Abstract
Techniques to improve the sensitivity of smear microscopy would facilitate early tuberculosis (TB) diagnosis and disease control, especially in low-income countries where the positive predictive value is high. C18-carboxypropylbetaine (CB-18) is a zwitterionic detergent that helps to compensate for the innate buoyancy of mycobacteria, potentially enhancing recovery by centrifugation. Previous data suggest that CB-18 may increase the sensitivity of smear, culture, and molecular amplification diagnostic testing. The goal of the present study was to evaluate if the sensitivity of the smear technique using light microscopy could be improved by treating respiratory samples with CB-18. In the first phase, respiratory specimens were collected consecutively from patients with suspected pulmonary tuberculosis in a tertiary-care hospital in Rio de Janeiro, Brazil (236 specimens were analyzed). After protocol modifications, another 120 respiratory specimens were evaluated. The standard technique was N-acetyl-l-cysteine with sodium hydroxide (NALC-NaOH) treatment, smear concentration with centrifugation, and Ziehl-Neelsen staining. Culture on Löwenstein-Jensen slants was performed on all specimens for use as the “gold standard.” No specimens from patients undergoing active TB treatment were included. The initial protocol for CB-18 processing resulted in a sensitivity of 59.6% and specificity of 96.8% compared to standard processing with a sensitivity of 66.0% and specificity of 96.8%. Using the modified protocol, the sensitivity of CB-18 increased to 71.4% with a specificity of 97.0% versus standard processing with a sensitivity of 61.9% and a specificity of 99.0%. The diagnostic yield of acid-fast bacillus smear with CB-18 in the absence of fluorescence microscopy and PCR compared to standard processing with NALC-NaOH was not significantly different, although the power to detect a difference by the modified assay was low.
Tuberculosis (TB) remains one of the deadliest diseases worldwide, with an estimated 8.4 million new cases and over 2 million deaths each year (21). Smear microscopy is currently the most feasible microbiological method for diagnosis of pulmonary TB in developing countries due to its rapidity, low cost, and high positive predictive value for Mycobacterium tuberculosis (3). However, sputum smear microscopy with Ziehl-Neelsen staining is only 60 to 70% sensitive for the diagnosis of pulmonary TB compared with sputum culture (2, 8). Among human immunodeficiency virus-infected persons the sensitivity of sputum Ziehl-Neelsen smears has been reported to be even lower (2, 6, 10). Delayed or missed diagnoses contribute to M. tuberculosis transmission and mortality due to TB (9, 11).
Improvements in the sensitivity of smear microscopy would allow earlier diagnosis. TB programs could then optimize disease control from a public health standpoint as well as improve individual patient management (20). In previously published reports, homogenization and concentration of respiratory specimens by centrifugation have been shown to improve acid-fast bacillus (AFB) smear sensitivity (14, 20). Recently, another method for processing respiratory specimens using N,N-dimethyl-N-(n-octadecyl)-N-(3-carboxypropyl)ammonium inner salt, also known as C18-carboxypropylbetaine (CB-18), has been reported to result in a higher sputum smear sensitivity. CB-18 is a zwitterionic detergent that is taken up by viable mycobacteria, making them more dense and thereby increasing their recovery by centrifugation. It has been shown that CB-18 smear processing combined with the use of fluorescent stains significantly improves the sensitivities of AFB smears for M. tuberculosis (16-18). The purpose of this study was to compare light microscopy results of respiratory specimens processed by standard techniques and by CB-18 treatment for the diagnosis of pulmonary tuberculosis in a high- prevalence setting.
MATERIALS AND METHODS
Subjects and setting.
Study participants were drawn from the Outpatient and Inpatient Units of the Hospital Universitário Clementino Fraga Filho (HUCFF), Rio de Janeiro, Brazil. The first phase of the study included samples collected between October 1999 and March 2000, and the second phase included samples collected between July and September 2000. Consecutive respiratory samples sent to the laboratory from adult patients with suspected active pulmonary TB were included in the study. The reference standard for the diagnosis of pulmonary tuberculosis was culture growth of M. tuberculosis.
Approval for this study was granted by the Institutional Review Boards of HUCFF, the Brazilian Ministry of Health, and the Johns Hopkins Medical Institutions. Written informed consent was obtained from all study subjects.
Specimen collection.
Respiratory specimens submitted to the mycobacteriology laboratory with culture request were included. A total of 236 specimens were analyzed during the initial phase, and an additional 120 specimens were analyzed using the modified protocol. Each specimen with a volume of 5 ml or greater was split and processed by the standard digestion-decontamination method that combines N-acetyl-l-cysteine (NALC) and sodium hydroxide (NALC-NaOH) (3, 10, 15) and by the CB-18 method.
