Abstract
Background: Cognitive therapy (CT) may reduce depressive relapse and recurrence when patients learn and use the associated skills. Reported relapse and recurrence rates after CT discontinuation vary widely. The factors that determine when CT is preventive remain unidentified. We developed continuation-phase CT (C-CT) to teach responders skills to prevent relapse. This is the first randomized trial comparing CT with and without a continuation phase in responders to CT who were vulnerable, given their history of recurrent unipolar depression.
Methods: Patients aged 18 to 65 years (n=156) with recurrentDSM-IV major depressive disorder (MDD) entered 20 sessions of acute-phase CT (A-CT). Unmedicated responders (ie, no MDD and 17-item Hamilton Rating Scale for Depression score ≤9; n=84) were randomized to either 8 months (10 sessions) of C-CT or control (evaluation without CT). Follow-up lasted an additional 16 months. A clinician blind to assignment evaluated relapse and recurrence (ie, DSM-IV MDD).
Results: Over an 8-month period, C-CT significantly reduced relapse estimates more than control (10% vs 31%). Over 24 months, including the CT-free follow-up, age of onset and quality of remission during the late phase of A-CT each interacted with condition assignment to influence durability of effects. In patients with early-onset MDD, C-CT significantly reduced relapse and recurrence estimates (16% vs 67% in control). When patients had unstable remission during late A-CT, C-CT significantly reduced relapse and recurrence estimates to 37% (vs 62% in control).
Conclusions: Findings suggest that 8 months of C-CT significantly reduces relapse and recurrence in the highest-risk patients with recurrent MDD. Risk factors influenced the necessity for C-CT.
PATIENTS WHO have recovered from recurrent major depressive disorder (MDD) face an 80% rate of recurrence in the absence of prophylactic treatment.1 To reduce risk, psychopharmacologists prescribe continuation-phase antidepressant medication to prevent relapse (ie, the index episode continued) and maintenance-phase medication to prevent recurrence (ie, a new episode distinct from the index episode). In contrast, continued preventive therapy is not routine practice after response to the acute phase of depression-specific psychosocial interventions.
Prevention in the absence of ongoing treatment would distinguish cognitive therapy (CT) from pharmaco-therapy. Acute-phase CT (A-CT) could reduce the likelihood of relapse and recurrence even after it is discontinued,2 perhaps because patients learn and continue to use effective tools of symptom reduction. For example, prevention may follow discontinued therapy if the patient has developed a constructive thinking pattern and/or activates skills that reduce symptoms effectively.
Estimates of relapse and recurrence after A-CT lasting approximately 16 to 20 weeks have been as high as 74% and as low as 21% over 2 years.3,4 The extent of and conditions affecting reliable and effective prevention remain unidentified.
The durability of gains achieved with various types, rates, and phases of psychosocial interventions for depression is not well established. Only a few studies have evaluated the longer-term effects of discontinued A-CT,3-7 discontinued interpersonal psychotherapy,8 or either treatment in its continuation or maintenance phase.1,3,9-11 Study designs have consisted of acute-phase treatments identical to the continuation-phase treatment3,10,11 or different from the continuation and maintenance phases. 1,9,11-14
PATIENTS AND METHODS
PATIENTS
The recruitment and protocol were approved by the institutional review board. Patients (n>3500; recruited through media, announcements, and referrals) were triaged by telephone. If potentially eligible, patients were scheduled for evaluation (n>1200).
Outpatients (n=608) presented for evaluation at the Department of Psychiatry at The University of Texas Southwestern Medical Center at Dallas with the complaint of depression. Inclusion criteria involved the following: (1) DSMIV16 nonpsychotic unipolar MDD; (2) recurrent MDD with clear interepisode recovery (ie, 2 or more episodes of MDD separated by at least 2 months of a return to more-or-less normal functioning); (3) 17-item Hamilton Rating Scale for Depression17 (HRSD-17) score of 16 or higher at initial diagnostic evaluation and at a follow-up; and (4) written informed consent.
Trained evaluators, using the Structured Clinical Interview forDSM-III-R (SCID outpatient version)18 and strictly applyingDSM-III-R19 andDSM-IV criteria for MDD and other disorders, referred 361 patients (59%) for nonprotocol treatment. Exclusion criteria were (1) did not meet criteria for MDD (n=91); (2) MDD not recurrent (n=116); (3) HRSD-17 score less than 16 (n=66); (4) contraindicated medical condition or medication (n=28); (5) exclusionary comorbid psychiatric disorder (MDD with psychotic features, n=2; current alcohol or other drug abuse, n=9; primary sleep, eating, or sexual disorders, n=1 each; and borderline personality disorder, n=7); (5) imminent suicide risk at triage (n=2); or (6) inability to comply with the protocol (n=24). Thirteen patients were lost to follow-up after the initial clinic contact and were mailed referrals.
The remaining 247 patients completed the SCID and supplemental questions to allow evaluation ofDSM-IV diagnoses, entry criteria, and the characteristics described in the Table.
A faculty-level diagnostician reassessed eligibility criteria at a follow-up interview. Fifty patients were excluded because they (1) did not meet criteria for MDD (n=13); (2) had nonrecurrent MDD (n=12); (3) had a concurrent medical disorder or received treatment that might cause depressive symptoms (n=11); or (4) had comorbid exclusionary psychiatric disorders (MDD with psychotic features, n=7; delusional disorder, n=1; current alcohol or other drug abuse, n=6). An additional 36 patients were excluded because they (1) had an HRSD-17 score less than 16 at follow-up (n=16); (2) had primary panic disorder with agoraphobia (n=1); (3) had primary bulimia nervosa (n=1);(4) had borderline personality disorder (n=6); (5) could not complete questionnaires or comply with the protocol (n=10); or (6) preferred alternative treatment (n=2).
