Abstract
Rationale
Delta-opioid agonists produce a number of behavioral effects, including convulsions, anti-nociception, locomotor stimulation, and antidepressant-like effects. The development of these compounds as treatments for depression is limited by their convulsive effects. Therefore, determining how to separate the convulsive and antidepressant-like characteristics of these compounds is essential for their potential clinical use.
Objective
The present study tests the hypothesis that the rate of delta-opioid agonist administration greatly contributes to the convulsive properties, but not the anti-depressant-like effects, of delta-opioid agonists.
Materials and methods
The delta-opioid agonist SNC80 (1, 3.2, and 10 mg kg−1 or vehicle) was administered to Sprague–Dawley rats by intravenous infusion over different durations of time (20 s, 20, or 60 min). Convulsions were measured by observation prior to determining antidepressant-like effects in the forced swim test.
Results
Slowing the rate of SNC80 administration minimized delta agonist-induced convulsions without altering the effects of SNC80 in the forced swim test.
Conclusions
These data suggest that delta agonist-induced antidepressant properties are independent of convulsive effects, and that it may be possible to eliminate the convulsions produced by delta agonists, further promoting their potential clinical utility.
Keywords: Delta-opioid receptor, Antidepressants, Convulsions, Forced swim test, Rats, SNC80, Infusion rate
Introduction
Previous studies have shown that disregulation of the delta-opioid receptor system may be related to depression or depressive symptoms and, therefore, may be a useful therapeutic target for treating depression. It was demonstrated that mice lacking the delta-opioid receptor displayed altered emotional responses that are consistent with a depressive-like profile of behaviors (Filliol et al. 2000). In addition, compounds that activated the delta-opioid receptor have been shown to have antidepressant-like properties in a number of animal models. The selective delta-opioid agonist Tyr-d-Ser-(O–C(CH3)3)-Gly-Phe-Leu-Thr-(O–C(CH3)3 (BUBU) produced antidepressant-like effects in the learned helplessness model of depression (Tejedor-Real et al. 1998). Similarly, increasing levels of endogenous delta-opioid peptides with enkephalinase inhibitors such as RB101 revealed antidepressant-like effects in models of depression in mice and rats (Baamonde et al. 1992; Tejedor-Real et al. 1998). More recently, the nonpeptidic delta-opioid agonists SNC80 and (+)BW373U86 displayed naltrindole (NTI)-sensitive antidepressant-like properties in the forced swim test in rats (e.g., Broom et al. 2002a; Jutkiewicz et al. 2004). Overall, these findings suggest that activation of delta-opioid receptors may have therapeutic potential to treat depression.
In addition to antidepressant-like effects, delta-opioid receptor agonists produce convulsions, antinociception, and locomotor stimulation. Delta-opioid agonist-induced convulsions have been observed in mice, rats, and monkeys (Broom et al. 2002a; Comer et al. 1993; Dykstra et al. 1993; Hong et al. 1998; Negus et al. 1994; Pakarinen et al. 1995). It has been suggested that delta-opioid agonists may produce antidepressant-like effects through a mechanism similar to electroconvulsive shock (Comer et al. 1993). However, Broom et al. (2002b) demonstrated that preventing convulsions with the anticonvulsant benzodiazepine, midazolam, did not alter the antidepressant-like properties of the delta-opioid agonist (+)BW373U86, suggesting that the antide-pressant-like effects did not require a convulsive event. Thus, minimizing the undesirable convulsive effects while retaining the antidepressant-like properties appears feasible. In particular, the latency to convulsion shortens with increasing the dose of agonist (Broom et al. 2002a) and with different routes of administration (Jutkiewicz et al. 2004), indicating that the speed with which drug enters the circulation and/or the central nervous system (CNS) may play a role in delta-opioid-mediated convulsions. These preliminary data suggested that it might be possible to alter the time to onset of convulsion and, possibly, other characteristics of this behavior.
Previous studies have reported that varying the rate of administration alters the effects of different CNS drugs. For example, it was demonstrated that pharmacokinetic variables are an important determinant of the functional response of cocaine as measured by route-dependent neuronal circuit activation (Porrino 1993). Varying the rate of intravenous drug administration also altered the subjective effects of cocaine, but not some of the physiological effects of cocaine (Abreu et al. 2001). Similarly, the rate of intravenous cocaine administration changed the susceptibility to cocaine-induced locomotor sensitization in rats (Samaha et al. 2002). Likewise, oral administration of immediate-release methylphenidate produced subjective and cardiovascular effects, while the sustained-release methylphenidate formulation only produced changes in cardiovascular function (Kollins et al. 1998). In another drug class, rapid-onset diazepam produced more euphoria, observable signs of intoxication, psychomotor impairment, and longer-lasting sedation as compared with slow-onset diazepam (de Wit et al. 1993). In general, these studies demonstrate that rate of administration can alter the physiological, behavioral, and subjective effects of various classes of drugs.
The experiments, described herein, manipulated the rate at which the delta-opioid agonist SNC80 was administered intravenously to investigate how rate of administration changes its convulsive and antidepressant-like properties. It was hypothesized that reducing the rate of SNC80 administration would minimize the convulsive effects while preserving the antidepressant-like properties of the delta-opioid agonist. Multiple factors might contribute to the potential differences between these behavioral effects, including the quantity and the rate of drug accessing the site of action, tolerance development, and the time at which the behavioral effects are measured. The quantity of SNC80 and rate of drug administration were directly evaluated in the present study.
