Abstract
Conjugated linoleic acid (CLA) isomers, a group of positional and geometric isomers of linoleic acid [18:2(n-6)], have been studied extensively due to their ability to modulate cancer, atherosclerosis, obesity, immune function and diabetes in a variety of experimental models. The purpose of this review was to examine CLA’s isomer-specific regulation of adiposity and insulin sensitivity in humans and in cultures of human adipocytes. It has been clearly demonstrated that specific CLA isomers or a crude mixture of CLA isomers prevent the development of obesity in certain rodent and pig models. This has been attributed mainly to trans-10, cis-12 CLA, both in vivo and in vitro. However, CLA’s ability to modulate human obesity remains controversial because data from clinical trials using mixed isomers are conflicting. In support of some studies in humans, our group demonstrated that trans-10, cis-12 CLA prevents triglyceride (TG) accumulation in primary cultures of differentiating human preadipocytes. In contrast, cis-9, trans-11 CLA increases TG content. Closer examination has revealed that CLA’s antiadipogenic actions are due, at least in part, to regulation of glucose and fatty acid uptake and metabolism. This review presents our current understanding of potential isomer-specific mechanisms by which CLA reduces human adiposity and insulin sensitivity.
Keywords: conjugated linoleic acid, human adipocytes, obesity, insulin sensitivity, peroxisome proliferator-activated receptor-γ
Therapeutic effects of CLA
In 1987, Michael Pariza’s group made the seminal observation that conjugated linoleic acid (CLA),4 isolated from grilled beef or from a base-catalyzed isomerization of linoleic acid, inhibited chemically induced skin neoplasia in mice (1). In addition to their anticarcinogenic properties [reviewed in (2)], CLA isomers have been shown to modulate immune function [reviewed in (3)], as well as markers of atherosclerosis [reviewed in (4)], diabetes [reviewed in (5)], and obesity risk [reviewed in (6)]. The purpose of this review was to examine CLA’s ability to modulate human adiposity and insulin sensitivity, leading to our current hypothesis on how this isomer-specific phenomenon occurs.
Human studies with CLA
There is compelling evidence that feeding mixed isomers of CLA attenuates obesity in several animal models (6). However, replicating these findings in humans has been inconsistent, and the data are conflicting. For example, several studies demonstrated that consuming 3–3.4 g/d of mixed CLA isomers for 6 mo had no effect on body fat, body weight, fat-free mass, the percentage of body fat or blood lipids in healthy adults (7–9). In contrast, 7–13 wk of supplementation of mixed CLA isomers (1.4–6.8 g/d) reduced fat mass or the percentage of body fat in exercising adults (10), healthy adults (11), obese/overweight adults (12) and middle-aged adults (13). This discrepancy is likely due to isomer-specific mechanisms, with the trans-10, cis-12 isomer responsible for most of the antiadipogenic effects. In addition, the level of CLA given in these studies was approximately five times lower than levels given to animals, when expressed per unit of body weight.
More recently, a clinical trial demonstrated that obese subjects with syndrome X-like symptoms administered 3.4 g/d of the trans-10, cis-12 isomer of CLA for 12 wk weighed less, had smaller girths and had lower BMI than their own baseline measurements (14). In contrast, the weight, girth and BMI of subjects administered 3.4 g/d of a crude mixture of cis-9, trans-11 and trans-10, cis-12 CLA for 12 wk did not differ from baseline. Intriguingly, that study (14) found that obese subjects supplemented with trans-10, cis-12 CLA developed insulin resistance compared with those administered mixed CLA isomers or placebo. This finding is in agreement with several studies in mice, demonstrating that dietary CLA induces hyperinsulinemia (15), insulin resistance (16) and lipodystrophy (15,16). In contrast to these findings, there is evidence that mixed CLA isomers may reverse insulin resistant states in rodents (17,18) and may be associated with favorable alterations of several metabolic variables of human subjects with type II diabetes (5). These paradoxical findings may arise from the differential effects of cis-9, trans-11 and trans-10, cis-12 CLA, different ratios of the two isomers, the different levels of CLA used or the species and metabolic status of the experimental model. Collectively, evidence in humans is still inconclusive regarding the ability of the different CLA isomers to influence body composition and insulin sensitivity. Future isomer-specific clinical trials in normal weight, overweight and obese participants should provide much needed insight into this discrepancy.
Evidence of CLA’s isomer-specific regulation of metabolism in human adipocytes
In 1997, Pariza’s group first demonstrated the ability of a crude mixture of CLA isomers to regulate body composition in mice (19), an effect that has been extremely reproducible in most animal models tested (6). In humans, however, CLA’s ability to modulate adiposity has not been demonstrated consistently. Therefore, our group set out to determine which CLA isomers decreased the triglyceride (TG) content and modulated the differentiation program of human adipocytes using a primary human preadipocyte model system.
Human preadipocyte model
Primary cultures of (pre)adipocytes isolated from human adipose tissue more closely resemble in situ conditions in human adipose tissue than cultures from other species or from cell lines. These cultures also contain myoblasts, chondroblast and epithelial cells, as occurs normally in intact adipose tissue. In contrast, cell lines may exhibit significant alterations in their biological and physiologic properties that may not be operative in body tissue. Some alterations among cells lines include immortalization, altered cell morphology, aneuploidy, loss of contact inhibition, dedifferentiation and genetic drift. Therefore, properties of primary cells isolated from human adipose tissue make them a relatively good model for examining how CLA influences the growth, differentiation and metabolism of human adipose tissue. Furthermore, human adipocytes have the capacity to synthesize fatty acids de novo, albeit ~80% lower than the capacity of rodent adipocytes under similar conditions (20). In support of this concept, we recently determined that a portion of the newly synthesized intracellular lipid pool in primary cultures of human adipocytes is derived from glucose (21). However, preformed fatty acids were taken up and utilized for energy production and storage to a much greater extent than was glucose (22).