A total of 161 patients were eligible for the study and 5 refused consent; thus, 156 patients consented to enter A-CT.
STUDY PROCEDURES
Pharmacotherapy was not a study procedure. From entry through follow-up, patients agreed to postpone or report the use of psychotropic medication, nonprotocol psychotherapy, or other psychiatric or psychosocial treatment.
Acute-Phase Cognitive Therapy
Acute-phase cognitive therapy was conducted as described by Beck et al,2 within a 12- to 14-week protocol (twenty 50- to 60-minute individual sessions held twice weekly for the first 8 weeks and once weekly for the last 4 weeks). The therapists completed clinician rating scales. Strategies were focused on symptom reduction but could include relapse prevention.
Five experienced therapists provided CT. Each had completed 1 or more years of CT training, achieving and maintaining Cognitive Therapy Scale24 (CTS) scores of 40 or more before treating any study patients.
An off-site consultant (see the acknowledgments at the end of the article) used the CTS to evaluate competence and provide feedback. Therapists received weekly group supervision.
Randomization to the Experimental Phase
Only responders (no MDD and HRSD-17 score of 9 or less by a blind evaluator) who completed 20 sessions of A-CT and consented to randomization to either C-CT or evaluation only (control) entered the 8-month experimental phase. Responders were randomized using PROC PLAN in SAS statistical software, version 6.04 (SAS Institute Inc, Cary, NC) by strata that included the following: (1) number of episodes (ie, 2 vs ≥3); (2) HRSD-17 score less than 6 and 6 to 9 based on the blind evaluator’s score collected within 7 days of session 20; and (3) double depression (presence or absence ofDSM-IV dysthymia before onset of the presenting episode). Research personnel and patients concealed assignment from blind evaluators.
In both conditions, patients agreed to remain unmedicated and were scheduled for 10 sessions that occurred biweekly for the first 2 months and monthly for the remaining 6 months. The clinician collected self-report questionnaires, assessed diagnostic status according to DSM-IV MDD, recorded any medication use, and completed rating scales. Regardless of diagnostic status, all patients proceeded with C-CT or evaluation until consent was withdrawn or through month 8. All patients were instructed to telephone the evaluator if they became symptomatic between visits. When patients experienced relapse or recurrence, they were referred for additional treatment (eg, pharmacotherapy).
Continuation-Phase Cognitive Therapy
The same therapist provided A-CT and 10 sessions of C-CT as described by Jarrett (unpublished manual available on request) and elaborated by Jarrett and Kraft.15 Patients were taught to use emotional distress or symptoms to trigger coping skills learned in A-CT. The purpose of C-CT is to prevent relapse and recurrence, review strategies associated with effective symptom reduction, maintain skills acquired during A-CT, and develop coping strategies in preparation for identified or anticipated vulnerabilities. Whereas A-CT emphasized reducing symptoms and acquiring skills, C-CT emphasized preventing relapse and recurrence, reducing residual symptoms, and generalizing skills.
Most sessions lasted 60 minutes, although 90 minutes was allowable. Patients received no monetary incentives.
Evaluation Only (Control)
An evaluator who had not provided A-CT conducted 10 assessment visits scheduled at the same frequency as C-CT for 8 months. Prescribed control procedures prohibited the use of CT or other psychosocial interventions before relapse. When patients described psychosocial problems, the evaluator did not intervene. Self-reports and clinician ratings were collected. Sessions lasted approximately 20 to 30 minutes, and patients were paid $25 for each visit.
Longitudinal Follow-up Phase
All consenting patients attended follow-up assessments for 16 months or until they withdrew consent. When clinic assessments were not feasible, evaluations were conducted by telephone. An evaluator followed up the patient monthly for the first 4 months and bimonthly for the remaining 12 months. Procedures were identical to the control cell.
OUTCOME MEASURES
The primary dependent variables were the proportion of DSM-IV diagnoses of MDD (ie, relapse or recurrence) as specified by an evaluator who was blind to cell assignment and used the Longitudinal Interval Follow-up Evaluation25 (LIFE). Blind evaluations were conducted at (1) the end of A-CT; (2) any time the patient, therapist, or follow-up evaluators suspected relapse or recurrence; (3) early exit; (4) months 4 and 8 of the experimental phase; and (5) months 12 and 24 of follow-up. Assessment for months 13 to 20 was conducted by unblinded evaluators because of the high cost of blind evaluations. If relapse or recurrence was suspected, a blind evaluation occurred.
The following definitions were used to conceptualize and define change points in the course of unipolar MDD.26
Response, the extent of patient response to A-CT, was defined by the blind evaluator within 7 days after session 20 as (1) absence ofDSM-IV MDD and (2) an HRSD-17 score of 9 or less. Remission, when the patient is no longer fully symptomatic, was defined by a diagnostician (ie, therapist, blind evaluator, or other clinic evaluator) specifying for 6 consecutive weeks that (1) DSM-IV criteria for MDD were not met and (2) HRSD-17 score was 6 or less (during the acute phase) or the LIFE Psychiatric Rating Scale (PSR) rating was 1 or 2 (during experimental and follow-up phases). Recovery, the end of an episode, was defined as any project diagnostician declaring for 8 consecutive months that (1) DSM-IV criteria for MDD were not met and (2) HRSD-17 score was 6 or less (during the acute phase) and/or the LIFE PSR rating was 1 or 2 (during experimental and follow-up phases). Relapse, a continuation of the presenting episode, was defined by the blind evaluator as symptoms meetingDSM-IV criteria for MDD (ie, LIFE PSR score of 5 or 6 for 2 weeks) before the criteria for recovery were met. Recurrence, the emergence of a new episode distinct from the presenting episode, was defined by the blind evaluator as meeting DSM-IV criteria for MDD after the criteria for recovery were met.