NALC-NaOH processing.
The respiratory samples were incubated for 20 min with 2.0% NALC-2.0% NaOH, and then centrifuged at 3,000 × g for 20 min. The samples were decanted, and the sediment was resuspended in the remaining supernatant. All specimens were then stained for AFB and cultured as described below.
CB-18 processing.
The CB-18 smear kit provided by the manufacturer (Integrated Research Technology, LLC, Baltimore, Md.) included 20× Tris-citrate buffer, CB-18 stock, and NALC. In the initial manufacturer's protocol, respiratory specimens were treated with a buffered solution of 1 mM CB-18 in Tris-citrate buffer (50 mM Tris-HCl, 12.5 mM citrate [pH 7.6], 1.5 mM NaCl) with 15 mM NALC. The samples were mixed with buffered CB-18 and immediately subjected to centrifugation at 3,000 × g for 20 min at 25°C. The samples were decanted, resuspended in the remaining supernatant, and then used to prepare smears for diagnostic evaluation. In the second phase of the study, the manufacturer introduced modifications that included (i) increasing the concentration of CB-18 to 4 mM, (ii) lowering the pH of the buffer to pH 6.0, and (iii) incubating specimens in the CB-18 solution for 90 min. In addition, in the second phase of the study, samples were initially homogenized in an equal volume of 15 mM NALC-50 mM NaOH-2.9% sodium citrate for 15 min prior to addition of the CB-18 solution. Following the incubation, samples were again subjected to centrifugation at 3,000 × g for 20 min, decanted, and then resuspended in the remaining supernatant for smear analysis.
AFB smears.
Smears made from the NALC-NaOH and CB-18 protocols were heat fixed and stained using the Ziehl-Neelsen (ZN) technique for AFB staining. A small number of specimens (85 specimens) processed with CB-18 were also stained with the Kinyoun staining technique as well as ZN. The smears were scanned at ×1,000 magnification with a light microscope. The results were quantified in accordance with published standards (7). For quality assurance, an experienced scientist in the Mycobacteriology Laboratory at HUCFF reread 10% of the negative smears and all the positive smears.
Culture.
A portion of each processed sediment was removed for smear analysis. The specimen was then resuspended in 2.5 ml of distilled water. Löwenstein-Jensen slants were inoculated with approximately 0.4 ml of the resuspended sediment and incubated at 37°C for 8 weeks. All specimens that were culture positive for mycobacteria were tested by standard biochemical methods to distinguish M. tuberculosis from nontuberculous mycobacteria, as described elsewhere (7, 13).
Statistical analyses.
Analyses were carried out using STATA 6.0 statistical software. Sensitivity, specificity, and positive and negative predictive values were calculated for each method. Fisher's test and McNemar's test were used for comparisons of results. Agreement between the two AFB staining methods was evaluated using the kappa score.
RESULTS
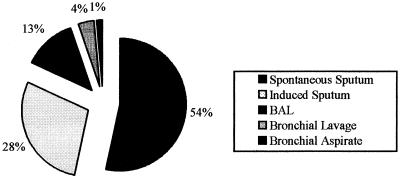
During the course of the study, 494 samples collected from patients suspected of having active pulmonary tuberculosis were processed by the NALC-NaOH and CB-18 processing methods. In the initial phase of the project, 368 specimens were collected; of those, 132 were not included in the analysis. Reasons for exclusion were flaking and washing away of the smear during staining after CB-18 processing (n = 53), anti-TB treatment started more than 4 weeks before sample collection (n = 18), culture contamination (n = 15), and failure to culture (n = 46). For the modified protocol, 126 specimens were collected; of those, 6 were lost and were not included in the analysis: flaking and washing away of the smear during staining with CB-18 processing (n = 1) and anti-TB treatment started more than 4 weeks before sample collection (n = 5). The 417 specimens evaluated included different types of respiratory specimens, including spontaneous sputum (54%), induced sputum (28%), bronchoalveolar lavage (13%), bronchial lavage (4%), and bronchial aspirate (1%) (Fig. 1). Samples other than spontaneous sputum showed no significant differences among techniques (standard, original and modified CB-18 protocols [data not shown]). Overall, 68 samples (16%) had cultures that grew M. tuberculosis.
FIG. 1.
Types and distribution of respiratory specimens from 356 patients (236 for the initial phase and 120 for the second phase of the study) at HUCFF. All samples were processed using both the standard method and the CB-18 method for bacillary recovery.
Comparison of processing methods.
