Abstract
Nonpeptidic δ-opioid agonists produce a number of behaviors, such as antidepressant-like effects, locomotor stimulation, antinociception, and convulsions. To consider this class of compounds as potential therapeutics for humans, the effects of δ-opioid agonists after repeated administration must be evaluated. Therefore, the present study investigated the effects of repeated δ-opioid agonist, SNC80 ([(+)-4-[(αR)-α-[(2S,5R)-2,5-dimethyl-4-(2-propenyl)-1-piperazinyl]-(3-methoxyphenyl)-methyl]-N,N-diethylbenzamide), administration on its antidepressant-like effects in the forced swim test, locomotor activity, and convulsions in male Sprague-Dawley rats. Tolerance developed rapidly to the convulsive and locomotor-stimulating effects of SNC80 but not to the antidepressant-like effects. In addition, tolerance was evaluated at the level of the receptor-G protein interaction by measuring 5′-O-(3-[35S]thio)triphosphate binding in brains from rats that were pretreated with SNC80. With various exposure durations to SNC80, some brain regions demonstrated tolerance at different times, suggesting that adaptations in the δ-opioid system may occur during agonist exposure. Overall, the lack of observable tolerance to the antidepressant-like effects of SNC80 indicates that this class of compounds has potential as a novel antidepressant therapy.
ABBREVIATIONS: (+)BW373U86, [(+)-4-[(αR)-α-[(2S, 5R)-2,5-dimethyl-4-(2-propenyl)-1-piperazinyl]-(3-hydroxyphenyl)methyl]-N,N-diethylbenzamide; SNC80, [(+)-4-[(αR)-α-[(2S,5R)-2,5-dimethyl-4-(2-propenyl)-1-piperazinyl]-(3-methoxyphenyl)methyl]-N,N-diethylbenzamide; [35S]GTPγS, 5′-O-(3-[35S]thio)triphosphate; ANOVA, analysis of variance; NSB, nonspecific binding
Nonpeptidic δ-opioid agonists have antidepressant-like effects in the forced swim test in rats (Broom et al., 2002b; Jutkiewicz et al., 2004). The selective δ-opioid antagonist, naltrindole, attenuates the antidepressant-like effects of nonpeptidic δ-opioid agonists, demonstrating that these effects are mediated through the δ-opioid receptor. As compared with typical antidepressants such as desipramine and fluoxetine, these compounds produce a greater magnitude of antidepressant-like activity in the forced swim test (Broom et al., 2002b). Moreover, the nonpeptidic δ-opioid agonists produce antidepressant-like effects after acute agonist administration, suggesting a faster onset of action than known anti-depressants. In addition, δ-opioid receptor agonists produce a number of other behavioral effects, including convulsions in mice, rats, and monkeys (Comer et al., 1993; Dykstra et al., 1993; Negus et al., 1994; Pakarinen et al., 1995; Hong et al., 1998; Broom et al., 2002a,b), antinociception in hyperalgesic or allodynia models (Butelman et al., 1995; Hong et al., 1998; Brandt et al., 2001a; Broom et al., 2002c), locomotor stimulation in rats (Spina et al., 1998; Fraser et al., 2000; Broom et al., 2002b; Jutkiewicz et al., 2004), and discriminative and other control of operant behavior in rats and monkeys (Dykstra et al., 1993; Negus et al., 1994; Pakarinen et al., 1995; Stevenson et al., 2002).
After repeated administration of peptidic or nonpeptidic δ-opioid agonists, tolerance develops to convulsive effects in mice and rats (Comer et al., 1993; Broom et al., 2002c), antinociception in mice (Tseng et al., 1997; Zhao and Bhargava, 1997; Broom et al., 2002c), and rate-decreasing effects on food-maintained responding in monkeys (Brandt et al., 2001b). In vitro and ex vivo studies demonstrate that prolonged δ-opioid agonist treatment rapidly desensitizes and down-regulates δ-opioid receptors, providing possible mechanisms for decreased behavioral and cellular responses (Bot et al., 1997; Breivogel et al., 1997; Remmers et al., 1998; Law et al., 2000; Okura et al., 2000; 2003). For example, mice treated with intracerebroventricular [d-Pen2,d-Pen5]-enkephalin or [d-Ala2,Glu4]-deltorphin II (deltorphin II) for 2 or 4 days displayed diminished antinociceptive responses and decreased receptor density in brain homogenates as measured by [3H]-[d-Pen2,d-Pen5]-enkephalin binding (Zhao and Bhargava, 1997). Likewise, repeated administration of the δ-opioid receptor-preferring ligand [d-Ala2,d-Leu5]-enkephalin decreased δ-opioid receptor density in several brain regions (Tao et al., 1993).
The effect of repeated administration of δ-opioid agonists could be critical in the development of an antidepressant therapy based on δ-opioid receptor activation. Depressed patients need to take antidepressants on a chronic basis; therefore, tolerance development would limit the therapeutic utility of δ-opioid agonists. As previously proposed (Jutkiewicz et al., 2004), the behavioral effects associated with δ-opioid receptor activation may have different efficacy requirements and, therefore, different receptor reserves (Broom et al., 2002c). Drawing from receptor theory, the present study tests the hypothesis that repeated administration of nonpeptidic δ-opioid agonists produces tolerance to high efficacy-requiring behaviors, such as convulsions, but not to the low efficacy-requiring behaviors, such as locomotor activity and antidepressant properties. For example, a previous study demonstrated that, after a single treatment in rats, tolerance developed to convulsive effects of (+)BW373U86 but not to its antidepressant-like effects (Broom et al., 2002a).
The present study investigated the effects of repeated administration of the nonpeptidic δ-opioid agonist SNC80 on convulsive, locomotor-stimulating, and antidepressant-like effects in the forced swim test in rats. In addition, SNC80-induced brain region-specific loss of receptor function was evaluated using ex vivo [35S]GTPγS binding in brain slices from rats pretreated with SNC80. Overall, these data demonstrated that differential tolerance developed to the behavioral effects and that δ-opioid agonist exposure produced cellular changes across brain regions. However, after repeated SNC80, changes in G protein activation alone do not account for all of the tolerance observed to the behavioral effects of SNC80. These findings may identify receptor populations in different brains regions that contribute to the δ-opioid agonist-induced behaviors as well as receptor populations that are differentially regulated.
Materials and Methods
Subjects
Male Sprague-Dawley rats (250–350 g) were obtained from Harlan (Indianapolis, IN) and housed in groups of three rats per cage. All animals were fed a standard laboratory diet and maintained on a 12-h light/dark cycle with lights on at 6:30 AM at an average temperature of 21°C. Experiments were conducted during the light cycle between 9:00 AM and 5:00 PM. Studies were performed in accordance with the Declaration of Helsinki and with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. The experimental protocols were approved by the University of Michigan University Committee on the Use and Care of Animals.