Materials and methods
Subjects
Male Sprague–Dawley rats (250–350 g) were obtained from Harlan Sprague Dawley (Indianapolis, IN) and were housed in groups of three to four rats per cage prior to surgery. The rats were implanted with intravenous catheters 3–7 days after arrival and were then housed singly until termination of the study. All animals were fed a standard laboratory diet, and the housing room was maintained on a 12-h light/dark cycle, with lights on at 7:00 a.m. Studies were performed in accordance with the Declaration of Helsinki and with the Guide for the Care and Use of Laboratory Animals, as adopted and promulgated by the National Institutes of Health, and were approved by the University of Michigan University Committee on the Use and Care of Animals.
Procedures
Surgical procedures
Catheters were constructed from approximately 15 cm of Micro-Renathane tubing (MRE-040, Braintree Scientific, Inc, Braintree, MA). Intravenous catheters were implanted as described previously (Baird et al. 2000). Briefly, rats were anesthetized with ketamine hydrochloride (100 mg kg−1, i.p.) and xylazine hydrochloride (10 mg kg−1, i.p.). The right jugular vein was isolated, and approximately 3 cm of the catheter was inserted into it and secured to the surrounding tissues. The remaining tubing was threaded subcutaneously to a dorsal incision point and secured to the musculature directly below the incision. The 2–3 cm of tubing that remained exposed outside the rat’s body was plugged with a stainless steel pin (McMaster–Carr, Chicago, IL). Immediately after, and every day following the surgery, the catheters were flushed with approximately 0.5 ml of heparinized saline (50 U ml−1). On average, rats were allowed 4–5 days of recovery from surgery before being used in an experiment.
Intravenous drug infusions
Rats were administered vehicle or drug solutions either s.c. or i.v. Intravenous infusions of either 1, 3.2, or 10 mg kg−1 SNC80 or vehicle were administered over one of three infusion durations: 20 s, 20, or 60 min. For the 20-s infusion, the catheter was simply attached to a syringe, and the experimenter infused a single dose over 20 s. For the 20- and 60-min infusion rates, the catheter was attached to a syringe on an infusion pump (Harvard Apparatus, Natick, MA), and the pump administered a single dose (1, 3.2, or 10 mg kg−1 or vehicle) at a constant speed over 20 or 60 min. During pump-operated drug infusions, the catheter was attached to a swivel, which allowed the rat to move freely around the cage. For antagonism studies, NTI (0.1, 1, or 10 mg kg−1) was administered s.c. 30 min prior to the start of an infusion of SNC80.
Convulsion observation
The observation of convulsant activity was performed as previously described for rats (Broom et al. 2002a). Briefly, rats were placed individually in plastic observation cages with bedding and observed for 20 min after the s.c. injection or during the i.v. infusion and for 20 min following the termination of the i.v. infusion. In general, convulsant activity occurred as clonic movements of the head, face, and forepaws. Postconvulsion catalepsy was identified as failing to remove its paws from a horizontal rod within 15 s and by the loss of the righting reflex. The qualitative nature of the convulsive events (in terms of intensity) was determined by behavioral observation and recorded by observers blind to treatment.
Convulsion threshold determination
The method used to determine the threshold dose required for a convulsion in rats was derived from studies measuring the threshold dose of pentylenetetrazol (Pollack and Shen 1985). For these experiments, rats with implanted i.v. catheters (described above) were injected s.c. with vehicle or one dose of SNC80 1 h prior to receiving an i.v. infusion of 0.5 mg kg−1 min−1 SNC80 (10 mg kg−1 SNC80 over 20 min). The infusion was terminated at the first sign of repeated clonic muscle contractions of the head or forelimbs. The amount of drug infused was recorded and used to calculate the convulsive threshold dose (i.e., the dose of drug that produced a convulsion). Based on these studies, a shift in the convulsive threshold dose following different SNC80 pretreatments can be determined. For example, if SNC80 pretreatment induces tolerance, then a larger threshold dose would be required to produce a convulsion. Infusions were limited to 1 h (to conserve compound), such that the total dose received did not exceed 30 mg kg−1.
Forced swim test
Rats were evaluated in the 1-day forced swim test as previously described (Broom et al. 2002a,b) 30 min following the termination of any i.v. drug infusion or 60 min after an s.c. injection following convulsion observation. Briefly, male Sprague–Dawley rats were placed in a clear acrylic, cylindrical container (46-cm tall×20-cm diameter) filled with 30 cm of 25°C (±1°C) water. The 15-min swim session was videotaped from above the tank for later scoring. Swim tank water was changed after every rat. Following the swim session, rats without i.v. catheters were removed from the water, towel-dried, placed in a heated cage for 15 min, and sacrificed by 100 mg kg−1 s.c. administration of pentobarbital. After swimming, rats with i.v. catheters were killed by i.v. administration of pentobarbital (100 mg kg−1) to insure catheter patency; only data from rats with patent catheters were used.