STATISTICAL ANALYSES
The hypothesized relapse rates over the 8-month experiment were 47% for control and 19% for C-CT based on early analyses of previous samples.3 A minimum sample size of 72 was necessary to detect a significant difference.27 Recurrence estimates were reported as secondary measures during the 24-month follow-up.
All analyses were of an intention-to-treat strategy (N=84; n for C-CT=41; n for controls=43). The end point in all the survival analyses was either relapse or recurrence. Patients who dropped out or survived were censored. Survival curves and relapse and recurrence rates were estimated using Kaplan-Meier product-limit methods.28 The survival rates for the 2 cells were compared using the log-rank test.29 The Cox proportional hazard regression was used to evaluate the influence of each candidate covariate (age of onset, age, sex, number of episodes, length of current episode, comorbid lifetime diagnoses, and definite Research Diagnostic Criteria21 endogenous diagnosis) as described by Collett.30 Covariates were compared with the null model to determine their influence on relapse over 8 months or on relapse and recurrence over 24 months without regard to condition assignment.
Categorical variables were reported as percent frequency (eg, number and percentage) and continuous variables as mean and SE. Significance was defined as P≤.05; Fisher exact tests (FETs) were 2-tailed.
We have focused on evaluating relapse and recurrence rates in patients who respond to 3 to 4 months of CT. We reported high rates of relapse and recurrence over a 2-year follow-up.3 After discontinuing CT, relapse and recurrence rates were 45% over 8 months, 50% over 1 year, and 74% over 2 years. These data motivated us to develop and test continuation-phase CT (C-CT)15 in a second cohort of patients who responded to A-CT. The cohort receiving C-CT had reduced relapse rates of 20% after 8 months of A-CT, 27% after 1 year, and 36% after 2 years.3 Since the patients were not randomized, the results were inconclusive, which prompted us to design the current trial.
To our knowledge, this is the first randomized clinical trial to compare CT with and without a continuation phase in CT responders who had presented with recurrent MDD. The hypothesis was that C-CT provided for 8 months would reduce relapse rates significantly more than the control (assessment only). Secondary aims included examining effects for 2 years to evaluate the durability of A-CT and C-CT and to identify predictors of both relapse and recurrence.
RESULTS
SAMPLE CHARACTERISTICS
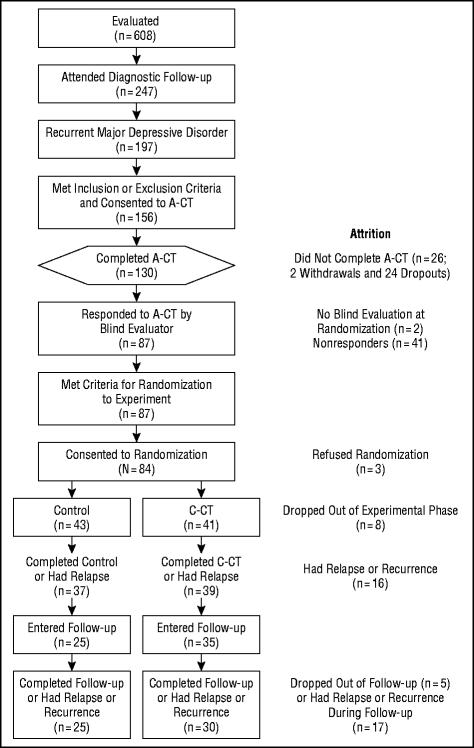
Figure 1 enumerates the sample composition. Eligible outpatients (n=156) with recurrent MDD and clear interepisode recovery entered A-CT. Eligible responders (n=84) consented to randomization. The demographic and clinical characteristics of responders randomized to the 2 cells, and tests of differences are reported in the Table.
Figure 1.
Flow diagram for patients through all study phases, including attrition. Subjects (n=156) were registered in the study on consenting to acute-phase cognitive therapy (A-CT). Eligible responders were randomized to the clinical trial (N=84). C-CT indicates continuation-phase CT.
PATIENT DISPOSITION
Of the 156 patients who entered A-CT, 130 (83%) completed 20 sessions (2 patients became psychotic or homicidal and were withdrawn; 24 dropped out). Of these 130 patients, 128 (98%) consented to the first blind evaluation. Of these 128, 87 (68%) were judged to have responded to A-CT. Eighty-four (97% of those eligible) consented to randomization (1 patient refused to discontinue treatment with the A-CT therapist, 1 sought alternative treatment, and 1 had scheduling conflicts).
Seventy-six (90%) of the 84 randomized patients completed 10 sessions of the 8-month experiment or had a relapse or recurrence. When dropouts (n=8) were compared with completers (ie, had 10 sessions or had a relapse or a recurrence; n=76), 2 significant differences emerged. Dropouts had fewer previous episodes of depression (ǀtǀ 15.5=4.03, P=.001) and more had double depression (FET, P=.04). There was no significant difference in the number of patients who completed C-CT (95%; 39 of 41) compared with controls (86%; 37 of 43; FET, P=.28). One patient dropped out of C-CT because of a scheduling conflict and 1 failed to attend the first session. The 6 patients who dropped out of the control cell reported that the study was taking too much time.