Procedures
Locomotor Activity Measurements
To measure changes in locomotor activity, rats were surgically implanted with transmitters (model ER-4000 E-Mitter; Mini Mitter Co. Inc., Bend, OR) under ketamine (100 mg/kg i.m.) and xylazine (10 mg/kg i.m.) anesthesia. The transmitter was implanted inside the peritoneal cavity of a rat at least 6 days before conducting an experiment, and after the surgical procedure, rats were singly housed. The transmitter broadcasted changes in location that were sent to a receiver (model ER-4000 Receiver, Mini Mitter Co., Inc.) placed under the home cage of each rat. Data were collected and processed simultaneously by the Vital View data acquisition system (Mini Mitter Co., Inc.). Counts of locomotor activity were summed every 5 min for each rat and recorded by Vital View software (Mini Mitter Co., Inc.). Baseline locomotor activity counts were collected for at least 40 min before handling or injection. After an injection of vehicle or the δ-opioid agonist SNC80 (n = 6 per treatment), locomotor activity measurements were collected for each rat for approximately 5 h, but graphical representations of the data include postinjection locomotor counts until activity levels returned to baseline. For tolerance studies, baseline and postinjection locomotor activity counts were recorded at the same time daily for 7 days.
Forced Swim Test
To measure antidepressant-like activity, six to eight rats per condition (n = 6–8/condition) were subjected to a modified forced swim test as described previously (Broom et al., 2002b). In brief, rats were placed gently in a clear cylindrical acrylic container (46 cm tall × 20 cm in diameter) filled with 30 cm of 25°C (±1°C) water for a single 15-min swim session. SNC80 (3.2, 10, or 32 mg/kg) was administered as a single subcutaneous injection 60 min before the forced swim test. For tolerance studies, rats were injected once daily with the same dose (0.32, 1.0, 3.2, 10, or 32 mg/kg SNC80) for 1 to 7 days and were then evaluated in the forced swim test 60 min after the last injection. In other studies, rats were administered once daily injections of increasing doses of SNC80 over 3 consecutive days (3.2, 10, and then 32 mg/kg SNC80) and were then evaluated in the forced swim test 60 min after the last injection. Cylinder water was changed after every rat. After the swim period, rats were removed from the water, towel-dried, and placed in a heated cage for 15 min.
Videotaped 15-min test swims were scored for immobility, swimming, and climbing behaviors (Detke et al., 1995). The individuals scoring the videotapes were blind to the drug treatments received by each rat. Every 5 s, the observer scored the rat’s behavior as one of the three categories: immobility, swimming, or climbing. The total counts of each behavior during the 15-min swim were averaged within treatment groups. These behaviors were defined as: immobility, floating in the water without struggling, and using only small movements to keep the head above water; swimming and moving limbs in an active manner (more than required to keep head above water), and causing movement between quadrants of the cylinder; and climbing, making active movements with the forepaws in and out of the water, often directed at the wall of the swim tank.
Convulsion Observations
Immediately after subcutaneous injection, rats were placed in an observation chamber for 20 min to observe for convulsions and cataleptic behaviors. Time to onset of convulsion, duration of convulsion, and duration of catalepsy were recorded. Catalepsy duration was defined as the time to remove forelimbs from a rod elevated approximately 2 inches off the ground. After the 20-min observation period, rats were returned to the home cage. For tolerance studies, rats were injected daily as described above.
Ex Vivo Agonist-Stimulated [35S]GTPγS Autoradiography
Agonist-stimulated [35S]GTPγS autoradiography was performed as previously described (Sim et al., 1995). Rats (n = 6–8/condition) were decapitated 24 h after a single injection of vehicle or 32 mg/kg SNC80 or 24 h after the last of six daily injections of vehicle or 32 mg/kg SNC80. Brains were removed and immediately frozen in 2-methylbutane (Sigma-Aldrich, St. Louis, MO) on dry ice. Coronal sections (20 μm) were cut throughout the brain on a cryostat maintained at −18°C, mounted on gelatin-subbed slides, and stored at −80°C for less than 4 weeks until use. Coronal sections were rinsed in TME buffer (50 mM Tris-HCl, 3 mM MgCl2, 0.2 mM EGTA, 100 mM NaCl, and 0.1% bovine serum albumin, pH 7.4) at room temperature for 10 min. After this first incubation, slices were incubated in TME buffer containing 2 mM GDP and 9.5 mU/ml adenosine deaminase for 15 min at room temperature. Then, slices were incubated in TME buffer with GDP, adenosine deaminase, 0.04 nM [35S]GTPγS, and 1 nM (+)BW373U86 or 10 μM cold GTPγS (to determine non-specific binding) for 2 h at room temperature. As determined from previous studies (Jutkiewicz et al., 2005), 1 nM (+)BW373U86 was determined to be the EC50. For some experiments, frontal cortex slices only were incubated with 0.32 nM (+)BW373U86, and some hippocampal slices were incubated with 10 μM (+)BW373U86. After the 2-h incubation, slices were rinsed twice for 2 min in cold 50 mM Tris buffer (pH 7.4) and once in distilled water for 1 min. Slides were air-dried for a few hours and exposed to film for 48 h in film cassettes together with 14C standards (GE Healthcare, Little Chalfont, Buckinghamshire, UK). Images were digitized, and densitometric analysis was conducted using NIH Image J software.
Data Handling and Statistical Analysis
Data from the forced swim test were averaged within each treatment. Totals for each behavioral measure (immobility, swimming, or climbing) were analyzed by one-way ANOVA, and Dunnett’s post hoc tests were used to compare drug-treated groups to vehicle-treated rats (Prism Software; GraphPad Software Inc., San Diego, CA). For studies involving increasing doses of SNC80, data were analyzed by one-way ANOVA with a significance level of p < 0.05 and Tukey’s post hoc test.
Locomotor activity data were summed over 5-min intervals beginning at least 40 min before injection with drug and continuing for at least 5 h after an injection of a test compound. Locomotor activity counts were averaged across rats in each treatment group and were calculated as time after injection ± 2 min. Time course data for each rat were also converted to a positive area under curve value relative to the average vehicle data for the corresponding test day. These values were subsequently averaged for each dose (or vehicle treatment) (Prism). Positive area under curve values were compared for each dose across days of injections or for each test day across drug treatments by one-way ANOVA with Tukey’s post hoc test.
For the [35S]GTPγS autoradiography experiments, nonspecific binding (NSB) [35S]GTPγS binding was subtracted from basal- and drug-stimulated levels, and drug-stimulated [35S]GTPγS binding was averaged across rats for each brain region in each treatment group and calculated as percent stimulation over basal. Stimulation levels between vehicle- and SNC80-treated rats were compared by Student’s t test for each brain region evaluated.
Drugs
All test compounds were injected subcutaneously in a volume of 1 ml/kg. SNC80 was dissolved in an 8% of 1 M HCl solution. (+)BW373U86 was dissolved in sterile water. Ketamine hydrochloride (Vedco Inc., St. Joseph, MO) and xylazine hydrochloride (Fermenta Animal Health Co., Kansas City, MO) were all dissolved in sterile water.
Results
Acute (subcutaneous) injection of 32 mg/kg SNC80 produced convulsions in 25 to 33% of rats (i.e., two rats of six or two rats of eight in two separate determinations). The SNC80-induced convulsions were brief, occurring 6 to 14 min after injection and lasted 10 to 30 s. The convulsions were manifested as clonic contractions involving the head, forelimbs, and neck. After a convulsion, rats were cataleptic for approximately 2 min, during which the rats also failed to demonstrate a righting reflex. These data are consistent with previous studies measuring the convulsive effects of SNC80 (Jutkiewicz et al., 2004). With subsequent administrations, SNC80 did not produce convulsions in any rats at any dose tested (data not shown), such that rats either convulsed after the first administration or they did not convulse at all.
