, J. P. MacGregor, B. C. Hill, F. C. Tenover, D. J. Brenner, and R. S. Weyant. 2001. Assignment of CDC weak oxidizer group 2 (WO-2) to the genus Pandoraea and characterization of three new Pandoraea genomospecies. J. Clin. Microbiol. 39:1819-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Baere, T., S. Steyaert, G. Wauters, P. De Vos, J. Goris, T. Coenye, T. Suyama, G. Verschraegen, and M. Vaneechoutte. 2001. Classification of Ralstonia pickettii biovar 3/“thomasii” strains (Pickett, 1994) and of new isolates related to nosocomial recurrent meningitis as Ralstonia mannitolytica sp. nov. Int. J. Syst. E vol. Microbiol. 51:547-558. [DOI] [PubMed] [Google Scholar]
- 11.Drancourt, M., C. Bollet, A. Carta, and P. Rousselier. 2001. Phylogenetic analyses of Klebsiella and Raoultella gen. nov., with description of Raoultella ornitholytica comb. nov., Raoultella terrigena comb. nov., and Raoultella planticola comb. nov. Int. J. Syst. E vol. Microbiol. 51:925-932. [DOI] [PubMed] [Google Scholar]
- 12.Edmond, M. B., S. A. Riddler, C. M. Baxter, B. M. Wicklund, and A. W. Pasculle. 1993. Agrobacterium radiobacter: a recently recognized opportunistic pathogen. Clin. Infect. Dis. 16:388-391. [DOI] [PubMed] [Google Scholar]
- 13.Fain, M. G., and J. D. Haddock. 2001. Phenotypic and phylogenetic characterization of Burkholderia (Pseudomonas) sp. strain LB400. Curr. Microbiol. 42:269-275. [DOI] [PubMed] [Google Scholar]
- 14.Farmer, J.J., III. 1995. Enterobacteriaceae: introduction and identification, p. 438-449. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology. ASM Press, Washington, D.C.
- 15.Funke, G., T. Hess, A. von Graevenitz, and P. Vandamme. 1996. Characteristics of Bordetella hinzii strains isolated from a cystic fibrosis patient over a 3-year period. J. Clin. Microbiol. 34:966-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilligan, P. H. 1991. Microbiology of airway disease in patients with cystic fibrosis. Clin. Microbiol. Rev. 4:35-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauben, L., L. Vauterin, J. Swings, and E. R. B. Moore. 1997. Comparison of 16S ribosomal DNA sequences of all Xanthomonas species. Int. J. Syst. Bacteriol. 47:328-335. [DOI] [PubMed] [Google Scholar]
- 18.Henry, D. A., M. E. Campbell, J. J. LiPuma, and D. P. Speert. 1997. Identification of Burkholderia cepacia isolates from patients with cystic fibrosis and use of a simple new selective medium. J. Clin. Microbiol. 35:614-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hulse, M., S. Johnson, and P. Ferrieri. 1993. Agrobacterium infections in humans: experience at one hospital and review. Clin. Infect. Dis. 16:112-117. [DOI] [PubMed] [Google Scholar]
- 20.Kattar, M. M., J. F. Chavez, A. P. Limaye, S. L. Rassoulian-Barret, S. L. Yarfitz, L. C. Carlson, Y. Houze, S. Swanzy, B. L. Wood, and B. T. Cookson. 2000. Application of 16S rRNA gene sequencing to identify Bordetella hinzii as the causative agent of fatal septicemia. J. Clin. Microbiol. 38:789-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirchhof, G., B. Eckert, M. Stoffels, J. I. Baldani, V. M. Reis, and A. Hartmann. 2001. Herbaspirillum frisingense sp. nov., a new nitrogen-fixing bacterial species that occurs in C4-fibre plants. Int. J. Syst. E vol. Microbiol. 51:157-168. [DOI] [PubMed] [Google Scholar]
- 22.Kiska, D. L., A. Kerr, M. C. Jones, J. A. Caracciolo, B. Eskridge, M. Jordan, S. Miller, D. Hughes, N. King, and P. Gilligan. 1996. Accuracy of four commercial systems for identification of Burkholderia cepacia and other gram-negative, nonfermenting bacilli recovered from patients with cystic fibrosis. J. Clin. Microbiol. 34:886-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lannigan, R., and Z. Hussain. 1993. Wound isolate of Salmonella typhimurium that became chlorate resistant after exposure to Dakin's solution: concomitant loss of hydrogen sulfide production, gas production, and nitrate reduction. J. Clin. Microbiol. 31:2497-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LiPuma, J. J. 1998. Burkholderia cepacia: management issues and new insights. Clin. Chest Med. 19:473-486. [DOI] [PubMed] [Google Scholar]
- 25.LiPuma, J. J. 1998. Burkholderia cepacia epidemiology and pathogenesis: implications for infection control. Curr. Opin. Pulm. Med. 4:337-441. [DOI] [PubMed] [Google Scholar]