Sixty responders survived the experiment and entered follow-up. Of these, 92% (n=55) completed 16 months of follow-up or experienced a relapse or recurrence. The percentage of patients completing follow-up in the 2 cells did not differ significantly (C-CT=86%, 30 of 35; control=100%, 25 of 25; FET, P=.08).
PATIENT COMPLIANCE
When relapse status was disregarded, the Wilcoxon rank test showed that neither the number of sessions (z=1.8, P=.07) nor the number of months (z=0.43, P=.67) differed in the 2 conditions. The 41 responders randomized to C-CT completed an average of 9.49 ± 0.30 (mean ± SE; mode = 10) sessions during an average of7.26±0.25 months (mode=7.66). The 43 responders randomized to the control cell completed an average of 9.05±0.29 sessions (mode=10) during an average of 7.03±0.26 months (mode=7.59).
At randomization, all patients planned to defer psychiatric treatment until they experienced a relapse or recurrence or the study ended. Only 10 patients reported seeking psychiatric “treatment” lasting 2 or more weeks before relapse or recurrence. During the experimental phase, 2 patients reported “treatment”: 1 C-CT patient took methylphenidate hydrochloride (Ritalin) for 8 weeks and 1 control patient had 4 sessions of “peer support” for grief. None reported depressive symptoms (ie, PSR score ≥4) 4 weeks before or during the interval in which they received treatment.
During the follow-up phase and before relapse or recurrence, 8 patients (4 in C-CT and 4 in control) reported “treatment” for 2 or more weeks. One patient from C-CT who reported depressive symptoms also received 4 sessions of psychotherapy. The following treatment occurred during intervals in which the patients did not report depressive symptoms (ie, PSR score ≥4): 1 C-CT patient sought marital therapy with the cognitive therapist during longitudinal follow-up (36 sessions); 1 C-CT patient attended 2 sessions in a “women’s employment support group”; 2 patients (1 C-CT and 1 control) received 2 to 3 psychotherapy or family therapy sessions; 1 control patient had 19 sessions of marital therapy; 1 control patient took 5 conflict resolution classes with his partner; and 1 control patient reported taking melatonin for 3 weeks.
THERAPIST COMPETENCE
Of 210 total CTS ratings during A-CT, only 18 scores(8.6%) fell below 40. The mean A-CT CTS score was 47.10±0.35 (mode=52) with all therapists achieving mean scores of more than 40 (when CTS ratings included more than 1 A-CT session from the same patient, a mean was calculated and used in the analysis). Although an analysis of variance (ANOVA) showed statistically significant differences in therapists’ scores (F4=4.51, P=.002), the mean score for each therapist was more than 46. The ANOVA produced nonsignificant effects across years (F3=1.98, P=.12). A Cox regression indicated no relation between therapist’s mean A-CT CTS score and patient’s relapse status (χ21=.09, P=.77).
Of 40 total CTS ratings during C-CT, only 6 scores (15%) were below 40. The mean C-CT CTS score was 46.3±1.17 (mode=46) with all therapists achieving mean scores of more than 40. The ANOVAs showed no statistically significant differences in mean CTS scores across therapists (F4=1.21, P=.32) or across years (F3=2.56, P=.07).
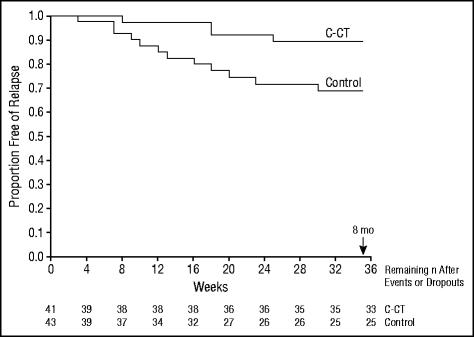
The primary aim of the trial was to test the hypothesis that C-CT reduced relapse more than control (ie, assessment only) during the 8 months in which C-CT was provided. Patients who did not relapse or drop out during the 8 months were censored after 8 months. A log-rank test showed that during the 8 months of C-CT, it reduced the proportion of relapse significantly more than the control (relapse estimate: 10.3% vs 30.9%; χ21=5.26, P=.02; Figure 2).
Figure 2.
Kaplan-Meier survival curves estimating time until relapse (DSM-IV major depressive disorder diagnosed by blind evaluation) during the 8-month (35-week) experiment. A log-rank test showed that continuation-phase cognitive therapy (C-CT) reduced the proportion of relapse significantly more than control (CT-free evaluation) (χ21=5.26, P=.02). Estimated relapse was 10% for C-CT patients and 31% for controls (n=41 and n=43, respectively).
SECONDARY ANALYSES
Both age of onset and patient age were identified as potential covariates (P=.10) by influencing relapse over 8 months (age, χ21=3.17, P=.09; age of onset, χ21=9.32, P=.002) and relapse or recurrence over 24 months (age, χ21=6.43, P=.01; age of onset, χ21=4.40, P=.04). The correlation between age and age of onset for the randomized sample (n=84) was 0.5 (P<.001). Age of onset was used as the covariate because previous studies show that it predicted relapse and recurrence.31,32 A comparison of Akaike Information Criteria30 verified that age of onset by itself was as good a predictor as both age and the 2 variables combined.