In rats implanted with locomotor activity probes, baseline levels of activity before time 0 were low (Fig. 1a). Rats receiving vehicle injections demonstrated increased locomotor activity immediately after the injection, but activity levels returned to baseline within approximately 40 min. With an acute dose of 3.2 mg/kg SNC80, locomotor activity was stimulated for 100 to 120 min. Single doses of 10 and 32 mg/kg SNC80 increased initial activity counts above vehicle levels immediately after the injection, and locomotor activity remained elevated for approximately 160 to 180 min. In Fig. 1b, locomotor stimulation is graphed as positive area under curve relative to locomotor activity levels induced by vehicle injection for each day tested. A dose of 10 mg/kg SNC80 produced a robust increase in locomotor activity, more than doubling the level of locomotor activity produced by 3.2 mg/kg; however, 32 mg/kg SNC80-induced locomotor activity was slightly lower than that produced by 10 mg/kg. With 32 mg/kg SNC80, all rats became unstable and demonstrated uncoordinated movements and some rats convulsed and experienced catalepsy, potentially contributing to the slightly decreased but more variable activity observed.
Fig. 1.
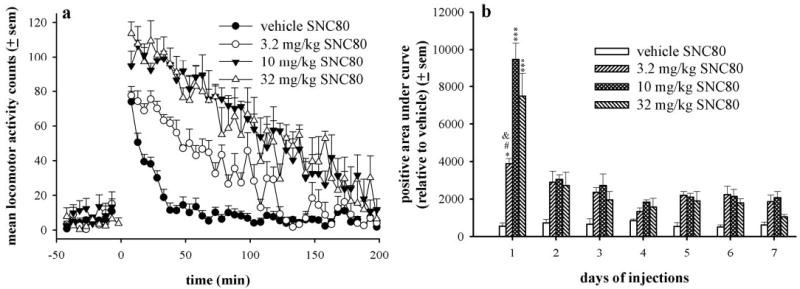
a, time course of effects of SNC80 on locomotor activity in Sprague-Dawley rats (n = 6 per dose) after an acute injection of vehicle, 3.2, 10, or 32 mg/kg. Locomotor activity counts before time 0 were baseline levels of activity before the injection (subcutaneously) that occurred at time 0. b, effects of repeated administration of SNC80 on locomotor activity in Sprague-Dawley rats. Data are represented as positive area under curve relative to averaged vehicle activity counts for each day, and statistical analysis is compared across subsequent days of injections for a single dose of SNC80 by one-way ANOVA with Tukey’s post hoc test.***, p < 0.001 as compared with all subsequent days of injections with either 10 or 32 mg/kg SNC80 by post hoc comparison. *, p < 0.05 as compared with days 2, 3, 5, and 6 of 3.2 mg/kg SNC80 by post hoc comparison; #, p < 0.001 as compared with day 4 with 3.2 mg/kg SNC80 by post hoc comparison and p < 0.01 as compared with day 7 with 3.2 mg/kg SNC80 by post hoc comparison.
With successive vehicle injections, injection-induced activity decreased, demonstrating adaptation or behavioral tolerance to the injection effect over time. Locomotor activity produced by subsequent daily injections of the same dose of SNC80 was represented as positive area under curve relative to averaged vehicle levels for each day (Fig. 1b). All of the tested doses of SNC80 produced less locomotor activity after repeated injections compared with the first injection [for 3.2 mg/kg, F(6,35) = 5.3, p < 0.001; for 10 mg/kg, F(6,35) = 35.42, p < 0.001; for 32 mg/kg, F(6,35) = 12.59, p < 0.001); however, 3.2 and 10 mg/kg SNC80 produced small but significant increases above vehicle levels even after 7 days of administration (p < 0.05 and p < 0.01, respectively). For example, with a second injection of 10 mg/kg SNC80, the level of activity was decreased more than 300% from the initial injection. Day 4 was the only day where there were no statistical differences between the treatment conditions.
Antidepressant-like effects of SNC80 were evaluated in the forced swim test after an acute injection or after 2, 3, or 7 days of injections. An acute injection of SNC80 significantly decreased immobility [F(4,29) = 9.54, p < 0.001], increased swimming [F(4,29) = 3.33, p < 0.05], and increased climbing [F(4,29) = 4.08, p < 0.01] (Fig. 2a). At 3.2 mg/kg SNC80, immobility was significantly decreased as compared with vehicle (p < 0.01). A single injection of 10 mg/kg SNC80 decreased immobility (p < 0.01) and increased climbing (p < 0.05). With 32 mg/kg SNC80, immobility was significantly decreased (p < 0.01), and swimming (p < 0.05) and climbing (p < 0.01) were increased as compared with vehicle treatment. After 2 days of injections, SNC80 significantly decreased immobility [F(4,29) = 4.39, p < 0.01], produced a trend to increase swimming [F(4,29) = 2.35, p = 0.08], but did not alter climbing (Fig. 2b). At doses of 10 and 32 mg/kg, SNC80 significantly decreased immobility as compared with vehicle (p < 0.05 for both). In addition, SNC80 dose dependently increased swimming; however, this effect was not statistically reliable. After 3 days of injections, SNC80 decreased immobility [F(4,28) = 10.72, p < 0.001] and increased swimming [F(4,28) = 12.25, p < 0.001] but did not alter climbing behaviors (Fig. 2c). Doses of 10 and 32 mg/kg SNC80 significantly decreased immobility (p < 0.01) and increased swimming (p < 0.01). After 7 days of agonist administration (Fig. 2d), SNC80 decreased immobility [F(4,28) = 6.47, p < 0.001], increased swimming [F(4,28) = 6.54, p < 0.001], and increased climbing [F(4,28) = 3.04, p < 0.05]. Doses of 10 and 32 mg/kg SNC80 decreased immobility (p < 0.01) and increased swimming (p < 0.05). In contrast to other drug administration regimens, 1.0 mg/kg SNC80 decreased immobility (p < 0.05) and increased climbing (p < 0.05).
Fig. 2.