Trans-10, cis-12 CLA decreases TG content
We found that chronic supplementation with 3–30 μmol/L trans-10, cis-12 CLA prevented the accumulation of TG in primary cultures of differentiating preadipocytes isolated from human abdominal adipose tissue compared with vehicle controls (21). In contrast, cis-9, trans-11 CLA consistently increased TG accumulation compared with vehicle controls. Interestingly, the effective dose of CLA (3–30 μmol/L) for lowering TG levels in human preadipocytes (21,22) was lower than levels (50–100 μmol/L) used in murine 3T3-L1 preadipocytes (6,23). In addition, chronic supplementation up to 100 μmol/L CLA does not induce apoptosis, nor does it alter cellular protein or total nucleic acid levels in human adipocytes (unpublished observations). In contrast, CLA induces apoptosis in adipose tissue of mice (16), in mammary epithelium (24) and colonic mucosa (25) of rats, in murine 3T3-L1 preadipocytes (23) and in primary cultures of rat mammary cells (26).
Trans-10, cis-12 CLA decreases insulin-stimulated glucose uptake and utilization
We wanted to determine whether CLA decreased TG accumulation by influencing glucose utilization. Initially, we demonstrated that trans-10, cis-12 CLA inhibited de novo fatty acid synthesis, thereby limiting TG synthesis (21). Next, we found that chronic treatment of differentiating cultures of human preadipocytes with 3–30 μmol/L trans-10, cis-12 dose dependently decreased insulin-stimulated glucose uptake, oxidation and incorporation into cellular lipid (22). In contrast, the cis-9, trans-11 isomer of CLA had little effect on glucose uptake and utilization. Furthermore, chronic treatment with 30 μmol/L trans-10, cis-12 CLA, but not cis-9, trans-11 CLA, decreased the expression of the insulin-stimulated glucose transporter 4 (GLUT4) and acetyl-CoA carboxylase (ACC), the rate-determining enzyme in de novo fatty acid synthesis (22). Collectively, supplementation with trans-10, cis-12, but not cis-9, trans-11, CLA induced an insulin-resistant state in cultures of differentiating human adipocytes, thereby reducing the influx of glucose and subsequent de novo synthesis of fatty acids and glycerol for esterification into TG.
Trans-10, cis-12 CLA decreases fatty acid uptake and utilization
Although trans-10, cis-12 CLA dramatically reduced insulin-stimulated glucose uptake and GLUT4 gene expression, this is not likely the primary TG-lowering mechanism, because de novo fatty acid synthesis is not as robust in preadipocytes isolated from humans compared with preadipocytes from other mammals (20). In agreement with CLA’s isomer-specific regulation of TG accumulation, chronic supplementation with 30 μmol/L trans-10, cis-12 CLA suppressed 14C-oleic acid uptake and esterification into cellular lipid compared with control cultures (22). Similarly, the expression of genes associated with TG storage (i.e., perilipin) and fatty acid transport (i.e., adipocyte fatty acid binding protein, aP2; acyl-CoA-binding protein, ACBP) were dramatically decreased by trans-10, cis-12 CLA. Surprisingly, trans-10, cis-12 CLA decreased 14C-oleic acid oxidation, suggesting that the reduction in cellular TG is not due to increased β-oxidation. In contrast, the cis-9, trans-11 isomer did not greatly alter fatty acid uptake or metabolism, or gene expression compared with a vehicle control. Collectively, these data demonstrate that the trans-10, cis-12 CLA isomer specifically decreases fatty acid uptake and utilization of exogenously derived fatty acids, thereby limiting substrate for TG synthesis.
CLA-mediated insulin resistance and lipodystrophy
Although these data seem promising for CLA’s role as an anti-obesity nutrient, it is important to remember lessons learned from other models of diminished adipose TG stores (27–29). Under conditions in which adipose tissue is ablated or incapable of storing fatty acids, the nonesterified fatty acids remain elevated in the plasma and are deposited into peripheral tissues such as liver and muscle. This condition, known as lipodys-trophy, is highly correlated with insulin resistant states in the liver and muscle (27–29). In fact, by inhibiting glucose and fatty acid uptake into the adipocyte, trans-10, cis-12 CLA may have dramatic implications in vivo, by promoting whole-body insulin resistance, leading to hyperglycemia and hyperlipidemia. In support of this concept, several animal studies (15,16) and one clinical trial (14) clearly demonstrated that dietary CLA, particularly trans-10, cis-12 CLA, can induce lipoatrophic diabetes.
CLA’s regulation of peroxisome proliferator-activated receptor (PPAR)γ and its downstream target genes
CLA modulates adipocyte gene expression in a dose and isomer-specific manner in a variety of in vivo and in vitro models (6). It is generally accepted that this effect is mediated by the ability of CLA isomers to bind and activate members of the PPAR family of transcription factors. Belury and colleagues (30) first demonstrated that CLA isomers were high affinity ligands for PPARγ in a rat hepatoma cell line. Additionally, Yu et al. (31) demonstrated that CLA isomers could activate PPARγ in murine macrophages.