An observer, blind to the drug treatment conditions, analyzed the videotaped swim session and scored one behavioral measure every 5 s for the 15-min swim session. Every 5 s, the behavior was scored as immobility, swimming, or climbing (Detke et al. 1995). Immobility was described as floating in the water using only small movements to keep the head above water. Swimming was defined as traveling around the swim tank while actively moving paws and keeping paws below the water surface. Climbing was described as actively moving the paws into and out of the water.
Ex vivo [3H]NTI-binding experiments
Rats (N=3 per condition) were administered vehicle, 1.0, 3.2, or 10 mg kg−1 SNC80 over a 20-s, 20-, or 60-min i.v. infusion duration. Rats were sacrificed by decapitation 30 min after the infusion ended (at the time rats would normally be evaluated in the forced swim test); the cerebellum was removed, and the brains were submerged in 10 ml of ice-cold 50 mM Tris (pH 7.4). The brains were homogenized, divided into 1-ml aliquots, and flash-frozen. Competition-binding experiments were conducted in a 2-ml volume assay. For standard curve determinations, 400 μl of vehicle-treated rat brains were added to 20 μl of a known concentration of SNC80 (0.032–10 μM) and 20 μl of 0.1 nM [3H]NTI. Known concentrations of SNC80 diluted in 0.1% bovine serum albumin (BSA) were added to the assay tubes with Sigmacote-coated (Sigma, St. Louis, MO) pipette tips. All brain samples from drug-treated rats were evaluated simultaneously with the standard curves. To calculate brain concentrations of drug, 400 μl of treated rat brains were added to 20 μl of 0.1% BSA and 20 μl of 0.1 nM [3H]NTI. To attain the final assay volume, 1.56 ml 50 mM Tris (pH 7.4) was added to all tubes. All samples were vortexed and incubated in a 25°C water bath for 60 min. Following incubation, the samples were quickly filtered through glass fiber filters (Schleicher and Schuell no.32, Keene, NH) and soaked in 0.1% polyethylenimine (Sigma) that were mounted in a Brandel cell harvester (Biomedical Research and Development Laboratories, Gaithersburg, MD). Each filter was removed and placed in a 5-ml polypropylene scintillation vial with 4 ml scintillation cocktail for at least 5 h to allow equal distribution of radioactivity and then subjected to liquid scintillation counting. The drug concentrations that could be assayed ranged from 0.1 to 100 nM.
Data handling and statistical analyses
For all convulsion experiments, data were expressed as the percent of rats that convulsed in each treatment group of six to eight rats. For convulsion threshold experiments, the threshold dose of SNC80 was calculated by multiplying the infusion rate by the time necessary to produce convulsive activity. In the forced swim test experiments, the total counts for each behavior during the 15-min swim were averaged within each treatment group. Statistical tests were performed using one-way ANOVA with Dunnett’s post hoc analysis (Graphpad Prism software). p<0.05 was accepted as statistical significance. For [3H]NTI-binding studies, standard curves and extrapolated unknown values of drug-treated brains were calculated using Graphpad Prism software.
Drugs
[(+)-4-[(αR)-α-[(2S,5R)-2,5-dimethyl-4-(2-propenyl)-1-pi-perazinyl]-(3-methoxyphenyl)methyl]-N,N-diethylbenzamide (SNC80) was dissolved in 8% of 1N HCl. Naltrindole was dissolved in sterile water. All drugs were administered in a volume of 1 ml kg−1. For binding studies, a stock solution of 10 mM SNC80 was made as described above and diluted in 0.1% BSA solution for standard curve determinations.
Results
SNC80 administration by fast (20 s) i.v. infusion
Fast 20-s infusions of intravenous administration of SNC80 (IV SNC80) produced convulsions in 66% of rats at 1.0 mg kg−1 and 100% of rats at 3.2 and 10 mg kg−1 (Fig. 1a). Doses higher than 10 mg kg−1 IV SNC80 were lethal. Convulsions produced by 1.0 and 3.2 mg kg−1 IV SNC80 occurred during the infusion or immediately after the infusion and appeared similar in form to convulsions induced by subcutaneous administration of SNC80, as determined by behavioral observation alone. The convulsions manifested as clonic contractions of the musculature of the face, head, and forepaws, lasting 15–40 s, followed by a period of catalepsy lasting 2–4 min. However, convulsions produced by fast 20-s infusion of 10 mg kg−1 IV SNC80 were more severe as demonstrated by longer durations (30–60 s), contractions of the whole body, and myoclonic twitches observed throughout the catalepsy period.
Fig. 1.