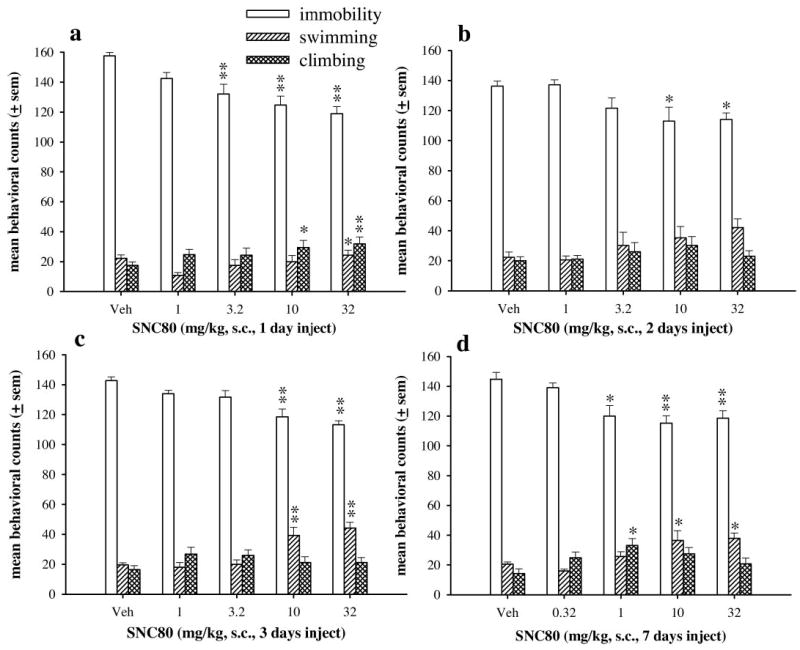
The effects of SNC80 in the forced swim test after acute (a), 2 (b), 3 (c), or 7 (d) days of drug administration in Sprague-Dawley rats (n = 6 per dose). Drug was injected 60 min before the forced swim test. The bars and vertical lines above each bar represent the mean and S.E.M. for immobility (open bars), swimming counts (single-hatched bars), and climbing counts (double-hatched bars). *, p < 0.05;**, p < 0.01 as compared with vehicle only as determined by Dunnett’s post hoc test.
Antidepressant-like effects of SNC80 were also evaluated in the forced swim test after administration of increasing doses of SNC80 over 3 days. Rats received either 3 days of vehicle injections, or 2 days of vehicle injections and 32 mg/kg SNC80 on day 3, or an injection of 3.2 mg/kg SNC80 on day 1, 10 mg/kg SNC80 on day 2, and 32 mg/kg on day 3 (Fig. 3). After 2 days of vehicle injections and 32 mg/kg SNC80 on day 3, five of six rats demonstrated convulsions; however, rats that received increasing doses of SNC80 over 3 days did not convulse. SNC80 administered either as a single injection of 32 mg/kg SNC80 or increasing doses over 3 days significantly decreased immobility [F(2,17) = 47.8, p < 0.001], increased swimming [F(2,17) = 11.22, p < 0.001], and increased climbing [F(2,17) = 6.36, p < 0.01] as compared with rats that received only vehicle injections. There were no statistical differences between rats receiving a single dose or three increasing doses of SNC80.
Fig. 3.
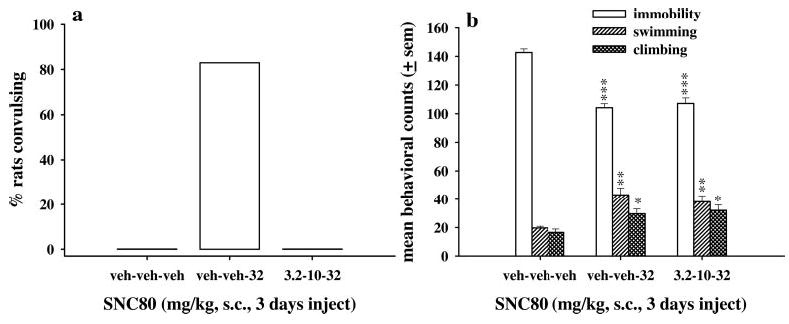
The effects of increasing doses of SNC80 on convulsions (a) and antidepressant-like effects (b) in Sprague-Dawley rats (n = 6 per dose). Either vehicle or one dose of SNC80 was injected once daily; 60 min after the final injection, rats were evaluated in the forced swim test. The bars and vertical lines above each bar represent the mean ± S.E.M. for immobility (open bars), swimming counts (single-hatched bars), and climbing counts (double-hatched bars). *, p < 0.05; **, p < 0.01;***, p < 0.001 as compared with vehicle only as determined by Dunnett’s post hoc test.
To measure G protein activation in different brain regions, brain slices from rats treated with vehicle or 32 mg/kg SNC80 24 h before sacrifice were compared in ex vivo [35S]GTPγS binding experiments. In brain slices from vehicle-pretreated rats, the approximate EC50 of (+)BW373U86 (1 nM; Jutkiewicz et al., 2005) produced high levels of [35S]GTPγS binding in forebrain regions, including the frontal cortex, nucleus accumbens, and caudate putamen, and in a one-hindbrain region, the pontine nuclei (Fig. 4a). Intermediate to low levels of [35S]GTPγS binding were measured in the prelimbic cortex, cingulate cortex, amygdala, piriform cortex, and thalamus in vehicle-pretreated rats. Some brain regions had minimal, if any, agonist-induced [35S]GTPγS binding at 1 nM (+)BW373U86, such as the hippocampus, hypothalamus, substantia nigra, and periaqueductal gray, in vehicle-pretreated rats. In brain slices from 32 mg/kg SNC80-pretreated rats, 1 nM (+)BW373U86 produced similar levels of [35S]GTPγS binding in all brains regions as compared with that observed in slices from vehicle-pretreated rats, except in the pontine nuclei. In the pontine nuclei, [35S]GTPγS binding was absent and, therefore, significantly lower in slices from SNC80-pretreated rats as compared with vehicle-treated rats (p < 0.05) (Fig. 4, a and e). In SNC80-pretreated rats, there was also a trend for less [35S]GTPγS binding in the nucleus accumbens as a whole as compared with slices from vehicle-treated rats (p = 0.07). More detailed analysis demonstrated that 1 nM (+)BW373U86 produced significantly less [35S]GTPγS binding in the nucleus accumbens core (p = 0.023) and a trend to less [35S]GTPγS binding in the nucleus accumbens shell (p = 0.096) from SNC80-treated rats as compared with vehicle-treated rats (Fig. 4b). Conversely, there was a trend of increased [35S]GTPγS binding in the hippocampus from SNC80-treated rats as compared with vehicle-treated rats (p = 0.09).
Fig. 4.