Both in vitro and in vivo experiments have unequivocally shown that PPARγ is the master adipogenic regulator (32). Interconnected to its role in adipocyte differentiation, PPARγ regulates insulin sensitivity by transcriptionally activating genes involved in insulin signaling, glucose uptake, and fatty acid uptake and storage (Fig. 1). In fact, the antidiabetic drug family known as thiazolidinediones (TZD) mediate their insulin-sensitizing effects by directly activating PPARγ. Because activation of PPARγ increases adipogenesis and insulin sensitivity (33), we hypothesized that trans-10, cis-12 CLA may exert its TG-lowering and insulin resistance–inducing effects by decreasing the expression or activity of PPARγ. To test this hypothesis, we first examined the effects of CLA isomers on PPARγ gene expression throughout the differentiation paradigm in human preadipocytes. Chronic, but not acute treatment with trans-10, cis-12 CLA dramatically decreased PPARγ1 and PPARγ2 expression compared with a vehicle control (22). The chronic trans-10, cis-12 CLA–mediated reduction of PPARγ expression was coupled to decreased expression of downstream targets such as ACBP, aP2, perilipin-A, lipoprotein lipase (LPL) and GLUT4 (22). In contrast, cis-9, trans-11 CLA increased the expression of PPARγ and its downstream targets compared with vehicle controls. In support of these findings, CLA’s ability to reduce PPARγ expression in murine adipocytes was demonstrated recently by two independent laboratories (34,35).
FIGURE 1.
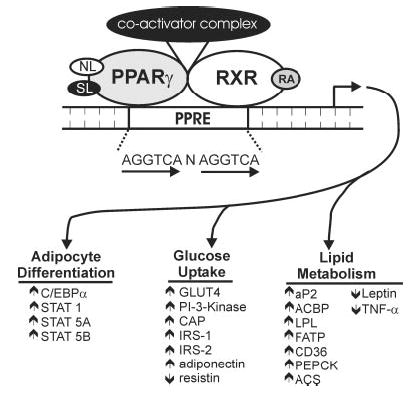
Schematic representation of the mechanism by which peroxisome proliferator-activated receptor (PPAR) γ activation promotes adipocyte differentiation, insulin sensitivity and lipid accumulation in adipocytes. Activation of PPARγ by natural ligands (NL; i.e., eicosanoids, oxidized lipids, PUFA) or synthetic ligands (SL; i.e., thiazolidinediones, tyrosine analogs) initiates heterodimerization with the retinoid X receptor (RXR), which requires ligand activation by 9-cis-retinoic acid (RA). This obligate heterodimeric complex binds to peroxisome proliferator-activated receptor response elements (PPRE) in the promoters of target genes. Once bound to the promoter, the recruitment of coactivator complexes, which contain histone acetyl-transferase activity, modifies the nucleosome structure to allow for the basal transcriptional machinery to gain access to target gene promoters. Subsequent transactivation of CAAT/enhancer binding protein-α (C/ EBPα) and signal transducers and activators of transcription (STAT) 1, 5A and 5B promotes adipocyte differentiation. Activation of PPARγ promotes insulin-stimulated glucose uptake by increasing the expression of downstream targets including the insulin-stimulated glucose transporter 4 (GLUT4), phosphatidylinositol 3-kinase (PI-3-Kinase), cbl-associated protein (CAP), insulin receptor substrate (IRS) 1 and 2, and adiponectin, and by decreasing the expression of resistin. Furthermore, PPARγ activation promotes lipid storage by increasing the expression of the adipocyte fatty acid binding protein (aP2), acyl-CoA binding protein (ACBP), lipoprotein lipase (LPL), acyl-CoA synthetase (ACS), fatty acid transport protein (FATP), fatty acid translocase (CD36) and phosphoenol pyruvate carboxykinase (PEPCK), and by decreasing the expression of leptin and tumor necrosis factor-α (TNFα).
In addition the ability of trans-10, cis-12 CLA to reduce the expression of PPARγ, we hypothesized that CLA directly affects PPARγ activity by competing with endogenous ligands, or diminishing endogenous ligand synthesis. To test this hypothesis, we transiently transfected 3T3-L1 adipocytes with a PPARγ-responsive reporter construct containing the intronic proliferator-activated receptor response elements from the rat ACBP gene (36). In the absence of exogenously added PPARγ ligand, both isomers slightly decreased reporter activity. Furthermore, when 100 nmol/L BRL 49653 was added in the presence of CLA isomers, both isomers antagonized ligand-dependent activation of the reporter construct, with trans-10, cis-12 CLA being the most robust antagonist (22). These data are in agreement with Granlund and colleagues (37), who recently reported that both CLA isomers antagonized the ligand-dependent transactivation of a PPARγ-responsive reporter, in both COS-1 and 3T3-L1 cells, with the trans-10, cis-12 isomer being the most robust antagonist.
Hence, there is mounting evidence in adipocytes that both the cis-9, trans-11 and the trans-10, cis-12 isomers of CLA antagonize PPARγ activity. However, the lack of strict isomer specificity indicates that this effect on PPARγ activity does not contribute significantly to CLA’s antiadipogenic actions, mediated solely by trans-10, cis-12 CLA. Therefore, our results currently support a model in which the trans-10, cis-12 isomer specifically down-regulates the expression of PPARγ, rather than activity, thereby decreasing both lipogenesis and adipogenesis.