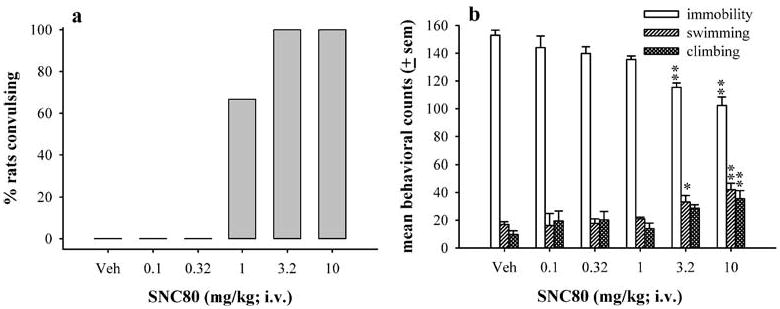
The effects of fast 20-s infusion of IV SNC80 on convulsive (a) and antidepressant-like effects in the forced swim test (b) in Sprague–Dawley rats (N=6 per dose). Drug was injected 30 min prior to the forced swim test. The bars and vertical lines above each bar represent the mean and standard error of the mean (SEM) for immobility (open bars), swimming counts (single-hatched bars), and climbing counts (double-hatched bars). * indicates p<0.05, and ** indicates p<0.01, as compared to vehicle as determined by Dunnett’s post hoc test
In the forced swim test, fast 20-s infusions of IV SNC80 significantly decreased immobility [F(5,28)=15.49, p<0.0001] and increased swimming and climbing behaviors [F(5,28)=5.70, p=0.0015 and F(5,28)=4.27, p=0.0068, respectively] (Fig. 1b). Doses of 3.2 (p<0.01) and 10 mg kg−1 (p<0.01) produced significant decreases in immobility. Significant increases were observed in swimming, with doses of 3.2 (p<0.05) and 10 mg kg−1 (p<0.01), and in climbing, with a dose of 10 mg kg−1 (p<0.01).
As shown in Fig. 2a and b, the convulsive and anti-depressant-like effects produced by fast 20-s infusion of 3.2 mg kg−1 IV SNC80 were blocked by 1.0 and 10 mg kg−1 NTI, but not by 0.1 mg kg−1 of NTI, similar to that previously observed with subcutaneous administration of SNC80 (SC SNC80) (Broom et al. 2002a). NTI alone did not produce convulsions and did not have an effect in the forced swim test. The effects of 10 mg kg−1 IV SNC80, administered by 20-s infusion, were not antagonized by any dose of NTI tested.
Fig. 2.
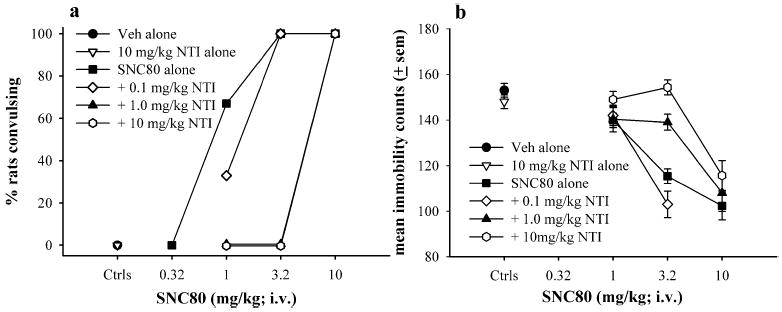
The effects of vehicle, 0.1, 1.0, or 10 mg kg−1 naltrin-dole (NTI) administered (s.c.) 30 min prior to vehicle or various doses of IV SNC80 on convulsive (a) and antidepressant-like effects in the forced swim test (b) in Sprague–Dawley rats (N=6 per condition). Antidepressant-like effects are expressed as mean immobility counts only
The dose–effect curve for convulsions produced by fast 20-s IV SNC80 was shifted approximately 30-fold to the left of the SC SNC80 dose–effect curve (Fig. 3a). In the forced swim test, similar doses of IV SNC80 and SC SNC80 produced significant decreases in immobility (Fig. 3b); however, fast 20-s infusions with 3.2 and 10 mg kg−1 SNC80 produced a larger decrease in immobility from control values as compared with SC SNC80.
Fig. 3.
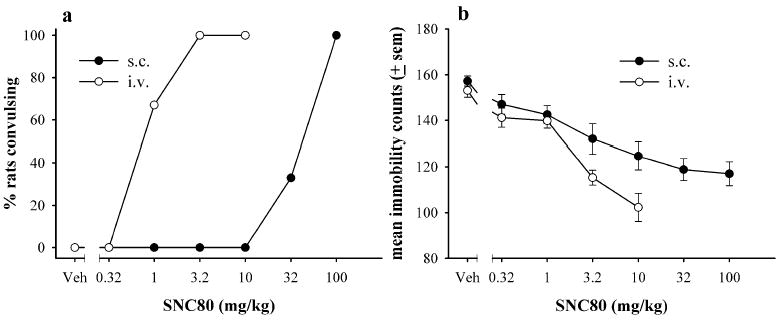
Comparison of the convulsive (a) and antidepressant-like effects (b) of SNC80 measured following s.c. administration or fast 20-s IV SNC80 infusion (N=6 per dose). Drug was injected 60 min prior to the forced swim test for s.c. administration or 30 min prior to the forced swim test for IV SNC80. Antidepressant-like effects are expressed as mean immobility counts only
SNC80 administration by slow i.v. infusions
The effects of slow IV SNC80 infusions (20 or 60 min) on convulsions and immobility, as compared to 20-s fast infusions, are shown in Fig. 4a and b. With a single SNC80 dose infused over 20 min (Fig. 4a), 33 and 83% of rats convulsed with 3.2 and 10 mg kg−1 IV SNC80, respectively. With a 60-min infusion of a single SNC80 dose (Fig. 4a), only one (17%) out of six rats convulsed with 10 mg kg−1 IV SNC80. All convulsions produced by slow infusions appeared similar to typical delta-opioid receptor-mediated convulsions (Broom et al. 2002a; Jutkiewicz et al. 2004). The dose–effect curve for convulsions was shifted to the right by increasing the duration of i.v. infusion. Downward shifts of the dose–effect curve were not thoroughly evaluated by testing slow infusions of higher doses. Although the rate of drug infusion affected the number of rats convulsing, changing the rate of i.v. infusion did not alter the decrease in immobility observed with 3.2 and 10 mg kg−1 IV SNC80 in the forced swim test (Fig. 4b).