In the first phase, M. tuberculosis culture growth was identified in 47 samples, and in the second phase another 21 samples were culture positive. The initial CB-18 protocol was slightly less sensitive than routine ZN smears, 59.6% (28 of 47) versus 66.0% (31 of 47) (P = 0.29). During the first phase of the study, there was no significant difference in the positive predictive value between NALC-NaOH decontamination and the CB-18 processing method (89.8 and 88.7%, respectively) using culture as the “gold standard.” The modified CB-18 protocol improved the sensitivity of CB-18 to 71.4% (15 of 21) over that of the routine protocol, which was 61.9% (13 of 21); however, this difference was not significant (P = 0.16). The specificities of both diagnostic techniques (CB-18 and NALC-NaOH) were both 96.8% (183 of 189) in the initial protocol and were 97.0% (96 of 99) and 99.0% (98 of 99), respectively, in the modified protocol. Even with the modification of the CB-18 processing method, the positive predictive value for CB-18 remained close to that of routine ZN due to the high specificity of both methods (Table 1). The modified CB-18 protocol showed an improvement in smear staining compared to the initial protocol, but the number of positive cultures was small (n = 21). The most notable difference between the two versions of CB-18 processing was in the readability of the slides. In the first phase, 14% of smears were not readable versus 1% (P = 0.00) using the modified protocol.
TABLE 1.
Evaluation of diagnostic methodsa
| Method (no. of samples) | No. of specimens culture positive | NALC-NaOH
|
CB-18
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Se (%) | Sp (%) | PPV (%) | NPV (%) | Se (%) | Sp (%) | PPV (%) | NPV (%) | ||
| NALC-NaOH/CB-18b initial protocol (n = 236) | 47 | 66.0 (31/47)c | 96.8 (183/189)d | 89.8 | 55.0 | 59.6 (28/47)c | 96.8 (183/189)d | 88.7 | 50.1 |
| NALC-NaOH/CB-18 modified protocol (n = 120) | 21 | 61.9 (13/21)c | 99.0 (98/99)d | 96.4 | 52.7 | 71.4 (15/21)c | 97.0 (96/99)d | 91.1 | 59.2 |
Se, sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value.
P values not statistically significant.
Value in parentheses is the number of specimens positive by smear/number of culture-positive specimens.
Value in parentheses is the number of specimens negative by smear/number of culture-negative specimens.
Comparison of AFB staining techniques.
A comparison of two staining techniques used in AFB smear analysis was performed on 85 of the samples processed using CB-18. Kinyoun staining was compared to ZN staining to determine if there was any difference that would affect the performance of CB-18 in diagnostic use. There was fair agreement between the two techniques (overall agreement = 72.9%; kappa test = 0.715). However, 22% of Kinyoun slides and 1% of ZN slides were unreadable due to technical problems (Table 2).
TABLE 2.
Evaluation of AFB techniques
| Stain | No. of samples
|
||
|---|---|---|---|
| Positive | Negative | Unreadable | |
| ZN | 11 | 73 | 1 |
| Kinyoun | 5 | 61 | 19 |
DISCUSSION
The CB-18 technique is based on the use of the zwitterionic detergent C18-carboxypropylbetaine. Previous reports of increases in both smear and culture sensitivity were believed to result from a reduced impact on viability, alteration of the buoyant density of the mycobacteria to enhance collection efficiency during centrifugation, and dispersion of those mycobacteria that cord (16, 17). In a previous study the CB-18 processing method used specimens derived from clinical laboratories in the United States. Those authors reported an increase in smear sensitivity for M. tuberculosis from 69.0% with NALC-NaOH to 93.1% with CB-18 (P < 0.01) (16).
We evaluated this technique under field conditions in a high-prevalence, resource-challenged setting. The initial CB-18 protocol was slightly less sensitive than routine smears, 59.6% (28 of 47) versus 66.0% (31 of 47). The modified CB-18 protocol improved the sensitivity of CB-18 to 71% (15 of 21) over that of the routine protocol, which was 62% (13 of 21). There were no significant differences in the sensitivity or specificity of the two methods for either version of CB-18 processing. The power of this study to detect differences in the two techniques was limited by the number of positive cultures (n = 68), especially in the second phase, where there were only 21 positive specimens.