Proposed mechanism by which CLA decreases adipogenesis and insulin sensitivity
On the basis of our data using primary cultures of differentiating human preadipocytes, we devised a working model of potential mechanisms by which trans-10, cis-12 CLA decreases adipogenesis and insulin sensitivity (Fig. 2). Our data suggest that physiologic levels (e.g., 10–30 μmol/L) of trans-10, cis-12 CLA inhibit the differentiation of human preadipocytes into mature insulin-sensitive adipocytes (21,22). We know that trans-10, cis-12 CLA can be taken up by differentiating human adipocytes and esterified into neutral lipid (primarily TG) and phospholipid cellular fractions (22). We have also demonstrated that trans-10, cis-12 CLA supplementation decreases the intracellular ratio of monounsaturated fatty acids (MUFA) to saturated fatty acids (SFA). Currently, we believe that trans-10, cis-12 CLA exerts its partial inhibition of adipocyte differentiation by reducing the expression of PPARγ and its downstream targets that are critical for fatty acid (i.e., ACBP, aP2, LPL, perilipin) and glucose metabolism (i.e., GLUT4; ACC; stearoyl-CoA desaturase 1, SCD-1). By reducing the expression of PPARγ in developing preadipocytes, trans-10, cis-12 CLA inhibits glucose (Fig. 3) and fatty acid uptake and metabolism (Fig. 2). The result is a fibroblast-like cell type that has some characteristics of adipocytes (e.g., leptin expression), but has significantly decreased insulin sensitivity and ability to store TG. These actions oppose those of PPARγ activators such as TZD. Currently, the mechanism by which trans-10, cis-12 CLA decreases the expression of PPARγ in human adipocytes is unclear.
FIGURE 2.
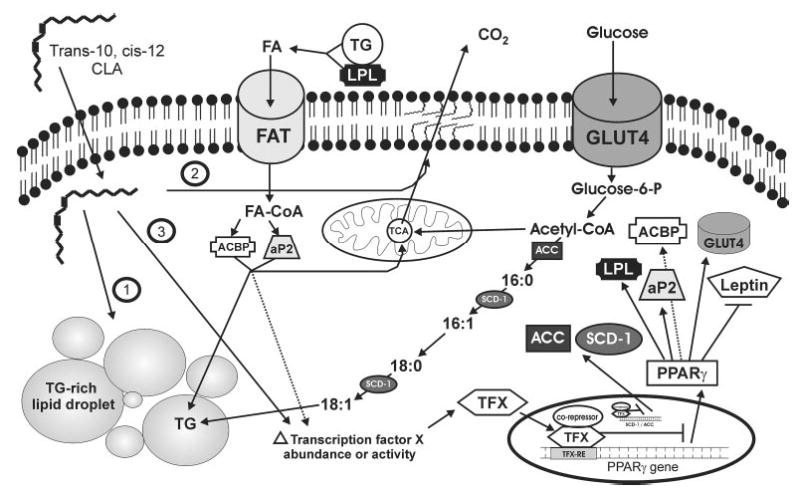
Proposed mechanism by which trans-10, cis-12 conjugated linoleic acid (CLA) decreases triglyceride (TG) accumulation in differentiating human (pre)adipocytes. CLA enters the cell through an unidentified mechanism of transport, and is shuttled into different regulatory compartments. Mechanism #1: CLA can be esterified into TG-rich lipid droplets, where it is likely to have little regulatory role, and could potentially increase TG stores. Mechanism #2: CLA can be esterified into the membrane phospholipids bi-layer, where it could alter membrane fluidity and membrane-associated signal transduction cascades. Mechanism #3: CLA can alter the abundance or activity of a currently unidentified transcription factor (TFX), which results in transcriptional repression of the peroxisome proliferator-activated receptor (PPAR) γ and its downstream targets lipoprotein lipase (LPL), acyl-CoA binding protein (ACBP), adipocyte fatty acid binding protein (aP2), the insulin-stimulated glucose transporter 4 (GLUT4) and leptin. In addition, by altering the activity or abundance of TFX, CLA decreases the expression of stearoyl-CoA desaturase 1 (SCD-1) and acetyl-CoA carboxylase (ACC), which is likely independent of CLA’s ability to reduce PPARγ signaling. By down-regulating GLUT4, ACC, and SCD-1, CLA attenuates insulin-stimulated glucose uptake, malonyl-CoA synthesis, and oleate synthesis, respectively, collectively decreasing de novo fatty acid synthesis. Decreased malonyl-CoA would impair fatty acid (FA) elongation into long-chain, unsaturated FA, limiting their availability for eicosanoid production and cell signaling, including PPARγ activation. By down-regulating LPL, ACBP and aP2, CLA attenuates FA uptake and alters intracellular trafficking to attenuate esterification into TG. Alternatively, CLA-induced alterations of FA metabolism could decrease synthesis of endogenous ligands (i.e., eicosanoids) for TFX or PPARγ.
FIGURE 3.