Fig. 4.
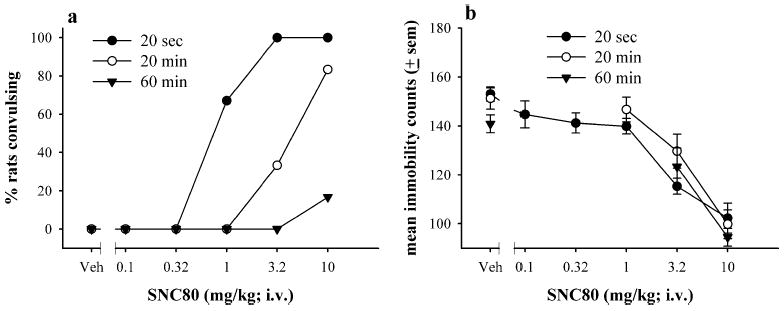
The effects of IV SNC80 by fast 20-s, slow 20-min, or slow 60-min infusions on convulsions (a) and antidepressant-like effects in the forced swim test (b). Vehicle or a single dose of IV SNC80 was infused over the duration listed above, and rats were exposed to the swim session 30 min after the termination of the infusion. Antidepressant-like effects are expressed as mean immobility counts only
As shown in Fig. 5a and b, 10 mg kg−1 NTI was administered prior to different infusion rates of 10 mg kg−1 IV SNC80. As described earlier, NTI did not antagonize the convulsions or antidepressant-like effects produced by fast 20-s infusions of 10 mg kg−1 IV SNC80. However, 10 mg kg−1 NTI completely attenuated the convulsions and antidepressant-like effects observed with 20- and 60-min slow infusions of 10 mg kg−1 IV SNC80 [F(2,17)=36.73, p<0.001 and F(2,17)=17.62, p=0.0001, respectively].
Fig. 5.
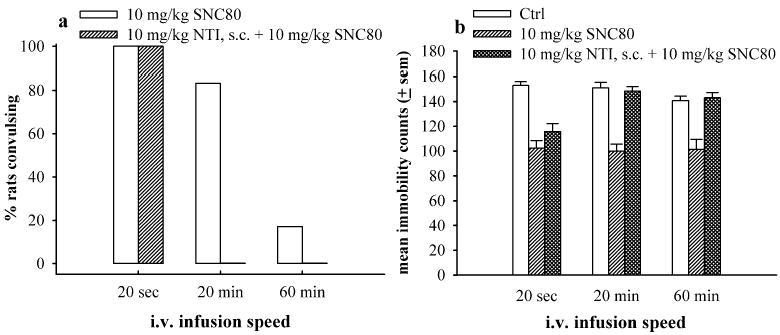
The effects of 10 mg kg−1 NTI administered (s.c.) 30 min prior to IV SNC80 by fast 20-s, slow 20-min, or slow 60-min infusions on the convulsive (a) and antidepressant-like effects in the forced swim test (b) in Sprague–Dawley rats (N=6 per condition). The bars and vertical lines above each bar represent the mean and standard error of the mean (SEM) for SNC80 alone (open bars), 10 mg kg−1 SNC80 alone (single-hatched bars), or 10 mg kg−1 NTI administered prior to 10 mg kg−1 SNC80 (double-hatched bars). ** indicates p<0.01 as compared to Ctrl determined by one-way ANOVA with Dunnett’s post hoc test
Convulsions, antidepressant-like effects, and brain concentrations of SNC80
With the slow infusions of IV SNC80, convulsions occurred during the infusion. The SNC80 dose that had been infused at the time of the convulsion was calculated by multiplying the infusion rate by the time that the convulsion occurred, shown in Table 1. The convulsions observed with a 20-min slow infusion of 3.2 and 10 mg kg−1 IV SNC80 occurred when 2.6 (±0.6) and 3.07 (±0.14) mg kg−1, respectively, were infused. With a 60-min infusion of 10 mg kg−1 IV SNC80, one out of six rats convulsed at a dose of 2.28 mg kg−1 IV SNC80. Overall, rats either convulsed with an average dose of 2.85± 0.18 mg kg−1 during slow infusions of IV SNC80, in-dependent of dose or infusion duration, or they did not convulse at all during the 20- or 60-min infusions.
Table 1.