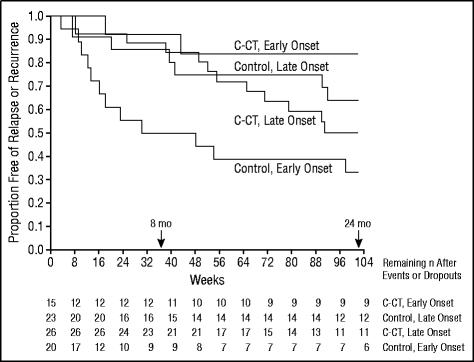
Since age of onset was identified as a covariate, the sample was divided into 2 strata (onset ≤18 years vs onset >18 years) using an approximate median split. A log-rank test for 8 months’ experimental phase revealed a significant interaction between age of onset and condition (χ23=14.22, P=.003; Figure 3). Post hoc results for 8 months’ experimental phase replicated the following pattern found throughout 24 months.
Figure 3.
Kaplan-Meier survival curves estimating time until relapse or recurrence (DSM-IV major depressive disorder diagnosed by blind evaluation) during 24 months (102 weeks). A log-rank test revealed significant interaction between condition (continuation-phase cognitive therapy [C-CT] or control [CT-free evaluation]) and age of onset (early, ≤18 years; late, >18 years) (χ23=10.09, P=.02).
For 24 months after randomization, a log-rank test revealed a significant interaction between age of onset and condition (χ23=10.09, P=.02; Figure 3). Control patients with an earlier age of onset were significantly more likely to experience a relapse than were those with later onset (67% vs 36%; χ21 = 4.34, P = .04). Patients with an earlier onset were significantly less likely to experience a relapse or recurrence if they received C-CT than if they did not (16% vs 67%; χ21= 6.78, P = .009). There was no difference in the relapse and recurrence estimates for patients with early and late onset who were treated with C-CT (16% vs 50%; χ21=2.71, P=.10). Similarly, there was no significant difference in the relapse and recurrence estimates for patients with later-onset MDD who did and did not receive C-CT (50% vs 36%; χ21=0.549, P=.47).
Since previous studies4,7,13,14,33-35 showed that quality of acute-phase remission predicted relapse or recurrence, we hypothesized that C-CT might reduce relapse or recurrence when remission was unstable in late-phase A-CT. Based on the work of Thase and associates,7 we formed 2 strata to determine whether the pattern of remission interacted with condition to influence the cumulative survival rates. Fifty-two (62%) of 84 patients showed “unstable” remission (1 or more HRSD-17 scores were ≥7 during the 6 final A-CT sessions and first blind evaluation spread over an average of 6.70±0.13 weeks). Thirty-two (38%) of 84 patients showed “stable” remission (all 7 HRSD-17 scores were <7 over an average of 6.34±0.15 weeks).
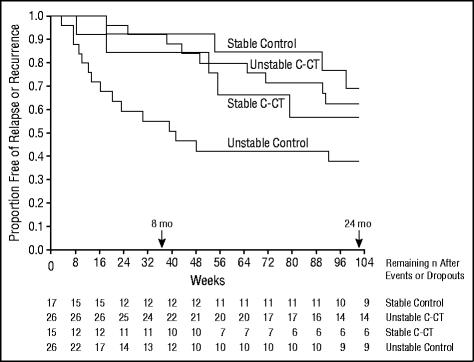
An overall log-rank test comparing the survival curves throughout 8 months for condition and stability of remission showed that the interaction between condition and stability was significant (χ23=14.96, P=.002; Figure 4). Pairwise comparisons using log-rank tests replicated the following pattern found throughout 24 months.
Figure 4.
Kaplan-Meier survival curves estimating time until relapse or recurrence (DSM-IV major depressive disorder diagnosed by blind evaluation) during 24 months (102 weeks). A log-rank test produced significant interaction between condition (continuation-phase cognitive therapy [C-CT] or control [CT-free evaluation]) and stability of acute-phase CT remission (stable and unstable) (χ23=8.67, P=.03).
Over 24 months, the log-rank test comparing survival curves for condition and stability of remission also showed a significant interaction (χ23=8.67, P=.03; Figure 4). Pairwise comparisons using log-rank tests showed that patients with an unstable remission were significantly more likely to experience a relapse or recurrence without C-CT (62%) than with C-CT (37%; χ21=5.36, P=.02). Of the patients without C-CT, those who had an unstable pattern of remission were more likely to experience a relapse or recurrence than were those with a stable pattern of remission (62% vs 30%; χ21=4.70, P=.03). All other comparisons were nonsignificant.
COMMENT
To our knowledge, this is the first randomized clinical trial that compares CT with and without a continuation phase in CT responders who remain at high risk for relapse and recurrence because of a history of recurrent depression. Eight months of C-CT focusing on residual symptoms, relapse prevention, and consolidation of skills reduced estimated relapse rates significantly more than the control. The 24-month analyses revealed that in patients at higher risk for relapse and recurrence (ie, those with early onset of their first depression or unstable remission late in the acute phase), C-CT reduced rates even after therapy was discontinued. This finding suggests that in vulnerable patients, the effects of C-CT may endure after discontinuation, distinguishing the effects of CT from those of pharmacotherapy. Furthermore, the estimates of relapse and recurrence during 2 years in the higher-risk patients treated with C-CT are comparable to rates observed during continuation and maintenance pharmacotherapy.36 When the higher-risk patients received C-CT, their estimated relapse or recurrence did not differ from those at lower risk who had only A-CT. Specifying these interactions between C-CT and unstable remission or age of onset in robust prediction of relapse or recurrence adds to similar, emerging literature.4,7,13,14,23,31-34,37
The major limitation of this study is that the sample size decreases over time. The findings require replication. Changing any combination of the parameters defining the sample or treatment may affect generalizability. This study design differs from studies with (1) CT in all 3 phases, (2) pharmacotherapy in all 3 phases, (3) acute-phase pharmacotherapy followed by continuation- and maintenance-phase CT (combination therapy), or (4) CT focused exclusively on residual symptoms.