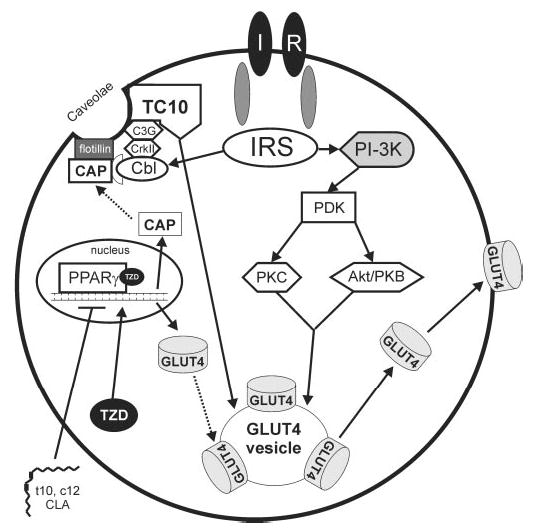
Schematic model of insulin-stimulated glucose uptake in adipocytes. Under normal conditions, insulin binds to the extracellular α subunits of the insulin receptor (IR), which leads to intracellular autophosphorylation of β subunits of the IR. Subsequent tyrosine phosphorylation of the insulin receptor substrate (IRS) family of proteins leads to the phosphorylation and activation of phosphatidylinositol 3-kinase (PI-3K). Phosphorylation of PI-3K leads to the activation of the phosphoinositide-dependent protein kinase (PDK), which phosphorylates and activates downstream serine/threonine kinases protein kinase C (PKC) and protein kinase B (Akt/PKB). This cascade results in the translocation of the insulin-stimulated glucose transporter 4 (GLUT4) to the plasma membrane, where it facilitates intracellular flux of glucose. In addition to this PI-3K–dependent pathway, the IR can signal independently of PI-3K to stimulate GLUT4 translocation. In this case, IR autophoshorylation can lead to the tyrosine phosphorylation of c-cbl, and association of the cbl-associated protein (CAP). Subsequent binding of the SH2/SH3-containing adapter protein CrkII and the guanyl nucleotide exchange factor (C3G) activities the Rho family TC10. Activation of the TC10 plays a crucial role in the translocation of GLUT4 to the plasma membrane. Activation of both the PI-3K dependent and independent pathways is necessary for insulin-stimulated glucose uptake in adipocytes. Thiazolidinediones (TZD) increase insulin sensitivity in part by activating peroxisome proliferator-activated receptor (PPAR) γ-dependent transactivation of GLUT-4 and CAP genes. We contend that trans-10, cis-12 CLA (t10,c12-CLA)–induced insulin resistance involves antagonism of PPARγ in human adipocytes. In support of this concept, t10, c12-CLA diminishes the expression and TZD-induced activity of PPARγ (22). By antagonizing PPARγ signaling, t10, c12-CLA may indirectly reduce GLUT4 expression and insulin-stimulated glucose uptake.
Alternatively, it is possible that trans-10, cis-12 CLA reduces human adipocyte TG content through mechanisms other than simply decreasing PPARγ abundance. Hence, as proposed by James Ntambi (38–42) in 3T3-L1 adipocytes, an alternative hypothesis is that trans-10, cis-12 CLA reduces the expression or activity of SCD-1, an enzyme responsible for the Δ-9 desaturation of palmitate (16:0) and stearate (18:0). Intriguingly, Ntambi’s group (38) demonstrated that loss of SCD-1 function protects mice from developing obesity. This is likely due to decreased synthesis of long-chain MUFA such as oleate, a preferred substrate for TG synthesis. The effects of CLA on SCD-1 have been consistent, regardless of the model or species. Treatment with mixed isomers, or more specifically trans-10, cis-12 CLA, decreases either the activity or abundance of SCD-1 in a human breast cancer cells, human hepatocytes and murine adipocytes (39–41).
We recently reported that supplementation with trans-10, cis-12 CLA, but not cis-9, trans-11 CLA, decreased SCD-1 gene expression in differentiating human preadipocytes (22). In parallel, trans-10, cis-12 CLA decreased the MUFA:SFA ratio, likely due to decreased functional SCD-1 protein (22,40,41). Additionally, trans-10, cis-12 CLA supplementation increased stearate (18:0) levels, which has been shown to have negative feedback on the activity of ACC. This effect could further potentiate CLA’s ability to decrease de novo lipogenesis, and limit synthesis of downstream elongation products such as arachidonic acid (20:4), due to a lack of sufficient malonyl-CoA required for fatty acid elongation, and subsequent eicosanoid production. In support of this concept, treatment of human preadipocytes with trans-10, cis-12 CLA decreased phospholipid-associated 20:4 by 35% (22). Furthermore, SCD-1 has been implicated in leptin-mediated weight loss (42). Interestingly, we found that trans-10, cis-12 CLA increased leptin gene expression in human preadipocytes (22). Therefore, it is tempting to speculate that leptin acts through an autocrine loop to further suppress SCD-1 expression, thereby preventing adiposity. However, more work must be done to test this hypothesis.
Conclusions and perspectives
To date there are >600 published papers on CLA’s diverse biological applications, and reported effects on body composition are overwhelmingly strong. However, the vast majority of these reports have come from rodent or porcine models, and CLA’s potential as an antiobesity nutrient for humans is still a matter of debate. It is very likely that this conflict persists due to the fact that CLA’s mechanism of action is isomer specific and that the level of intake is critical, as is the metabolic status of the participants. We found that trans-10, cis-12 CLA attenuates human adipocyte TG content and differentiation (21,22). In contrast the cis-9, trans-11 isomer of CLA increases TG accumulation, and adipocyte-specific gene expression in human adipocytes (21,22). Therefore, it is tempting to speculate that the inconclusive results in human supplementation trials are due to the use of mixed isomers, which may negate one another, resulting in no net change in adiposity. Furthermore, the levels used in human trials were much lower than those used in animal studies. In any event, isomer-specific dose-titrated clinical studies combined with mechanistic studies in cultures of primary cells should provide the much needed insight on potential human applications for CLA.