Average dose of IV SNC80 that produced convulsions in rats receiving slow infusions
| Infusion duration (min) | Dose (mg kg−1) | Number of convulsing rats (N) | Average convulsive dose (mg kg−1) (±SEM) |
|---|---|---|---|
| 20 | 3.2 | 2/6 | 2.60 (0.6) |
| 10 | 5/6 | 3.07 (0.14) | |
| 60 | 10 | 1/6 | 2.28 |
The average brain concentrations of SNC80 equivalents, presumably comprising of the parent compound and/or its delta-opioid receptor-selective metabolite, (+) BW373U86 (Schetz et al. 1996), were measured 30 min after administration of various doses of IV SNC80 at different infusion durations (or at the time of swim). Table 2 shows these calculated drug levels in brain homogenates from treated rats. Fast infusions of IV SNC80 over 20-s and 20-min slow infusions produced dose-dependent increases in drug concentrations and similar levels of delta-opioid ligands in the brain. Following a 60-min infusion of 1.0 mg kg−1 IV SNC80, brain levels of drug were low and could not be accurately measured; however, with 3.2 and 10 mg kg−1, drug levels were lower than that observed with other infusion durations.
Table 2.
Brain equivalents of the delta-opioid agonist SNC80 or its delta-opioid metabolite measured 30 min following the termination of a 20-s, 20-min, or 60-min infusion of 1.0, 3.2, or 10 mg kg−1 IV SNC80
| Infusion duration | Dose (mg kg−1) | Average brain equivalents (nM) (±SEM) |
|---|---|---|
| 20 s | 1 | 1.86 (0.71) |
| 3.2 | 12.1 (6.46) | |
| 10 | >100 | |
| 20 min | 1 | 0.87 (0.36) |
| 3.2 | 11.73 (0.87) | |
| 10 | >100 | |
| 60 min | 1 | <0.1 |
| 3.2 | 4.76 (1.56) | |
| 10 | 11.23 (2.36) |
Convulsive threshold and tolerance development
To investigate potential tolerance development following SNC80 exposure, rats were injected with vehicle or various doses of SC SNC80 1 h prior to SNC80 infusions of 0.5 mg kg−1 min−1 for convulsive threshold determinations (Table 3). As the SNC80 pretreatment dose increased, the number of rats convulsing during the SNC80 infusion decreased, the threshold dose for convulsions increased, and the maximum overall dose infused increased (maximum dose infused was limited to 30 mg kg−1). With higher pretreatment doses, more rats were infused with 30 mg kg−1 SNC80 and did not convulse; thus, these rats received a dose tenfold higher than the average convulsive threshold determined in vehicle-pretreated rats.
Table 3.
The effects of a SNC80 pretreatment (s.c.) 1 h prior to dose threshold determination for SNC80-induced convulsions
| SNC80 pretreatment | Number of convulsing rats per group | Average dose infused (mg kg−1) | Convulsive dose (mg kg−1) |
|---|---|---|---|
| Vehicle | 6/6 | 3.11 (0.19) | 3.11 (0.19) |
| 0.1 | 5/6 | 7.80 (4.44) | 3.36 (0.16) |
| 0.32 | 4/6 | 12.47 (5.55) | 3.70 (0.81) |
| 1.0 | 4/6 | 13.20 (5.32) | 4.80 (0.47) |
| 3.2 | 2/6 | 21.45 (5.41) | 4.36 (0.43) |
| 10 | 0/6 | 30 | N/A |
Discussion
Intravenous administration of SNC80 produced convulsions and antidepressant-like effects in the forced swim test in male Sprague–Dawley rats. In general, IV SNC80 and SC SNC80 produced similar behavioral effects; however, IV SNC80 was approximately 30-fold more potent in producing convulsions than SC SNC80. In the forced swim test, similar doses of IV SNC80 and SC SNC80 produced antidepressant-like effects, although IV SNC80 produced a larger magnitude of effect as compared to SC SNC80 at similar doses. At high doses, fast IV SNC80 produced nondelta-opioid receptor-mediated convulsions and anti-depressant-like effects, as these effects were not blocked by NTI. Importantly, slowing the rate of IV SNC80 infusions decreased the number of rats convulsing without altering the antidepressant-like effects of SNC80.
SNC80-induced convulsions
Slow i.v. infusions of SNC80 might have decreased convulsive episodes for multiple reasons. One obvious explanation was that slow infusions of SNC80 failed to reach the necessary levels of drug in the brain. As demonstrated in Table 1, the convulsive event appeared closely related to the level of SNC80 at the time of convulsion, such that convulsions occurred after an average dose of 2.85 mg kg−1 SNC80. However, not all rats convulsed after receiving this dose, especially with slow infusion rates. Additionally, fast infusions of the lowest dose (1.0 mg kg−1) produced convulsions. These data suggested that a certain dose of SNC80 might be necessary for convulsion but was not the only factor controlling this behavioral outcome.
Slower rates of SNC80 administration might decrease convulsions by leading to lower drug levels in the vicinity of the delta-opioid receptor and slower rates of receptor activation at any given moment as compared with fast i.v. infusions. It was previously reported that the temporal pattern of synaptic activation was important in producing cocaine sensitization (Samaha et al. 2002) and long-term potentiation (Larson et al. 1986; Tsukada et al. 1994; Greenstein et al. 1998). This theory suggested that the timing of receptor activation might be a critical component of the intracellular processes producing convulsions. For example, activation of delta-opioid receptors, depending on the number of receptors occupied at a single moment, might alter the pattern of neuronal firing (Law 2004) and thus decrease the manifestation of convulsions. Therefore, fast infusions of 1.0 mg kg−1 SNC80 might produce convulsions by rapid receptor activation, thus attaining the pattern of neuronal activity required for a convulsion. Likewise, 60-min slow infusions of IV SNC80 might not activate enough receptors simultaneously to produce convulsions. Therefore, slow infusions of IV SNC80 potentially minimized convulsions by reducing the rate of receptor activation.