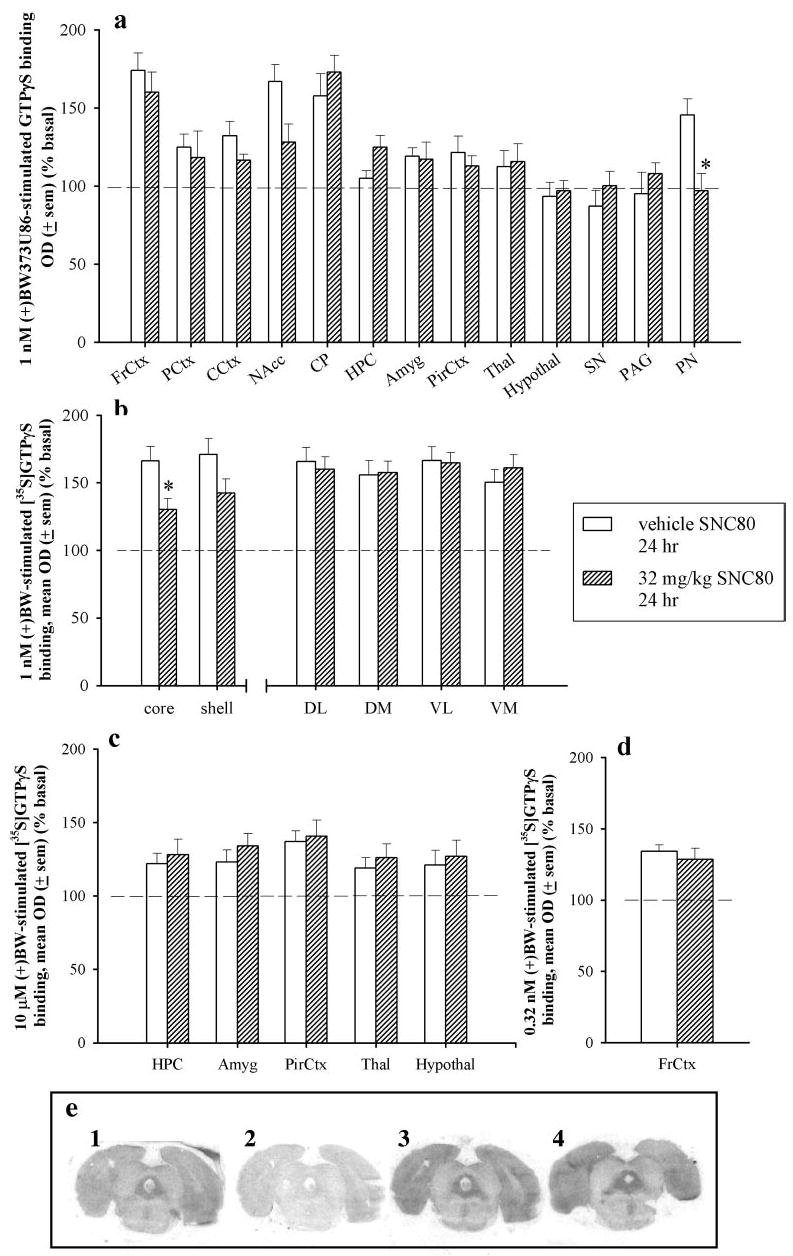
a, percent [35S]GTPγS stimulation by 1 nM (+)BW373U86 as compared with basal [35S]GTPγS stimulation in various brain regions from rats that received vehicle (open bars) or 32 mg/kg SNC80 (hatched bars) 24 h before sacrifice. FrCtx, frontal cortex; PCtx, prelimbic cortex; CCtx, cingulate cortex; NAcc, nucleus accumbens; CP, caudate putamen; HPC, hippocampus; Amyg, amygdala; PirCtx, piriform cortex; Thal, thalamus; hypothal, hypothalamus; SN, substantia nigra; PAG, periaqueductal gray; PN, pontine nuclei. *, p < 0.05 as compared with vehicle as determined by Student’s t test for each brain region. b, percent [35S]GTPγS stimulation by 1 nM (+)BW373U86 as compared with basal [35S]GTPγS stimulation in selected subregions from rats that received vehicle (open bars) or 32 mg/kg SNC80 (hatched bars) 24 h before sacrifice. core, nucleus accumbens core; shell, nucleus accumbens shell; DL, dorsolateral caudate putamen; DM, dorsomedial caudate putamen; VL, ventrolateral caudate putamen; VM, ventromedial caudate putamen. c, percent [35S]GTPγS stimulation by 10 μM (+)BW373U86 as compared with basal [35S]GTPγS stimulation in various brain regions from rats that received vehicle (open bars) or 32 mg/kg SNC80 (hatched bars) 24 h before sacrifice. Abbreviations are same as listed above. d, percent [35S]GTPγS stimulation by 0.32 nM (+)BW373U86 as compared with basal [35S]GTPγS stimulation in one brain region from rats that received vehicle (open bars) or 32 mg/kg SNC80 (hatched bars) 24 h before sacrifice. Abbreviations are same as listed above. e, autoradiograms of rat brain slices containing the pontine nuclei: 1, basal; 2, NSB; 3, 1 nM (+)BW (in vehicle-treated rat brain slice); and 4, 1 nM (+)BW373U86 (in 32 mg/kg SNC80-treated rat brain slice) treatments.
[35S]GTPγS binding was evaluated in the frontal cortex at the EC50 for (+)BW373U86 in this region (0.32 nM; Jutkiewicz et al., 2005), and no differences were observed in 0.32 nM (+)BW373U86-stimulated [35S]GTPγS binding between slices from vehicle- and SNC80-treated rats (Fig. 4d). A maximal concentration of (+)BW373U86 (10 μM) was used to evaluate [35S]GTPγS binding in brain regions with low stimulation at 1 nM (+)BW373U86 (hippocampus, amygdala, piriform cortex, thalamus, and hypothalamus) from vehicle-and SNC80-treated rats (Fig. 4c); however, [35S]GTPγS binding did not differ between these two pretreatment conditions in any brain region tested.
After 6 days of vehicle or 32 mg/kg SNC80 injections, lower levels of [35S]GTPγS binding were found only in the caudate putamen of SNC80-treated rats as compared with vehicle-treated rats (p < 0.05) (Fig. 5, a and c). Although all subregions of the caudate putamen demonstrated less [35S]GTPγS binding in slices from SNC80-treated rats as compared with vehicle-treated rats (Fig. 5b), a significant decrease in [35S]GTPγS binding was observed in the dorsolateral caudate putamen (p < 0.05), and a nearly significant decrease was observed in the dorsomedial caudate putamen (p = 0.059). There was no statistical difference between the rostral and caudal measurements of [35S]GTPγS binding in the caudate putamen (data not shown). The loss of [35S]GTPγS stimulation in the pontine nuclei observed 24 h after an acute injection was no longer observed after 6 days of SNC80 administration.
Fig. 5.