CLA’s ability to modulate insulin sensitivity in humans is not so clear, and characterization of CLA’s ability to modulate whole-body glucose homeostasis is required. Early reports demonstrated that mixed CLA isomers may reverse insulin-resistant states in rodents (17,18), and there was speculation that this may occur by activation of PPARγ (31,43). More recently, it was reported that these same CLA-mediated antidiabetic actions may hold true for type II diabetic subjects (5). In contrast, subsequent reports demonstrated that mixed isomers (16), and more specifically the trans-10, cis-12 isomer of CLA (14,15), induce insulin resistance. In support of this concept, we recently demonstrated that trans-10, cis-12 CLA decreased insulin-stimulated glucose uptake and metabolism in differentiating human preadipocytes (22). Our group (22) and others (34,35) also demonstrated that CLA decreases the expression of PPARγ in adipocytes, which could promote insulin resistance and oppose the hypoglycemic actions of TZD. Collectively, these paradoxical findings may arise from the use of mixed isomers of CLA or the difference in experimental models used. Currently, it is not clear whether CLA isomers are beneficial or deleterious in relation to insulin sensitivity.
Acknowledgments
We thank S. Mandrup at the University of Southern Denmark for her collaborations and critical review of this work.
Footnotes
Presented in part at Experimental Biology 03, April 2003, San Diego, CA [Brown, M., Fabiyi, O. & McIntosh, M. (2003) Trans-10, cis-12 conjugated linoleic acid (CLA) decreases glucose and fatty acid uptake and oxidation by altering adipocyte specific gene expression in primary cultures of human (pre)adipocytes. FASEB J. 17: A807 (abs.)].
Supported by grants from the National Institutes of Health (NIH)-DK 59289–01, NIH-DK 630070-01, the Office of Dietary Supplements, and the North Carolina Agricultural Research Service grant 06520 to M.K.M. and the American Society of Nutritional Sciences Predoctoral Fellowship to J.M.B.
Abbreviations: ACBP, Acyl-CoA binding protein; ACC, acetyl-CoA carboxylase; aP2, adipocyte fatty acid binding protein; CLA, conjugated linoleic acid; GLUT4, insulin-stimulated glucose transporter 4; LPL, lipoprotein lipase; MUFA, monounsaturated fatty acid; PPAR, peroxisome proliferator-activated receptor; SFA, saturated fatty acid; TG, triglyceride; TZD, thiazolidinedione.
References
- 1.Ha YL, Grimm NK, Pariza M. Anticarcinogens from fried ground beef: heat-altered derivatives of linoleic acid. Carcinogenesis. 1987;8:1881–1887. doi: 10.1093/carcin/8.12.1881. [DOI] [PubMed] [Google Scholar]
- 2.Belury M. Inhibition of carcinogenesis by conjugated linoleic acid: potential mechanisms of action. J Nutr. 2002;132:2995–2998. doi: 10.1093/jn/131.10.2995. [DOI] [PubMed] [Google Scholar]
- 3.Bassaganya-Riera J, Hontecillas &, Beitz DC. Colonic anti-inflammatory mechanisms of conjugated linoleic acid. Clin Nutr. 2002;2:451–459. doi: 10.1054/clnu.2002.0594. [DOI] [PubMed] [Google Scholar]
- 4.Kritchevsky D, Tepper SA, Wright S, Tso P, Czarnecki SK. Influence of conjugated linoleic acid (CLA) on establishment and progression of atherosclerosis in rabbits. J Am Coll Nutr. 2000;19:472S–477S. doi: 10.1080/07315724.2000.10718950. [DOI] [PubMed] [Google Scholar]
- 5.Belury MA. Dietary conjugated linoleic acid in health: physiological effects and mechanisms of action. Annu Rev Nutr. 2002;22:505–531. doi: 10.1146/annurev.nutr.22.021302.121842. [DOI] [PubMed] [Google Scholar]
- 6.Evans M, Brown J, McIntosh M. Isomer-specific effects of conjugated linoleic acid (CLA) on adiposity and lipid metabolism. J Nutr Biochem. 2002;13:508–516. doi: 10.1016/s0955-2863(02)00211-5. [DOI] [PubMed] [Google Scholar]
- 7.Berven G, Bye A, Hals O, Blankson H, Thom E, Wadstein J, Gudmundsen O. Safety of conjugated linoleic acid (CLA) in overweight or obese human volunteers. Eur J Lipid Sci Technol. 2000;102:455–462. [Google Scholar]
- 8.Zambell K, Keim N, Van Loan M, Gale B, Benito P, Kelly D, Nelson G. Conjugated linoleic acid supplementation in humans: effects on body composition and energy expenditure. Lipids. 2000;35:777–782. doi: 10.1007/s11745-000-0585-z. [DOI] [PubMed] [Google Scholar]
- 9.Medina E, Horn W, Keim N, Havel P, Benito P, Kelly D, Nelson G, Erickson K. Conjugated linoleic acid supplementation in humans: effect of circulating leptin concentrations and appetite. Lipids. 2000;35:783–788. doi: 10.1007/s11745-000-0586-y. [DOI] [PubMed] [Google Scholar]