An alternative or added explanation for decreased convulsive events with slow infusions was that the rats became tolerant to the convulsant effects of SNC80 following prolonged drug exposure. With long infusion durations, delta-opioid receptors can become desensitized and, perhaps, down-regulated. This hypothesis would suggest that there is a small receptor reserve for delta-opioid agonist-induced convulsions (Broom et al. 2002c). As demonstrated by the convulsive threshold data in Table 3, previous SNC80 exposure decreased the number of rats convulsing, increased the total amount of drug infused, and increased the threshold dose required for convulsions. These data suggested that exposure to SNC80 produced rapid tolerance to the convulsive effects of SNC80.
To summarize, SNC80-induced convulsions might be influenced by some level or pattern of delta-opioid receptor activation and the number of functional delta-opioid receptors. Therefore, low levels of simultaneous receptor activation and/or tolerance development might prevent this hypothetical pattern of receptor activation (or threshold dose) required for convulsions. Overall, the results suggested that brain concentrations of drug, the rate of receptor activation, and tolerance development might be contributing factors in the elimination of the SNC80-induced convulsion following slow rates of drug administration.
SNC80-induced antidepressant-like effects
Despite the nearly eliminated convulsion, the antidepressant-like effects of slow infusions of IV SNC80 did not change as compared to fast i.v. infusions, suggesting that the factors contributing to convulsive effects are not as relevant to antidepressant-like effects. With i.v. doses of 3.2 and 10 mg kg−1 SNC80, the antidepressant-like effects did not change in magnitude or absolute effect with the different infusion durations tested. Based on brain concentration studies measured at the time of the swim test (Table 2), the antidepressant-like effects appeared to be independent of whole-brain concentration of delta-opioid ligands. For example, following a 60-min infusion, there was less drug in the brain at any specific dose as compared with 20-s and 20-min infusions; however, the magnitude of the antidepressant-like effect did not change with different infusion durations. These data suggested that whole-brain concentrations at the time of the swim were not a critical factor in the antidepressant actions of SNC80 once certain levels were reached. However, SNC80 levels in specific brain regions might be a determining factor for antidepressant actions and should be considered in future studies. Additionally, tolerance development and patterns of receptor activation or neuronal firing did not alter antidepressant effects, as was observed with convulsions. These data suggested that there was a large receptor reserve for the SNC80-induced antidepressant-like effects, and that mechanisms downstream of the delta-opioid receptor might have a more significant role in the antidepressant actions of delta-opioid agonists.
Overall, these findings suggested that different mechanisms of action generate the nonpeptidic delta-opioid agonist-induced convulsions and antidepressant-like effects, as these behaviors were differentially influenced by whole-brain drug concentration, drug administration rate, and tolerance development. This study supported previous findings demonstrating that delta-opioid agonists display anti-depressant properties and may have therapeutic potential for treating depression. However, SNC80 also produced delta-opioid receptor convulsions, severely limiting its potential clinical utility. Slow infusions of delta-opioid receptor agonists nearly eliminated convulsions without altering the antidepressant properties of these compounds. These data highlighted the independent natures of the convulsive and antidepressant-like effects and suggested that these two behavioral outcomes can be separated. Based on these findings, slow-release formulations of delta-opioid agonists would be interesting to study and might provide therapeutic utility without negative side effects.
Acknowledgments
This work was supported by grants from the US Public Health Service Grants DA00254, T32 GM07767, and T32 DA07267.