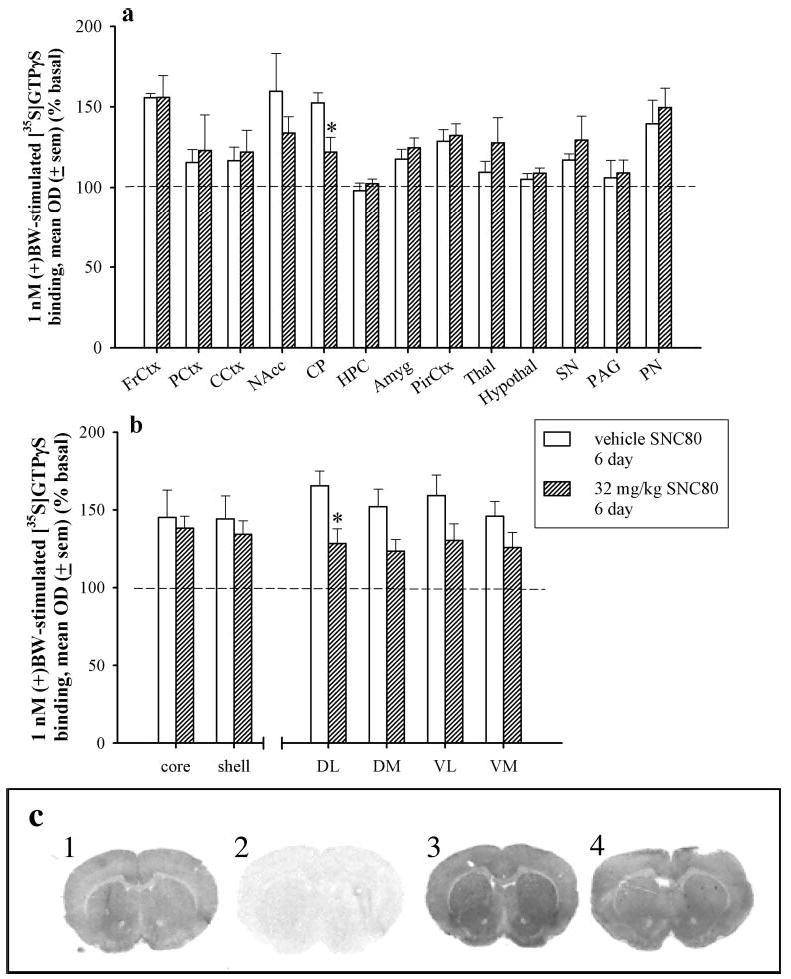
a, percent [35S]GTPγS binding by 1 nM (+)BW373U86 as compared with basal [35S]GTPγS binding in various brain regions from rats that received vehicle (open bars) or 32 mg/kg SNC80 (hatched bars) every 24 h for 6 days and were sacrificed 24 h after the sixth injection. FrCtx, frontal cortex; PCtx, prelimbic cortex; CCtx, cingulated cortex; NAcc, nucleus accumbens; CP, caudate putamen; HPC, hippocampus; Amyg, amygdala; PirCtx, piriform cortex; Thal, thalamus; hypothal, hypothalamus; SN, substantia nigra; PAG, periaqueductal gray; PN, pontine nuclei. *, p < 0.05 as compared with vehicle as determined by Student’s t test for each brain region. b, percent [35S]GTPγS binding by 1 nM (+)BW373U86 as compared with basal [35S]GTPγS binding in selected subregions from rats that received vehicle (open bars) or 32 mg/kg SNC80 (hatched bars) every 24 h for 6 days and were sacrificed 24 h after the sixth injection. core, nucleus accumbens core; shell, nucleus accumbens shell; DL, dorsolateral caudate putamen; DM, dorsomedial caudate putamen; VL, ventrolateral caudate putamen; VM, ventromedial caudate putamen. c, autoradiograms of striatal rat brain slices: 1, basal; 2, NSB; 3, 1 nM (+)BW (in vehicle-treated rat brain slice); and 4, 1 nM (+)BW373U86 (in 32 mg/kg SNC80-treated rat brain slice) treatments.
Discussion
The present study investigated the effects of repeated administration of the nonpeptidic δ-opioid agonist SNC80 on convulsive, locomotor-stimulating, and antidepressant-like effects in Sprague-Dawley rats. With repeated SNC80 treatment, tolerance developed to the convulsive and locomotor-stimulating effects of SNC80 but not to the antidepressant-like effects.
In the forced swim test, acute administration of SNC80 dose dependently decreased immobility, indicating an antidepressant-like effect. These antidepressant-like effects did not dissipate after 2, 3, or 7 days of once-daily injections. In contrast, tolerance developed to the convulsive effects of SNC80, such that 20 to 33% of rats convulsed after the first injection, but no rats convulsed with any subsequent injection. In the locomotor activity experiments, acute SNC80 dose dependently increased locomotor activity in the home cage. With repeated SNC80 administration, SNC80-induced locomotor activity was decreased as compared with acute activity levels at all doses tested. Finally, attenuated [35S]GTPγS binding suggests possible mechanisms for the observed tolerance to the behavioral effects of the δ-opioid agonist SNC80.
The results of the present study support previous findings demonstrating that tolerance to the convulsive effects of δ-opioid agonists developed rapidly (Comer et al., 1993; Broom et al., 2002a,c). Based on pharmacological principles, tolerance should develop to the behaviors requiring high drug efficacy, such as convulsions and antinociception (Broom et al., 2002c), before lower efficacy-related behaviors. Therefore, it might be expected that tolerance development to the locomotor-stimulating and antidepressant properties would require longer treatments or more agonist exposure, thereby causing increased receptor desensitization and/or down-regulation.
A single administration of SNC80 drastically decreased the amount of locomotor activity measured with subsequent SNC80 injections, suggesting rapid tolerance development to the locomotor-stimulating properties of SNC80. Each dose of SNC80 produced statistically similar levels of locomotor activity between days 2 and 7; however, SNC80-induced locomotor activity levels on days 2 to 7 were frequently, but not always, greater than that observed with vehicle-treated rats. For example, activity induced by 32 mg/kg SNC80 was statistically greater than vehicle levels on days 1 and 5 only, whereas activity induced by 3.2 mg/kg SNC80 was statistically greater than vehicle levels on days 1, 2, 5, 6, and 7. These findings are consistent with the idea that more tolerance occurs with administration of larger doses of SNC80. Locomotor activity after vehicle injection also decreased with subsequent injections, suggesting behavioral tolerance occurred with repeated handling and injection experiences. These data might suggest that the initial administration of SNC80 may have slowed or prevented the progression of behavioral tolerance with successive injections, such that SNC80-induced activity may not return to vehicle levels as vehicle-induced activity is continually changing. Alternatively, complete tolerance to SNC80-induced locomotor increases may never develop, potentially signifying that some portion of δ-opioid receptors related to the locomotor-stimulating properties fail to desensitize or down-regulate. For example, after a single SNC80 administration, (+)BW373U86-induced [35S]GTPγS binding did not change in the caudate putamen, a brain region generally associated with locomotor stimulation. However, after 6 days of SNC80 treatment, (+)BW373U86-induced [35S]GTPγS binding decreased by approximately 50% in the caudate putamen as compared with vehicle-treated rats. Overall, these data demonstrated that tolerance developed rapidly to the robust locomotor-stimulating properties of an acute injection of SNC80.
Although tolerance developed to the convulsive and locomotor-stimulating effects of the δ-opioid agonist SNC80, rapid tolerance did not develop to the antidepressant-like effects of SNC80. These data support previous findings that tolerance failed to develop to the antidepressant-like effects of the δ-opioid agonist (+)BW373U86 after 2 days of injections (Broom et al., 2002a; Torregrossa et al., 2004). In the present studies, the immobility level produced by 32 mg/kg SNC80 was similar across different treatment regimens, such that SNC80 engendered similar immobility levels between 113 and 118 counts after acute, two, three, or seven daily injections. Immobility levels differed slightly between the different vehicle-treated groups. Previously, various stressors have been shown to alter immobility levels in the forced swim test, such as restraint stress (Platt and Stone, 1982), and repeated injections might have had a similar effect in the present study. Despite these small differences, high doses of SNC80 still produced statistically significant and consistent decreases in immobility, indicating sustained antidepressant-like effects.
It might be suggested that the portion of locomotor stimulation remaining after repeated SNC80 contributed to the increased activity in the forced swim test, thus falsely identifying persistent antidepressant-like properties. However, previous studies demonstrated that δ-opioid agonist-induced antidepressant effects are separable from locomotor-stimulating effects of SNC80 (Broom et al., 2002b). In addition, the amount of locomotor stimulation was dramatically different between the first and subsequent injections; however, the highest dose of SNC80 produced similar levels of immobility in the forced swim test between acute, 2-, and 3-day treatments, as mentioned earlier. These data indicate that the locomotor stimulation was not related to increased activity in the forced swim test.