When combined with the literature, results suggest that CT used as a continuation- and maintenance-phase treatment deserves more evaluation and appears to offer patients safe, tolerable, and effective prevention for an extended period. To date, after response to the acute phase has been documented, C-CT and its variants have protected responders to A-CT,3,10,11 acute-phase pharmacotherapy,11,12-14 and acute-phase pharmaco-therapy plus CT.10
The appropriate number of CT sessions, as well as the months needed to prevent relapse and recurrence, likely depends on age of onset and the extent of remission that a patient achieves during A-CT. 4,7,13,14,33-35 Current results suggest that A-CT alone (a total of 20 sessions) can reduce depressive relapse when stable remission has been achieved or in patients with a later age of onset. On the other hand, A-CT plus C-CT (30 sessions total, including a focus on prevention) can reduce relapse and recurrence in patients with an earlier age of onset or unstable remission. Clinicians and third-party payers can easily use extent of acute-phase remission to identify patients who no longer meet DSM-IV criteria for MDD, yet continue to require additional CT because the risk of relapse or recurrence is high.
The finding that people who develop depression early in life are vulnerable to depressive relapse and recurrence after successful treatment highlights the importance of evaluating early intervention and preventive strategies for children and adolescents. Psychosocial treatments offer promise for this population.37,38
To reduce residual symptoms and relapse and recurrence, C-CT should be standard practice, not only in psychopharmacology, but also in cognitive therapy when acute-phase remission is unstable. Additional controlled studies are necessary to evaluate the relative efficacy and effectiveness of 8 months of C-CT vs continuation-phase pharmacotherapy in patients at highest risk for depressive relapse and recurrence. Future treatments that reduce residual symptoms, relapse, and recurrence further will increase the relevance of the findings for affected patients.
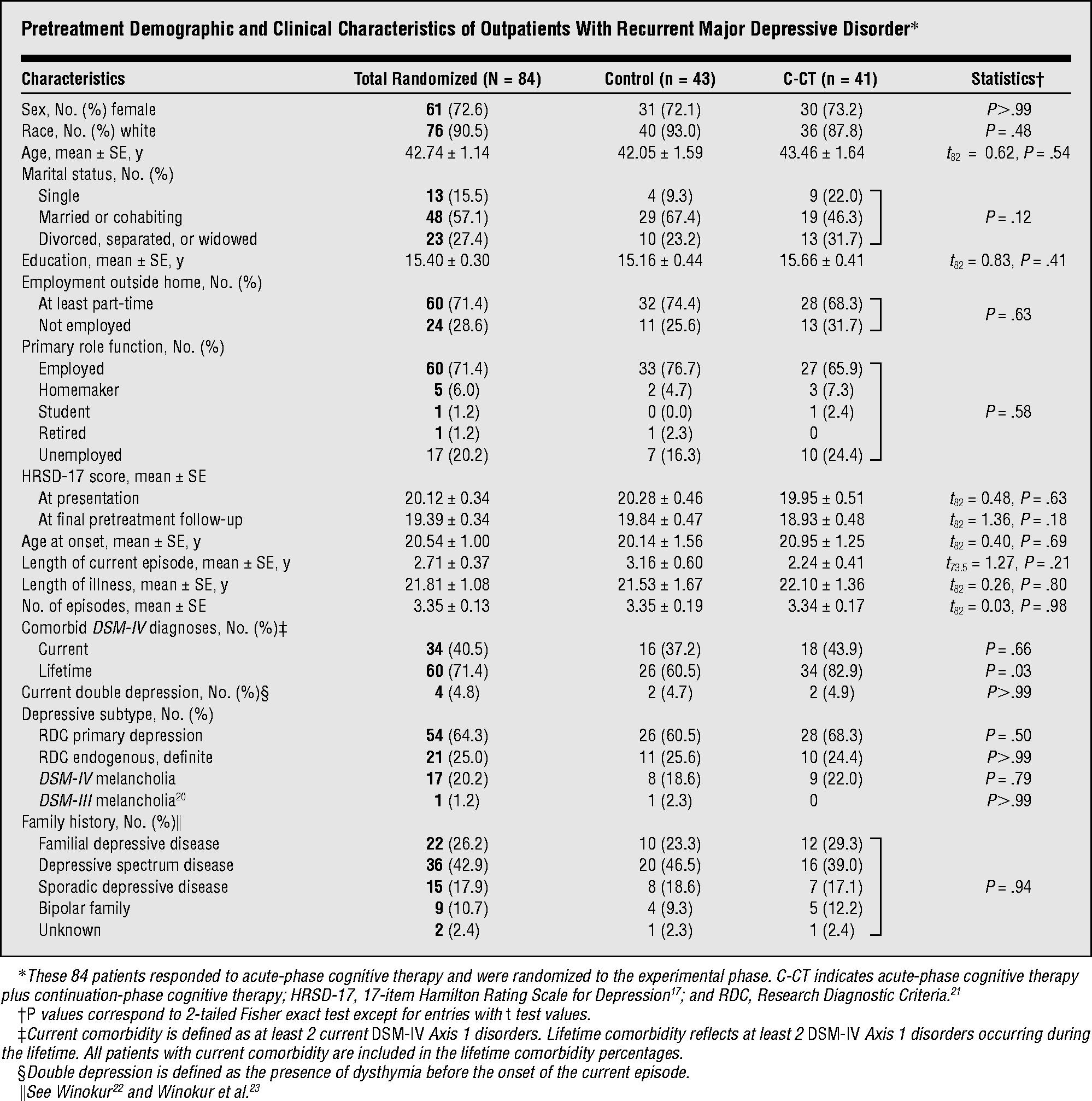
Pretreatment Demographic and Clinical Characteristics of Outpatients With Recurrent Major Depressive Disorder
Footnotes
Presented in part at the 33rd Annual Convention of the Association for Advancement of Behavior Therapy, Toronto, Ontario, November 13, 1999.