- 26.LiPuma, J. J., B. J. Dulaney, J. D. McMenamin, P. W. Whitby, T. L. Stull, T. Coenye, and P. Vandamme. 1999. Development of rRNA-based PCR assays for identification of Burkholderia cepacia complex isolates recovered from cystic fibrosis patients. J. Clin. Microbiol. 37:3167-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahenthiralingam, E., J. Bischof, S. K. Byrne, C. Radomski, J. E. Davies, Y. Av-Gay, and P. Vandamme. 2000. DNA-based diagnostic approaches for the identification of Burkholderia cepacia complex, Burkholderia vietnamiensis, Burkholderia multivorans, Burkholderia stabilis, and Burkholderia cepacia genomovars I and III. J. Clin. Microbiol. 38:3165-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMenamin, J. D., T. M. Zaccone, T. Coenye, P. Vandamme, and J. J. LiPuma. 2000. Misidentification of Burkholderia cepacia in U.S. cystic fibrosis treatment centers. Chest 117:1661-1665. [DOI] [PubMed] [Google Scholar]
- 29.Nemec, A., T. De Baere, I. Tjernberg, M. Vaneeckhoutte, T. J. K. van der Reijden, and L. Dijkshoorn. 2001. Acinetobacter ursingii sp. nov. and Acinetobacter schindleri sp. nov., isolated from human clinical specimens. Int. J. Syst. E vol. Microbiol. 51:1891-1899. [DOI] [PubMed] [Google Scholar]
- 30.Nemec, A., L. Dijkshoorn, and P. Jezek. 2000. Recognition of two novel phenons of the genus Acinetobacter among non-glucose-acidifying isolates from human specimens. J. Clin. Microbiol. 38:3937-3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearson, W. R., and D. J. Lipman. 1988. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 85:2444-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pitulle, C., D. M. Citron, B. Bochner, R. Barbers, and M. D. Appleman. 1999. Novel bacterium isolated from a lung transplant patient with cystic fibrosis. J. Clin. Microbiol. 37:3851-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pot, B., P. Vandamme, and K. Kersters. 1994. Analysis of electrophoretic whole-organism protein fingerprints, p. 493-521. In M. Goodfellow and A. G. J. O'Donell (ed.), Chemical methods in prokaryotic systematics. Wiley and Sons, Ltd., Chichester, United Kingdom.
- 34.Rosenstein, B. J., and P. L. Zeitlin. 1998. Cystic fibrosis. Lancet 351:277-282. [DOI] [PubMed] [Google Scholar]
- 35.Saitoh, S., T. Suzuki, and Y. Nishimura. 1998. Proposal of Craurococcus roseus gen. nov., sp. nov., and Paracraurococcus ruber gen. nov., sp. nov., novel aerobic bacteriochlorophyll a-containing bacteria from soil. Int. J. Syst. Bacteriol. 48:1043-1047. [DOI] [PubMed] [Google Scholar]
- 36.Shreve, M. R., S. Butler, H. J. Kaplowitz, H. R. Rabin, D. Stokes, M. Light, and W. R. Regelmann for the North American Scientific Advisory Group and Investigators for the Epidemiologic Study of Cystic Fibrosis. 1999. Impact of microbiology practice on cumulative prevalence of respiratory tract bacteria in patients with cystic fibrosis. J. Clin. Microbiol. 37:753-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sproer, C., U. Mendrock, J. Swiderski, E. Lang, and E. Stackebrandt. 1999. The phylogenetic positions of Serratia, Buttiauxella, and some other genera of the family Enterobacteriaceae. Int. J. Syst. Bacteriol. 49:1433-1438. [DOI] [PubMed] [Google Scholar]
- 38.Vandamme, P., M. Heyndrickx, M. Vancanneyt, B. Hoste, P. De Vos, E. Falsen, K. Kersters, and K. H. Hinz. 1996. Bordetella trematum sp. nov., isolated from wounds and ear infections in humans, and reassessment of Alcaligenes denitrificans Ruger and Tan 1983. Int. J. Syst. Bacteriol. 46:849-858. [DOI] [PubMed] [Google Scholar]
- 39.Vandamme, P., B. Holmes, M. Vancanneyt, T. Coenye, B. Hoste, R. Coopman, H. Revets, S. Lauwers, M. Gillis, K. Kersters, and J. R. W. Govan. 1997. Occurrence of multiple genomovars of Burkholderia cepacia in cystic fibrosis patients and proposal of Burkholderia multivorans sp. nov. Int. J. Syst. Bacteriol. 47:1188-1200. [DOI] [PubMed] [Google Scholar]
- 40.Vandamme, P., M. Vancanneyt, B. Pot, L. Mels, B. Hoste, D. Dewettinck, L. Vlaes, C. Van Den Borre, R. Higgins, J. Hommez, K. Kersters, J.-P. Butzler, and H. Goossens. 1992. Polyphasic taxonomic study of the emended genus Arcobacter with Arcobacter butzleri comb. nov. and Arcobacter skirrowii sp. nov., an aerotolerant bacterium isolated from veterinary specimens. Int. J. Syst. Bacteriol. 42:344-356. [DOI] [PubMed] [Google Scholar]
- 41.von Graevenitz, A. 1995. Acinetobacter, Alcaligenes, Moraxella, and other nonfermentative gram-negative bacteria, p. 520-532. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology. ASM Press, Washington, D.C.