The sensitivity of smear improved when the modified CB-18 protocol was used, but the increased length of time required (90-min incubation for CB-18) limits its routine utility in this setting. These results are consistent with the findings of another recent evaluation of CB-18. Comparison of a sodium dodecyl sulfate-sodium hydroxide processing method with a CB-18 processing protocol that included no incubation step failed to show improvements in smear sensitivity (12). When a 90-min incubation step was included in the procedure, there was a small improvement in the CB-18-based smear analyses. While CB-18 failed to show previously described improvements in the diagnostic evaluation of TB patients under field conditions, a potential limitation of this study is that M. tuberculosis culture (Löwerstein-Jensen medium) was used as the reference standard for determining diagnostic effectiveness, and culture may be imperfect in identifying all active TB cases. However, due to the high specificity of both techniques among our samples, it appears that using culture as the gold standard did not compromise our field evaluation of CB- 18.
The key difference between this study and those that have previously shown a higher smear sensitivity with CB-18 processing is the use of fluorochrome staining methods in the earlier studies. Auramine-rhodamine stain is a more sensitive technique, which could account for the discrepancy between the data presented here and those of Thornton et al. (16-18). We compared two fuchsin stain procedures and found that ZN staining identified twice as many positive smears as Kinyoun staining. ZN staining proved to be more efficient than Kinyoun staining for analyzing CB-18- processed specimens because many Kinyoun-stained slides were unreadable.
Although our study was not able to demonstrate an improvement in smear light microscopy using CB-18 compared to digestion-decontamination with NALC-NaOH followed by centrifugation, CB-18 may be more useful when used in conjunction with nucleic acid amplification, fluorescence staining, or other types of specimens. Published reports have shown an improvement in the recovery of mycobacteria other than tuberculosis among human respiratory specimens and also among animal products such as milk (5, 15, 16, 19). CB-18 has also been used for preparing samples for amplification and has been associated with an improvement in PCR results compared to other standard techniques (1, 4, 15).
In conclusion, in our setting neither the original nor the modified CB-18 protocol showed a significant increase in diagnostic yield of light microscopy smear compared to NALC-NaOH decontamination and concentration by centrifugation. A larger sample size may have detected a difference, however. CB-18 sample processing may be more useful when used in conjunction with more sensitive techniques for M. tuberculosis detection such as fluorescence microscopy and PCR amplification. Further field evaluation to address these issues is warranted.
Acknowledgments
This work was supported by the International Collaboration for Infectious Diseases Research, NIH grants AI 45432 and K24 AI 01637, and Fogarty Grant 2D43TW000010. CB-18 kits were kindly provided by Integrated Research Technologies, LLC.
We thank Aline Barros, Fábio C. Malta, and Elisabeth O. Santos for assistance with laboratory procedures.
REFERENCES
- 1.Antognoli, M. C., M. D. Salman, J. Triantis, J. Hernandez, and T. Keefe. 2001. A one-tube nested polymerase chain reaction for the detection of Mycobacterium bovis in spiked milk samples: an evaluation of concentration and lytic techniques. J. Vet. Diagn. Investig. 13:111-116. [DOI] [PubMed] [Google Scholar]
- 2.Colebunders, R., and I. Bastian. 2000. A review of the diagnosis and treatment of smear-negative pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 4:97-107. [PubMed] [Google Scholar]
- 3.Conde, M. B., C. M. Figueira, R. Moraes, L. S. Fonseca, K. Deriemer, and A. L. Kritski. 1999. Predictive value of the acid fast smear for detection of Mycobacterium tuberculosis in respiratory specimens in a reference center of HIV/AIDS in Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz 94:787-790. [DOI] [PubMed] [Google Scholar]
- 4.Cornejo, B. J., A. Sahagun-Ruiz, F. Suarez-Guemes, C. G. Thornton, T. A. Ficht, and L. G. Adams. 1998. Comparison of C18-carboxypropylbetaine and glass bead DNA extraction methods for detection of Mycobacterium bovis in bovine milk samples and analysis of samples by PCR. Appl. Environ. Microbiol. 64:3099-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dundee, L., I. R. Grant, H. J. Ball, and M. T. Rowe. 2001. Comparative evaluation of four decontamination protocols for the isolation of Mycobacterium avium subsp. paratuberculosis from milk. Lett. Appl. Microbiol. 33:173-177. [DOI] [PubMed] [Google Scholar]
- 6.Elliott, A. M., K. Namaambo, B. W. Allen, N. Luo, R. J. Hayes, J. O. Pobee, and K. P. McAdam. 1993. Negative sputum smear results in HIV-positive patients with pulmonary tuberculosis in Lusaka, Zambia. Tuberc. Lung Dis. 74:191-194. [DOI] [PubMed] [Google Scholar]
- 7.Kent, P. T., and G. P. Kubica. 1995. Public health mycobacteriology. Guide for the level III laboratory. Centers for Disease Control, Atlanta, Ga.