References
- Abreu ME, Bigelow GE, Fleisher L, Walsh SL. Effects of intravenous injection speed on responses to cocaine and hydromorphone in humans. Psychopharmacology (Berl) 2001;154:76–84. doi: 10.1007/s002130000624. [DOI] [PubMed] [Google Scholar]
- Baamonde A, Daugé V, Ruiz-Gayo M, Fulga IG, Turcaud S, Fournié-Zaluski MC, Roques BP. Antidepressant-type effects of endogenous enkephalins protected by systemic RB 101 are mediated by opioid δ and dopamine D1 receptor stimulation. Eur J Pharmacol. 1992;216:157–166. doi: 10.1016/0014-2999(92)90356-9. [DOI] [PubMed] [Google Scholar]
- Baird TJ, Deng S-X, Landry DW, Winger G, Woods JH. Natural and artificial enzymes against cocaine. I. Monoclonal antibody 15A10 and the reinforcing effects of cocaine in rats. J Pharmacol Exp Ther. 2000;295:1127–1134. [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Nonpeptidic delta-opioid receptor agonists reduce immobility in the forced swim assay in rats. Neuropsychopharmacology. 2002a;26(6):744–755. doi: 10.1016/S0893-133X(01)00413-4. [DOI] [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Convulsant activity of a nonpeptidic delta-opioid receptor agonist is not required for antidepressant-like effects. Psychopharmacology (Berl) 2002b;164(1):42–48. doi: 10.1007/s00213-002-1179-y. [DOI] [PubMed] [Google Scholar]
- Broom DC, Nitsche JF, Pintar JE, Rice KC, Woods JH, Traynor JR. Comparison of receptor mechanisms and efficacy requirements for δ-agonist-induced convulsive activity and antinociception in mice. J Pharmacol Exp Ther. 2002c;303:723–729. doi: 10.1124/jpet.102.036525. [DOI] [PubMed] [Google Scholar]
- Comer SD, Hoenicke EM, Sable AI, McNutt RW, Chang KJ, DeCosta BR, Mosberg HI, Woods JH. Convulsive effects of systemic administration of the delta opioid agonist BW373U86 in mice. J Pharmacol Exp Ther. 1993;267(2):888–895. [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- de Wit H, Dudish S, Ambre J. Subjective and behavioral effects of diazepam depend on its rate of onset. Psychopharmacology (Berl) 1993;112:324–330. doi: 10.1007/BF02244928. [DOI] [PubMed] [Google Scholar]
- Dykstra LA, Schoenbaum GM, Yarbrough J, McNutt R, Chang K-J. A novel δ-opioid agonist, BW373U86, in squirrel monkeys responding under a schedule of shock titration. J Pharmacol Exp Ther. 1993;267:875–882. [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HWD, Simonin F, Befort K, Gavériaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for δ- and μ-opioid receptors exhibit opposing alterations of emotional responses. Nature. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Greenstein YJ, Pavlides C, Winson J. Long-term potentiation in the dentate gyrus is preferentially induced at theta rhythm periodicity. Brain Res. 1998;438:331–334. doi: 10.1016/0006-8993(88)91358-3. [DOI] [PubMed] [Google Scholar]
- Hong EJ, Rice KC, Calderon S, Woods JH, Traynor JR. Convulsive behavior of nonpeptide δ-opioid ligands: comparison of SNC80 and BW373U86 in mice. Analgesia. 1998;3:269–276. [Google Scholar]
- Jutkiewicz EM, Eller EB, Folk JE, Rice KC, Traynor JR, Woods JH. δ-opioid agonists: differential efficacy and potency of SNC80, its 3-OH (SNC86) and 3-desoxy (SNC162) derivatives in Sprague–Dawley rats. J Pharmacol Exp Ther. 2004;309(1):173–181. doi: 10.1124/jpet.103.061242. [DOI] [PubMed] [Google Scholar]
- Kollins SH, Rush CR, Pazzaglia, Ali JA. Comparison of acute behavioral effects of sustained-release and immediate-release methylphenidate. Exp Clin Psychopharmacol. 1998;6(4):367–374. doi: 10.1037//1064-1297.6.4.367. [DOI] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368:347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- Law PY (2004) Delta opioid receptor signal trafficking. In: Chang K-J, Porreca F, Woods JH (eds) The delta receptor. Dekker, New York, pp 61–88
- Negus SS, Butelman ER, Chang K-J, DeCosta B, Winger G, Woods JH. Behavioral effects of the systemically active delta opioid agonist BW373U86 in rhesus monkeys. J Pharmacol Exp Ther. 1994;270:1025–1034. [PubMed] [Google Scholar]
- Pakarinen ED, Woods JH, Moerschbaecher JM. Repeated acquisition of behavioral chains in squirrel monkeys: comparisons of a mu, kappa and delta opioid agonist. J Pharmacol Exp Ther. 1995;272:552–559. [PubMed] [Google Scholar]
- Pollack GM, Shen DD. A timed intravenous pentylenetetrazol infusion seizure model for quantifying the anticonvulsant effect of valproic acid in the rat. J Pharmacol Methods. 1985;13:135–146. doi: 10.1016/0160-5402(85)90057-9. [DOI] [PubMed] [Google Scholar]
- Porrino LJ. Functional consequences of acute cocaine treatment depend on route of administration. Psychopharmacology (Berl) 1993;112:343–351. doi: 10.1007/BF02244931. [DOI] [PubMed] [Google Scholar]
- Samaha A-N, Li Y, Robinson TE. The rate of intravenous cocaine administration determines susceptibility to sensitization. J Neurosci. 2002;22(8):3244–3250. doi: 10.1523/JNEUROSCI.22-08-03244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schetz JA, Calderon SN, Bertha CM, Rice KC, Porreca F. Rapid in vivo metabolism of a methylether derivative of (±)-BW373U86: the metabolic fate of [3H]SNC121 in rats. J Pharmacol Exp Ther. 1996;279:1069–1076. [PubMed] [Google Scholar]
- Tejedor-Real P, Micó JA, Smadja C, Maldonado R, Roques BP, Gibert-Rahola J. Involvement of δ-opioid receptors in the effects induced by endogenous enkephalins on learned helplessness model. Eur J Pharmacol. 1998;354:1–7. doi: 10.1016/s0014-2999(98)00423-3. [DOI] [PubMed] [Google Scholar]
- Tsukada M, Aihara T, Mizuno M, Kato H, Ito K. Temporal pattern sensitivity of long-term potentiation in hippocampal CA1 neurons. Biol Cybern. 1994;70:495–503. doi: 10.1007/BF00198802. [DOI] [PubMed] [Google Scholar]