Gratitude is expressed to our colleagues for contributing to this research. Marjorie Woodruff, PhD, Bethany Hampton, PhD, Catherine Judd, PA-C, MS, Douglas Lisle, PhD, Regina Kinney, PhD, Maria Marwill-Magee, PhD, Andrew Clifford, PhD, Martin Schaffer, MD, and Rodger Kobes, MD, provided clinical support. Research support was provided by Michelle White, BS, Edna Christian, MA, Joseph Begue, BA, Julie Lowe, BA, Daisha Cipher, PhD, Patricia Green, MS, Demetria Clinton, BA, Paula Reese, and Benjamin McPhee, BS. Brian F. Shaw, PhD, rated the cognitive therapy. Janet Smith, BA, and Richard C. Risser, MS, provided programming support. Thanks are expressed to Lee Anna Clark, PhD, and Donald McIntire, PhD, who commented on an early draft of the manuscript. We appreciate the administrative support of Kenneth Z. Altshuler, MD (Stanton Sharp Professor and previous chairman), and Eric J. Nestler, MD, PhD (The Lou and Ellen McGinley Distinguished Chair in Psychiatric Research and current chairman).
This research was supported in part by grants MH-38238 and MH-01571 from the National Institute of Mental Health, Bethesda, Md (Dr Jarrett).
REFERENCES
- 1.Frank E, Kupfer DJ, Perel JM, Cornes C, Jarrett RB, Mallinger AG, Thase ME, McEachran AB, Grochocinski VJ. Three-year outcomes for maintenance therapies in recurrent depression. Arch Gen Psychiatry. 1990;47:1093–1099. doi: 10.1001/archpsyc.1990.01810240013002. [DOI] [PubMed] [Google Scholar]
- 2.Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. Guilford Press; New York, NY: 1979. [Google Scholar]
- 3.Jarrett RB, Basco MR, Risser RC, Ramanan J, Marwill M, Kraft D, Rush AJ. Is there a role for continuation phase cognitive therapy for depressed outpatients? J Consult Clin Psychol. 1998;66:1036–1040. doi: 10.1037//0022-006x.66.6.1036. [DOI] [PubMed] [Google Scholar]
- 4.Evans MD, Hollon SD, DeRubeis RJ, Piasecki JM, Grove WM, Garvey MJ, Tuason VB. Differential relapse following cognitive therapy and pharmacotherapy for depression. Arch Gen Psychiatry. 1992;49:802–808. doi: 10.1001/archpsyc.1992.01820100046009. [DOI] [PubMed] [Google Scholar]
- 5.Kovacs M, Rush AJ, Beck AT, Hollon SD. Depressed outpatients treated with cognitive therapy or pharmacotherapy: a one-year follow-up. Arch Gen Psychiatry. 1981;38:33–39. doi: 10.1001/archpsyc.1981.01780260035003. [DOI] [PubMed] [Google Scholar]
- 6.Shea MT, Elkin I, Imber S, Sotsky S, Watkins J, Collins J, Pilkonis P, Beckham E, Glass D, Dolan R, Parloff M. Course of depressive symptoms over follow-up. Arch Gen Psychiatry. 1992;49:782–787. doi: 10.1001/archpsyc.1992.01820100026006. [DOI] [PubMed] [Google Scholar]
- 7.Thase ME, Simons AD, McGeary J, Cahalane JF, Hughes C, Harden T, Friedman E. Relapse after cognitive behavior therapy of depression: potential implications for longer courses of treatment. Am J Psychiatry. 1992;149:1046–1052. doi: 10.1176/ajp.149.8.1046. [DOI] [PubMed] [Google Scholar]
- 8.Weissman MM, Klerman GL, Prusoff BA, Sholomskas D, Padian N. Depressed outpatients: results one year after treatment with drugs and/or interpersonal psychotherapy. Arch Gen Psychiatry. 1981;38:51–55. doi: 10.1001/archpsyc.1981.01780260053005. [DOI] [PubMed] [Google Scholar]
- 9.Klerman GL, DiMascio A, Weissman M, Prusoff B, Paykel E. Treatment of depression by drugs and psychotherapy. Am J Psychiatry. 1974;131:186–192. doi: 10.1176/ajp.131.2.186. [DOI] [PubMed] [Google Scholar]
- 10.Blackburn IM, Eunson KM, Bishop S. A two-year naturalistic follow-up of depressed patients treated with cognitive therapy, pharmacotherapy, and a combination of both. J Affect Disord. 1986;10:60–75. doi: 10.1016/0165-0327(86)90050-9. [DOI] [PubMed] [Google Scholar]
- 11.Blackburn IM, Moore RG. Controlled acute and follow-up trial of cognitive therapy and pharmacotherapy in outpatients with recurrent depression. Br J Psychiatry. 1997;171:328–334. doi: 10.1192/bjp.171.4.328. [DOI] [PubMed] [Google Scholar]
- 12.Fava GA, Grandi S, Zielezny M, Rafanelli C, Canestrari R. Four-year outcome for cognitive behavioral treatment of residual symptoms in major depression. Am J Psychiatry. 1996;153:945–947. doi: 10.1176/ajp.153.7.945. [DOI] [PubMed] [Google Scholar]
- 13.Fava G, Rafanelli C, Grandi S, Conti S, Bellurado P. Prevention of recurrent depression with cognitive behavioral therapy: preliminary findings. Arch Gen Psychiatry. 1998;55:816–820. doi: 10.1001/archpsyc.55.9.816. [DOI] [PubMed] [Google Scholar]