- 8.Levy, H., C. Feldman, H. Sacho, H. van der Meulen, J. Kallenbach, and H. Koornhof. 1989. A reevaluation of sputum microscopy and culture in the diagnosis of pulmonary tuberculosis. Chest 95:1193-1197. [DOI] [PubMed] [Google Scholar]
- 9.Lienhardt, C., J. Rowley, K. Manneh, G. Lahai, D. Needham, P. Milligan, and K. P. McAdam. 2001. Factors affecting time delay to treatment in a tuberculosis control programme in a sub-Saharan African country: the experience of The Gambia. Int. J. Tuberc. Lung Dis. 5:233-239. [PubMed] [Google Scholar]
- 10.Long, R., M. Scalcini, J. Manfreda, M. Jean-Baptiste, and E. Hershfield. 1991. The impact of HIV on the usefulness of sputum smears for the diagnosis of tuberculosis. Am. J. Public Health 81:1326-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacIntyre, C. R., A. J. Plant, J. Hulls, J. A. Streeton, N. M. Graham, and G. J. Rouch. 1995. High rate of transmission of tuberculosis in an office: impact of delayed diagnosis. Clin. Infect. Dis. 21:1170-1174. [DOI] [PubMed] [Google Scholar]
- 12.Manterola, J. M., C. G. Thornton, E. Padilla, J. Lonca, I. Corea, E. Martinez, and V. Ausina. Comparison of the sodium dodecyl sulfate-sodium hydroxide specimen processing method with the C18-carboxypropylbetaine specimen processing method using the MB/BacT liquid culture system. Eur. J. Clin. Microbiol. Infect. Dis., in press. [DOI] [PubMed]
- 13.Nolte, F. S., and B. Metchock. 1995. Mycobacterium, p. 400-437. In P. R. Murray, E. J. Baron, and M. A. Pfaller (ed.), Manual of clinical microbiology, 6th ed. American Society for Microbiology, Washington, D.C.
- 14.Peterson, E. M., A. Nakasone, J. M. Platon-DeLeon, Y. Jang, L. M. de La Maza, and E. Desmond. 1999. Comparison of direct and concentrated acid-fast smears to identify specimens culture positive for Mycobacterium spp. J. Clin. Microbiol. 37:3564-3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thornton, C. G., M. R. Cranfield, K. M. MacLellan, T. L. Brink, Jr., J. D. Strandberg, E. A. Carlin, J. B. Torrelles, J. N. Maslow, J. L. Hasson, D. M. Heyl, S. J. Sarro, D. Chatterjee, and S. Passen. 1999. Processing postmortem specimens with C18-carboxypropylbetaine and analysis by PCR to develop an antemortem test for Mycobacterium avium infections in ducks. J. Zoo Wildl. Med. 30:11-24. [PubMed] [Google Scholar]
- 16.Thornton, C. G., K. M. MacLellan, T. L. Brink, Jr., D. E. Lockwood, M. Romagnoli, J. Turner, W. G. Merz, R. S. Schwalbe, M. Moody, Y. Lue, and S. Passen. 1998. Novel method for processing respiratory specimens for detection of mycobacteria by using C18-carboxypropylbetaine: blinded study. J. Clin. Microbiol. 36:1996-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thornton, C. G., K. M. MacLellan, T. L. Brink, Jr., and S. Passen. 1998. In vitro comparison of NALC-NaOH, Tween 80, and C18-carboxypropylbetaine for processing of specimens for recovery of mycobacteria. J. Clin. Microbiol. 36:3558-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thornton, C. G., K. M. MacLellan, T. L. Brink, Jr., D. M. Wolfe, O. J. Llorin, and S. Passen. 1998. Processing respiratory specimens with C18-carboxypropylbetaine: development of a sediment resuspension buffer that contains lytic enzymes to reduce the contamination rate and lecithin to alleviate toxicity. J. Clin. Microbiol. 36:2004-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thornton, C. G., K. M. MacLellan, J. R. Stabel, C. Carothers, R. H. Whitlock, and S. Passen. 2002. Application of the C(18)-carboxypropylbetaine specimen processing method to recovery of Mycobacterium avium subsp. paratuberculosis from ruminant tissue specimens. J. Clin. Microbiol. 40:1783-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkinson, D., and A. W. Sturm. 1997. Diagnosing tuberculosis in a resource-poor setting: the value of sputum concentration. Trans. R. Soc. Trop. Med. Hyg. 91:420-421. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization Global Tuberculosis Programme. 2001. WHO Report 2001: global tuberculosis control. World Health Organization, Geneva, Switzerland.