Ex vivo [35S]GTPγS binding experiments were used to evaluate tolerance development at the level of δ-opioid receptors in various brain regions (Fig. 4), such that tolerance would be manifested as reduced [35S]GTPγS binding as a result of desensitized receptors (i.e., decreased functional coupling of receptors and G proteins) or of a down-regulated receptor population. In these experiments, agonist-induced G protein activation was completely eliminated in the region of the pontine nuclei in rats that received 32 mg/kg SNC80 24 h earlier as compared with vehicle-treated rats. The pontine nuclei were demonstrated to have a high δ-opioid receptor density (Mansour et al., 1995) and are involved in relaying information between the cortex and the cerebellum. Additionally, this brain region was demonstrated to be involved in simple motor tasks, motor conditioning, and motor and spatial learning, as demonstrated by lesions in the pontocerebellar pathway that impaired motor coordination and equilibrium (Gasbarri et al., 2003). In relation to the present studies, acute administration of high SNC80 doses produced unstable and uncoordinated activity even in rats that did not convulse; however, this effect was not observed after subsequent drug administrations. Therefore, δ-opioid receptor activation in the pontine nuclei might contribute to the SNC80-induced impaired motor function, but with repeated agonist exposure, δ-opioid receptors in the pontine nuclei became uncoupled from the G protein, resulting in less [35S]GTPγS binding in brain slices and potentially more coordinated motor behavior in rats. However, after 6 days of SNC80 treatment, [35S]GTPγS binding in the pontine nuclei recovered, suggesting an adaptation of the system to repeated SNC80 exposure.
In addition to the pontine nuclei, a 24-h pretreatment with SNC80 decreased G protein activation in the nucleus accumbens. The nucleus accumbens is generally associated with the reinforcing effects of drugs. Under some conditions, rats intracranially self-administered the peptidic δ-opioid agonist [d-Pen2,d-Pen5]-enkephalin (Devine and Wise, 1994), but neither monkeys nor rats (L. F. Gomez and J. H. Woods, unpublished data) self-administered the nonpeptidic δ-opioid agonist SNC80 (Negus et al., 1998) or BW373U86 (Negus et al., 1994). Rapid tolerance development in the nucleus accumbens may contribute to the lack of self-administration of δ-opioid agonists. In addition to the reinforcing effects of drugs, striatal brain regions are generally associated with locomotor stimulation. The initial decrease in [35S]GTPγS binding in the nucleus accumbens followed by a subsequent decrease in the caudate putamen might contribute to the loss of SNC80-induced locomotor activity after repeated SNC80 administration.
Finally, in rats pretreated with SNC80, there was either no change or a trend to increase [35S]GTPγS binding in the hippocampus. The hippocampus is generally thought to be involved in δ-opioid agonist-induced convulsive activity (Haffmans and Dzoljic, 1983; DeSarro et al., 1992). Based on these findings, it was predicted that [35S]GTPγS binding would be decreased in rats that received SNC80 24 h earlier. However, at the maximal concentration tested (10 μM), no difference was observed between vehicle- and SNC80-treated rats. More recently, the piriform cortex was proposed as a brain region related to δ-opioid agonist-induced convulsion (Torregrossa et al., 2004); however, the present study did not observe differences in [35S]GTPγS binding between vehicle-and SNC80-treated rats. The present data suggest that another brain region, cellular mechanism, or downstream effector must contribute to observed tolerance to the convulsive effects of SNC80.
In conclusion, the present study investigated the effects of repeated administration of the δ-opioid agonist SNC80 on antidepressant-like effects, convulsions, and locomotor activity. Rapid tolerance developed to the convulsive and locomotor-stimulating effects of SNC80 but not to the antidepressant-like effects. In addition, uncoupling of the receptor from the G protein might contribute to some of the tolerance observed, but clearly, tolerance to the convulsive and locomotor effects of SNC80 observed in this study cannot be explained solely by G protein-receptor uncoupling. Finally, these results suggest that the antidepressant-like effects of the δ-opioid agonist SNC80 are separable from other SNC80-induced behavioral effects as measured by differential tolerance development. Moreover, a lack of observable tolerance to the antidepressant activity of SNC80 indicates that δ-opioid agonists could be effective on a long-term basis for anti-depressant therapy.
Footnotes
This work was supported by U. S. Public Health Service Grants DA00254, T32 GM07767, and T32 DA07267.
References
- Bot G, Blake AD, Li S, Reisine T. Opioid regulation of the mouse δ-opioid receptor expressed in human embryonic kidney 293 cells. Mol Pharmacol. 1997;52:272–281. doi: 10.1124/mol.52.2.272. [DOI] [PubMed] [Google Scholar]
- Brandt MR, Furness MS, Mello MK, Rice KC, Negus SS. Antinociceptive effects of delta-opioid agonists in Rhesus monkeys: effects on chemically induced thermal hypersensitivity. J Pharmacol Exp Ther. 2001a;296:939–946. [PubMed] [Google Scholar]
- Brandt MR, Furness MS, Rice KC, Fischer BD, Negus SS. Studies of tolerance and dependence with the δ-opioid agonist SNC80 in rhesus monkeys responding under a schedule of food presentation. J Pharmacol Exp Ther. 2001b;299:629–637. [PubMed] [Google Scholar]
- Breivogel CS, Selley DE, Childers SR. Acute and chronic effects of opioids on δ and μ receptor activation of G proteins in NG108–15 and SK-N-SH cell membranes. J Neurochem. 1997;68:1462–1472. doi: 10.1046/j.1471-4159.1997.68041462.x. [DOI] [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Convulsant activity of a nonpeptidic delta-opioid receptor agonist is not required for antidepressant-like effects. Psychopharmacology. 2002a;164:42–48. doi: 10.1007/s00213-002-1179-y. [DOI] [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Nonpeptidic delta-opioid receptor agonists reduce immobility in the forced swim assay in rats. Neuropsychopharmacology. 2002b;26:744–755. doi: 10.1016/S0893-133X(01)00413-4. [DOI] [PubMed] [Google Scholar]
- Broom DC, Nitsche JF, Pintar JE, Rice KC, Woods JH, Traynor JR. Comparison of receptor mechanisms and efficacy requirements for δ-agonist-induced convulsive activity and antinociception in mice. J Pharmacol Exp Ther. 2002c;303:723–729. doi: 10.1124/jpet.102.036525. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Negus SS, Gatch MB, Chang KJ, Woods JH. BW373U86, a delta-opioid receptor agonist, reverses bradykinin-induced thermal allodynia in rhesus monkeys. Eur J Pharmacol. 1995;277:285–287. doi: 10.1016/0014-2999(95)00134-7. [DOI] [PubMed] [Google Scholar]