- 10.Thom E, Wadstein J, Gudmundsen O. Conjugated linoleic acid reduces body fat in healthy, exercising humans. J Int Med Res. 2001;29:392–396. doi: 10.1177/147323000102900503. [DOI] [PubMed] [Google Scholar]
- 11.Mougious V, Matsakas A, Petridou A, Ring S, Sagredos A, Melissopoulou A, Tsigilis N, Nikolaidis M. Effect of supplementation with conjugated linoleic acid on human serum lipids and body fat. J Nutr Biochem. 2001;12:585–594. doi: 10.1016/s0955-2863(01)00177-2. [DOI] [PubMed] [Google Scholar]
- 12.Blankson H, Stakkestad J, Fagertum H, Thom E, Wadstein J, Gudmundsen O. Conjugated linoleic acid reduces body fat mass in overweight and obese humans. J Nutr. 2000;130:2943–2948. doi: 10.1093/jn/130.12.2943. [DOI] [PubMed] [Google Scholar]
- 13.Smedman A, Vessby G. Conjugated linoleic acid supplementation in humans: metabolic effects. Lipids. 2001;36:773–781. doi: 10.1007/s11745-001-0784-7. [DOI] [PubMed] [Google Scholar]
- 14.Riserus U, Arner P, Brismar K, Vessby B. Treatment with dietary trans-10 cis-12 conjugated linoleic acid causes isomer-specific insulin resistance in obese men with the metabolic syndrome. Diabetes Care. 2002;25:1516–1521. doi: 10.2337/diacare.25.9.1516. [DOI] [PubMed] [Google Scholar]
- 15.Clement L, Poirier H, Niot I, Bocher V, Guerre-Millo M, Krief S, Staels B, Besnard P. Dietary trans-10, cis-12 conjugated linoleic acid induces hyperinsulinemia and fatty liver in the mouse. J Lipid Res. 2002;43:1400–1409. doi: 10.1194/jlr.m20008-jlr200. [DOI] [PubMed] [Google Scholar]
- 16.Tsuboyama-Kasaoka N, Takahashi M, Tanemura K, Kim HJ, Tange T, Okuyama H, Kasai M, Ikemoto S, Ezaki O. Conjugated linoleic acid supplementation reduces adipose tissue by apoptosis and develops lipo-dystrophy in mice. Diabetes. 2000;49:1534–1542. doi: 10.2337/diabetes.49.9.1534. [DOI] [PubMed] [Google Scholar]
- 17.Houseknecht KL, Vanden Heuvel JP, Moya-Camerena SY, Portocarrero CP, Peck LW, Nickel KP, Belury M. Dietary conjugated linoleic acid normalizes impaired glucose tolerance in the Zucker diabetic fa/fa rat. Biochem Biophys Res Commun. 1998;244:678–682. doi: 10.1006/bbrc.1998.8303. [DOI] [PubMed] [Google Scholar]
- 18.Ryder JW, Portacarrero CP, Song XM, Cui L, Yu M, Cobatsiaris T, Galuska D, Bauman DE, Barbano DM, Charron MJ, Zierath JR, Houseknecht KL. Isomer-specific antidiabetic properties of conjugated linoleic acid. Improved glucose tolerance, skeletal muscle insulin action, and UCP-2 gene expression. Diabetes. 2001;50:1149–1157. doi: 10.2337/diabetes.50.5.1149. [DOI] [PubMed] [Google Scholar]
- 19.Park Y, Albright KJ, Storkson J, Cook ME, Pariza MW. Effect of conjugated linoleic acid on body composition in mice. Lipids. 1997;32:853–858. doi: 10.1007/s11745-997-0109-x. [DOI] [PubMed] [Google Scholar]
- 20.Swierczynski J, Goyke L, Wach A, Pankiewicz Z, Kochan W, Adamonis Z, Sledzinski Z, Aleksandrowicz A. Comparative study of the lipogenic potential of human and rat adipose tissue. Metabolism. 2000;49:594–599. doi: 10.1016/s0026-0495(00)80033-5. [DOI] [PubMed] [Google Scholar]
- 21.Brown JM, Halvorsen YD, Lea-Currie YR, Geigerman C, McIntosh M. Trans-10, cis-12, but not cis-9, trans-11, conjugated linoleic acid attenuates lipogenesis in primary cultures of stromal vascular cells from human adipose tissue. J Nutr. 2001;131:2316–21. doi: 10.1093/jn/131.9.2316. [DOI] [PubMed] [Google Scholar]
- 22.Brown JM, Boysen MS, Jensen SS, Morrison RF, Storkson J, Currie RL, Pariza M, Mandrup S, McIntosh MK. Isomer-specific regulation of metabolism and PPARγ signaling by CLA in human preadipocytes. J Lipid Res. 2003;44:1287–1300. doi: 10.1194/jlr.M300001-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans M, Geigerman C, Cook J, Curtis L, Kuebler B, McIntosh M. Conjugated linoleic acid suppresses triglyceride accumulation and induces apoptosis in 3T3–L1 preadipocytes. Lipids. 2000;35:899–910. doi: 10.1007/s11745-000-0599-6. [DOI] [PubMed] [Google Scholar]
- 24.Ip C, Dong Y, Thompson H, Bauman D, Ip MM. Control of rat mammary epithelium proliferation by conjugated linoleic acid. Nutr Cancer. 2001;39:233–238. doi: 10.1207/S15327914nc392_12. [DOI] [PubMed] [Google Scholar]
- 25.Park H, Ryu J, Ha Y, Park JHY. Dietary conjugated linoleic acid (CLA) induces apoptosis of colonic mucosa in 1, 2-dimethyl-hydrazine treated rats: a possible mechanism of the anticarcinogenic effect of CLA. Br J Nutr. 2001;86:549–555. doi: 10.1079/bjn2001445. [DOI] [PubMed] [Google Scholar]