- 14.Paykel ES, Scott J, Teasdale JD, Johnson AL, Garland A, Moore R, Jenaway A, Cornwall PL, Hayhurst H, Abbott R, Pope M. Prevention of relapse in residual depression by cognitive therapy: a controlled trial. Arch Gen Psychiatry. 1999;56:829–835. doi: 10.1001/archpsyc.56.9.829. [DOI] [PubMed] [Google Scholar]
- 15.Jarrett RB, Kraft D. Prophylactic cognitive therapy for major depressive disorder. In Session. 1997;3:65–79. [Google Scholar]
- 16.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 17.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;12:52–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R–Outpatient Version (With Psychotic Screen) New York State Psychiatric Institute, Biometrics Research Dept; New York: 1989. [Google Scholar]
- 19.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Revised Third Edition American Psychiatric Association; Washington, DC: 1987. [Google Scholar]
- 20.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Third Edition American Psychiatric Association; Washington, DC: 1980. [Google Scholar]
- 21.Spitzer RL, Endicott J, Robins E. Research Diagnostic Criteria: rationale and reliability. Arch Gen Psychiatry. 1978;35:773–782. doi: 10.1001/archpsyc.1978.01770300115013. [DOI] [PubMed] [Google Scholar]
- 22.Winokur G. Familial (genetic) subtypes of pure depressive disease. Am J Psychiatry. 1979;136:911–913. doi: 10.1176/ajp.136.7.911. [DOI] [PubMed] [Google Scholar]
- 23.Winokur G, Behar D, Van Valkenberg C, Lowry M. Is a familial definition of depression both feasible and valid? J Nerv Mental Dis. 1978;166:764–768. doi: 10.1097/00005053-197811000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Young J, Beck AT. Cognitive Therapy Scale: Rating Manual. Center for Cognitive Therapy; Philadelphia, Pa: 1980. [Google Scholar]
- 25.Keller MD, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, Andreasen N. The Longitudinal Interval Follow-up Evaluation: a comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- 26.Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Rush AJ, Weissman MM. Conceptualization and rationale for consensus definitions of terms in major depressive disorder: remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48:851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- 27.Freedman LS. Tables of the number of patients required in clinical trials using the log rank test. Stat Med. 1983;1:121–129. doi: 10.1002/sim.4780010204. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 29.Cox DR, Oakes D. Analysis of Survival Data. Chapman & Hall; London, England: 1984. [Google Scholar]
- 30.Collett D. Modeling Survival Data in Medical Research. Chapman & Hall; New York, NY: 1994. [Google Scholar]
- 31.Kessing LV, Andersen PK, Mortensen PB, Bolwig TG. Recurrence in affective disorder, I: case register study. Br J Psychiatry. 1998;172:23–28. doi: 10.1192/bjp.172.1.23. [DOI] [PubMed] [Google Scholar]
- 32.Sargeant JK, Bruce ML, Florio LP, Weissman MM. Factors associated with 1-year outcome of major depression in the community. Arch Gen Psychiatry. 1990;47:519–526. doi: 10.1001/archpsyc.1990.01810180019004. [DOI] [PubMed] [Google Scholar]
- 33.Judd LL, Paulus MP, Zeller P. The role of residual subthreshold depressive symptoms in early episode relapse in unipolar major depressive disorder [letter] Arch Gen Psychiatry. 1999;56:764–765. doi: 10.1001/archpsyc.56.8.764. [DOI] [PubMed] [Google Scholar]
- 34.Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, Paulus MP, Kunovac JL, Leon AC, Mueller TI, Rice JA, Keller MB. Major depressive disorder: a prospective study of residual subthreshhold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998;50:97–108. doi: 10.1016/s0165-0327(98)00138-4. [DOI] [PubMed] [Google Scholar]
- 35.Fava GA, Rafanelli C, Grandi S, Conti S, Belluardo P. In reply [letter to editor] Arch Gen Psychiatry. 1999;56:764–765. doi: 10.1001/archpsyc.55.9.816. [DOI] [PubMed] [Google Scholar]
- 36.Depression Guideline Panel Clinical Practice Guideline Number 5: Depression in Primary Care, 2: Treatment of Major Depression 1993Agency for Health Care Policy and Research, US Dept of Health and Human Services; Rockville, Md: AHCPR publication 93-0551 [Google Scholar]
- 37.Lewinsohn PM, Clarke GN. Psychosocial treatments for adolescent depression. Clin Psychol Rev. 1999;19:329–342. doi: 10.1016/s0272-7358(98)00055-5. [DOI] [PubMed] [Google Scholar]
- 38.Jaycox LH, Reivich KJ, Gillham J, Seligman ME. Prevention of depressive symptoms in school children. Behav Res Ther. 1994;32:801–816. doi: 10.1016/0005-7967(94)90160-0. [DOI] [PubMed] [Google Scholar]