- Comer SD, Hoenicke EM, Sable AI, McNutt RW, Chang K-J, De Costa BR, Mosberg HI, Woods JH. Convulsive effects of systemic administration of the δ-opioid agonist BW373U86 in mice. J Pharmacol Exp Ther. 1993;267:888–895. [PubMed] [Google Scholar]
- DeSarro GB, Marra R, Spagnolo C, Nisticò G. Delta opioid receptors mediate seizures produced by intrahippocampal injection of ala-deltorphin in rats. Funct Neurol. 1992;7:235–238. [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and nonadrenergic antidepressants. Psychopharmacology. 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Devine DP, Wise RA. Self-administration of morphine, DAMGO, and DPDPE into the ventral tegmental area of rats. J Neurosci. 1994;14:1978–1984. doi: 10.1523/JNEUROSCI.14-04-01978.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra LA, Schoenbaum GM, Yarbrough J, McNutt R, Chang K-J. A novel δ-opioid agonist, BW373U86, in squirrel monkeys responding under a schedule of shock titration. J Pharmacol Exp Ther. 1993;267:875–882. [PubMed] [Google Scholar]
- Fraser GL, Parenteau H, Tu T-M, Ducharme J, Perkins MN, Clarke PBS. The effects of δ agonists on locomotor activity in habituated and non-habituated rats. Life Sci. 2000;67:913–922. doi: 10.1016/s0024-3205(00)00690-1. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Pompili A, Pacitti C, Cicirata F. Comparative effects of lesions to the ponto-cerebellar and olivo-cerebellar pathways on motor and spatial learning in the rat. Neuroscience. 2003;116:1131–1140. doi: 10.1016/s0306-4522(02)00780-7. [DOI] [PubMed] [Google Scholar]
- Haffmans J, Dzoljic MR. Differential epileptogenic potentials of selective μ and δ opiate receptor agonists. J Neural Transmission. 1983;57:1–11. doi: 10.1007/BF01250043. [DOI] [PubMed] [Google Scholar]
- Hong EJ, Rice KC, Calderon S, Woods JH, Traynor JR. Convulsive behavior of nonpeptide δ-opioid ligands: comparison of SNC80 and BW373U86 in mice. Analgesia. 1998;3:269–276. [Google Scholar]
- Jutkiewicz EM, Eller EB, Folk JE, Rice KC, Traynor JR, Woods JH. δ-Opioid agonists: differential efficacy and potency of SNC80, its 3-OH (SNC86) and 3-desoxy (SNC162) derivatives in Sprague-Dawley rats. J Pharmacol Exp Ther. 2004;309:173–181. doi: 10.1124/jpet.103.061242. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Walker NP, Folk JE, Rice KC, Portoghese PS, Woods JH, Traynor JR. Comparison of peptidic and nonpeptidic δ-opioid agonists on [35S]GTPγS binding in brain slices from Sprague-Dawley rats. J Pharmacol Exp Ther. 2005;312:1314–1320. doi: 10.1124/jpet.104.078741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law P-Y, Maestri-El Kouhen O, Solberg J, Wang W, Erickson LJ, Loh HH. Deltorphin II-induced rapid desensitization of δ-opioid receptor requires both phosphorylation and internalization of the receptor. J Biol Chem. 2000;275:32057–32065. doi: 10.1074/jbc.M002395200. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implication. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- Negus SS, Butelman ER, Chang K-J, DeCosta B, Winger G, Woods JH. Behavioral effects of the systemically active δ-opioid agonist BW373U86 in rhesus monkeys. J Pharmacol Exp Ther. 1994;270:1025–1034. [PubMed] [Google Scholar]
- Negus SS, Gatch MB, Mello NK, Zhang X, Rice KC. Behavioral effects of the δ-selective opioid agonist SNC80 and related compounds in rhesus monkeys. J Pharmacol Exp Ther. 1998;286:362–375. [PubMed] [Google Scholar]
- Okura T, Cowell SM, Varga E, Burkey TH, Roeske WR, Hruby VJ, Yamamura HI. Differential down-regulation of the human δ-opioid receptor by SNC80 and [d-Pen2,d-Pen5]enkephalin. Eur J Pharmacol. 2000;387:R11–R13. doi: 10.1016/s0014-2999(99)00761-x. [DOI] [PubMed] [Google Scholar]
- Okura T, Varga EV, Hosohata Y, Navratilova E, Cowell SM, Rice K, Nagase H, Hruby VJ, Roeske WR, Yamamura HI. Agonist-specific down-regulation of the human δ-opioid receptor. Eur J Pharmacol. 2003;459:9–16. doi: 10.1016/s0014-2999(02)02823-6. [DOI] [PubMed] [Google Scholar]
- Pakarinen ED, Woods JH, Moerschbaecher JM. Repeated acquisition of behavioral chains in squirrel monkeys: comparisons of a mu, kappa and delta opioid agonist. J Pharmacol Exp Ther. 1995;272:552–559. [PubMed] [Google Scholar]
- Platt JE, Stone EA. Chronic restraint stress elicits a positive antidepressant response on the forced swim test. Eur J Pharmacol. 1982;82:179–181. doi: 10.1016/0014-2999(82)90508-8. [DOI] [PubMed] [Google Scholar]
- Remmers AE, Clark MJ, Liu XY, Medzihradsky F. δ-Opioid receptor down-regulation is independent of functional G protein yet is dependent on agonist efficacy. J Pharmacol Exp Ther. 1998;287:625–632. [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Childers SR. In vitro autoradiography of receptor-activated G proteins in rat brain by agonist-stimulated guanylyl 5′[γ-[35S]thio]-triphosphate binding. Proc Natl Acad Sci USA. 1995;92:7242–7246. doi: 10.1073/pnas.92.16.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina L, Longoni R, Mulas A, Chang K-J, Chiara G. Dopamine-dependent behavioural stimulation by non-peptide delta opioids BW373U86 and SNC80: I. locomotion, rearing and stereotypies in intact rats. Behav Pharmacol. 1998;9:1–8. [PubMed] [Google Scholar]
- Stevenson GW, Canadas F, Gomez-Serrano M, Ullrich T, Zhang X, Rice KC, Riley AL. Delta opioid discrimination learning in the rat: assessment with the selective delta agonist SNC80. Pharmacol Biochem Behav. 2002;71:291–300. doi: 10.1016/s0091-3057(01)00658-x. [DOI] [PubMed] [Google Scholar]
- Tao P-L, Seybold VS, Loh HH. Autoradiographic evidence for decrease in binding of μ- and δ-opioid receptors after subchronic [D-Ala2,D-Leu5]enkephalin treatment in rats. Eur J Pharmacol. 1993;231:145–149. doi: 10.1016/0014-2999(93)90442-k. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Isgor C, Folk JE, Rice KC, Watson SJ, Woods JH. The δ-opioid receptor agonist (+)BW373U86 regulates BDNF mRNA expression in rats. Neuropsychopharmacology. 2004;29:649–659. doi: 10.1038/sj.npp.1300345. [DOI] [PubMed] [Google Scholar]
- Tseng LF, Narita M, Mizoguchi H, Kawai K, Mizusuna A, Kamei J, Suzuki T, Nagase H. Delta-1 opioid receptor-mediated antinociceptive properties of a nonpeptidic delta opioid receptor agonist, (−)TAN-67, in the mouse spinal cord. J Pharmacol Exp Ther. 1997;280:600–605. [PubMed] [Google Scholar]
- Zhao G-M, Bhargava HN. Effects of multiple intracerebroventricular injections of [D-Pen2,D-Pen5]enkephalin and [D-Ala2,Glu4]deltorphin II on tolerance to their analgesic action and on brain δ-opioid receptors. Brain Res. 1997;745:243–247. doi: 10.1016/s0006-8993(96)01156-0. [DOI] [PubMed] [Google Scholar]