- 26.Ip C, Ip M, Loftus T, Shoemaker S, Shea-Eaton W. Induction of apoptosis by conjugated linoleic acid in cultured mammary tumor cells and premalignant lesions of the rat mammary gland. Cancer Epidemiol Biomark Prev. 2000;9:689–696. [PubMed] [Google Scholar]
- 27.Moitra J, Mason MM, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, Feigenbaum L, Lee E, Aoyama T, Eckhaus M, Reitman M, Vinson C. Life without white fat: a transgenic mouse. Genes Dev. 1998;12:3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gavrilova O, Marcus-Samuels B, Graham D, Kim J, Shulman GI, Castle A, Vinson C, Eckhaus M, Reitman M. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Investig. 2000;105:271–278. doi: 10.1172/JCI7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reue K, Xy P, Wang XP, Slavin BG. Adipose tissue deficiency, glucose intolerance, and increased atherosclerosis result from mutation in the mouse fatty liver dystrophy (fld) gene. J Lipid Res. 2000;41:1067–1076. [PubMed] [Google Scholar]
- 30.Moya-Camerena SJ, Vanden Heuvel J, Blanchard S, Leesnitzer L, Belury M. Conjugated linoleic acid is a potent and naturally occurring ligand and activator of PPARα. J Lipid Res. 1999;40:1426–1433. [PubMed] [Google Scholar]
- 31.Yu Y, Correll PH, Vanden Heuvel JP. Conjugated linoleic acid decreases production of pro-inflammatory products in macrophages: evidence for a PPARgamma-dependent mechanism. Biochim Biophys Acta. 2002;158:89–99. doi: 10.1016/s1388-1981(02)00126-9. [DOI] [PubMed] [Google Scholar]
- 32.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 33.Picard F, Auwerx J. PPARγ and glucose homeostasis. Annu Rev Nutr. 2002;22:167–197. doi: 10.1146/annurev.nutr.22.010402.102808. [DOI] [PubMed] [Google Scholar]
- 34.Kang K, Liu W, Albright KJ, Park Y, Pariza MW. Trans-10, cis-12 CLA inhibits differentiation of 3T3–L1 adipocytes and decreases PPARgamma expression. Biochem Biophys Res Commun. 2003;303:795–799. doi: 10.1016/s0006-291x(03)00413-3. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi Y, Kushiro M, Shinohara K, Ide T. Dietary conjugated linoleic acid reduces body fat mass and affects gene expression of proteins regulating energy metabolism in mice. Comp Biochem Physiol Biochem Mol Biol. 2002;133:395–404. doi: 10.1016/s1096-4959(02)00164-1. [DOI] [PubMed] [Google Scholar]
- 36.Helledie T, Grontved L, Jensen SS, Kiilerich P, Reitveld L, Albrektsen T, Boysen MS, Nohr J, Larsen LK, Fleckner J, Stunnenberg K, Kristiansen K, Mandrup S. The gene encoding the Acyl-CoA binding protein is activated by peroxisome proliferators activated receptor gamma through an intronic response element functionally conserved between humans and rodents. J Biol Chem. 2002;277:26821–26830. doi: 10.1074/jbc.M111295200. [DOI] [PubMed] [Google Scholar]
- 37.Granlund L, Juvet L, Pedersen JI, Nebb H. Trans-10, cis-12 conjugated linoleic acid prevents triacylglycerol accumulation in adipocytes by acting as a PPARγ modulator. J Lipid Res. 2003;44:1441–1452. doi: 10.1194/jlr.M300120-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Ntambi JM, Miyazaki M, Store JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci USA. 2002;99:11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi Y, Park Y, Storkson J, Pariza MW, Ntambi JM. Inhibition of stearoyl-CoA desaturase activity by the cis-9, trans-11 isomer and the trans-10, cis-12 isomer of conjugated linoleic acid in MDA-MB-231 and MCF-7 human breast cancer cells. Biochem Biophys Res Commun. 2002;294:785–790. doi: 10.1016/S0006-291X(02)00554-5. [DOI] [PubMed] [Google Scholar]
- 40.Choi Y, Park Y, Pariza MW, Ntambi JM. Regulation of stearoyl-CoA desaturase activity by the trans-10, cis-12 isomer of conjugated linoleic acid in HepG2 cells. Biochem Biophys Res Commun. 2001;284:689–693. doi: 10.1006/bbrc.2001.5036. [DOI] [PubMed] [Google Scholar]
- 41.Choi Y, Kim YC, Han YB, Park Y, Pariza MW, Ntambi JM. The trans-10, cis-12 isomer of conjugated linoleic acid downregulates stearoyl-CoA desaturase 1 gene expression in 3T3–L1 adipocytes. J Nutr. 2000;130:1920–1924. doi: 10.1093/jn/130.8.1920. [DOI] [PubMed] [Google Scholar]
- 42.Cohen P, Miyazaki M, Socci ND, Hagge-Greenberg A, Liedtke W, Soukas AA, Sharma R, Hudgins LC, Ntambi JM, Friedman JM. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science (Washington, DC) 2002;297:240–243. doi: 10.1126/science.1071527. [DOI] [PubMed] [Google Scholar]
- 43.McCarty MF. Activation of PPARgamma may mediate a portion of the anticancer activity of conjugated linoleic acid. Med Hypotheses. 2000;55:187–188. doi: 10.1054/mehy.1999.1010. [DOI] [PubMed] [Google Scholar]


