Abstract
OBJECTIVE
The Diabetes Prevention Program demonstrated the ability to delay or prevent type 2 diabetes in participants with impaired glucose tolerance (IGT). Participants with IGT are at high risk for cardiovascular disease (CVD), with a marked increase in the number and severity of CVD risk factors. We prospectively assessed the impact of our interventions on hypertension, dyslipidemia, and CVD events.
RESEARCH DESIGN AND METHODS
The study group consisted of 3,234 individuals with IGT randomly assigned to receive intensive lifestyle intervention, metformin, or placebo. Annual assessment of blood pressure, lipids, electrocardiogram, and CVD events was undertaken.
RESULTS
Hypertension was present in 30% of participants at study entry and then increased in the placebo and metformin groups, although it significantly decreased with intensive lifestyle intervention. Triglyceride levels fell in all treatment groups, but fell significantly more with intensive lifestyle intervention. Total cholesterol and LDL cholesterol levels were similar among treatment groups. Intensive lifestyle intervention significantly increased the HDL cholesterol level and reduced the cumulative incidence of the proatherogenic LDL phenotype B. At 3 years of follow-up, the use for pharmacologic therapy to achieve established goals in the intensive lifestyle group was 27–28% less for hypertension and 25% less for hyperlipidemia compared with placebo and metformin groups. Over an average of 3 years, 89 CVD events from 64 participants were positively adjudicated studywide, with no differences among treatment groups.
CONCLUSIONS
Lifestyle intervention improves CVD risk factor status compared with placebo and metformin therapy. Although no differences in CVD events were noted after 3 years, achieved risk factor modifications suggest that longer intervention may reduce CVD event rates.
Abbreviations: ATP, Adult Treatment Panel; CVD, cardiovascular disease; DPP, The Diabetes Prevention Program; ECG, electrocardiogram; IGT, impaired glucose tolerance; NHANES, National Health and Nutrition Examination Survey
Impaired glucose tolerance (IGT), defined as a fasting glucose level < 7.0 mmol/l and a 2-h level postoral glucose challenge between 7.8 and 11.1 mmol/l, imparts a markedly increased risk of development of type 2 diabetes with a range from 3.58 to 8.73% per year (1). The Diabetes Prevention Program (DPP) demonstrated the effectiveness of both intensive lifestyle intervention and metformin therapy in delaying or preventing the development of type 2 diabetes in an ethnically diverse population with IGT (2). In this study, IGT was characterized by fasting glucose levels of 5.9 ± 0.5 mmol/l (mean ± SD) and 2-h postglucose load glucose levels of 9.1 ± 0.9 mmol/l. In addition, most participants were obese with BMI of 34.0 ± 7.0 kg/m2 and hyperinsulinemic with a fasting insulin value of 185.7 ± 105.3 pmol/l. Increased prevalence of cardiovascular disease (CVD) and levels of CVD risk factors are seen in participants with degrees of hyperglycemia not yet reaching the diagnostic cutoff for diabetes (3–8). Some argue that the glycemic threshold for the development of macrovascular disease is lower than that for the development of microvascular disease, which serves as the indicator for the diagnosis of diabetes (9). Others find the relationship to CVD to be stronger with either elevated insulin levels (10) or demonstrable insulin resistance (11). Finally, others contend that the clustering of CVD risk factors seen in insulin-resistant participants may explain the excess CVD risk (12).
There are limited data for the impact of glucose-lowering strategies on the development of CVD events or risk factors in patients with IGT or nondiabetic patients. We prospectively assessed the prevalence of CVD and its risk factors in DPP participants, comparing the impact of intensive lifestyle intervention or metformin therapy with that of a placebo.
RESEARCH DESIGN AND METHODS
The study design and baseline characteristics of the study participants have been reported (13,14). Briefly, 3,234 individuals with IGT were identified for randomization in 27 clinical centers throughout the U.S. Eligibility required a fasting plasma glucose level of 5.3–6.9 mmol/l and a 2-h value after a 75-g glucose load of 7.8–11 mmol/l. The minimum fasting glucose requirement was removed for American-Indian participants. Exclusion criteria included medicines known to alter glucose tolerance, an illness with reduced life expectancy, or inability to participate in physical activity. CVD events were exclusionary if they occurred ≤ 6 months before randomization.
Eligible participants were randomly assigned to one of three interventions: metformin at a dose of 850 mg twice daily, placebo twice daily, or an intensive program of lifestyle modification. Random treatment assignments were stratified according to clinical center and double blinded for the metformin and placebo groups. The goals of the intensive lifestyle modification program were to achieve and maintain a weight reduction of at least 7% of initial body weight through consumption of a healthy low-calorie, low-fat diet and to engage in physical activity of moderate intensity (such as brisk walking) for at least 150 min/week (15).
A detailed history and physical examination were performed before randomization and annually thereafter. A 12-lead electrocardiogram (ECG) was completed using MACPC-DT ECG acquisition units (Marquette Electronics, Milwaukee, WI) and transmitted to the Electrocardiographic Reading Center (Epicare, Department of Public Health Sciences, Bowman-Gray School of Medicine, Winston-Salem, NC) for classification using the Minnesota code system (16,17). All participants completed a CVD risk status report including updates on family history of CVD as well as interim adverse events. If the participant described a potential CVD event, efforts to obtain hospital records, including all ECGs for review by the central reading center, were undertaken for event classification.
Blood pressure measurements were performed with a standard mercury manometer by certified staff at baseline and quarterly visits using a standard protocol, with the participant seated for at least 5 min before the measurement. A second pressure measurements was obtained 30 s later after complete cuff deflation. The mean of the two measurements was used for analysis. Hypertension was defined as blood pressure ≥ 140/90 mmHg or the use of antihypertensive medication. Participants meeting the criteria for hypertension were treated according to protocol by either their primary physicians or by clinical site staff, with primary agents being ACE inhibitors, angiotensin receptor blockers, or calcium channel antagonists.
Blood was drawn after a 12-h fast at baseline and annually thereafter. Total plasma cholesterol and triglyceride levels were measured using enzymatic methods standardized to the Centers for Disease Control and Prevention reference methods (18). HDL fractions of cholesterol were measured using dextran sulfate-Mg2+ to precipitate all of the apolipoprotein B–containing lipoproteins (19). The LDL cholesterol level was calculated by the Friedewald equation (20) unless the triglyceride level was > 4.5 mmol/l, in which case lipoprotein fractions were separated by preparative ultracentrifugation (21) and the LDL subfraction was determined by density gradient ultracentrifugation. Cholesterol was measured in each of 38 fractions using an enzymatic assay (Boehringer, Mannheim, Germany). The LDL cholesterol fractions were pooled, and the LDL cholesterol was determined enzymatically. Relative flotation (Rf) was determined by dividing the LDL peak fraction by the total number of fractions (n = 38). The predominance of small-density LDL was defined by the laboratory as Rf < 0.263 (10/38). National Cholesterol Education Program Adult Treatment Panel (ATP) II diagnostic criteria for hyperlipidemia (22) were applied to the triglyceride, LDL, and coronary heart disease risk status to determine the classification of participants into normolipidemia, hyperlipidemia requiring diet therapy only, or hyperlipidemia qualifying for drug therapy. Further, participants taking statins, bile acid sequestrants, or fibrates were classified as hyperlipidemic. Elevated LDL was defined as LDL levels of ≥ 3.4 mmol/l or use of lipid-lowering pharmacologic therapy. Elevated triglyceride was defined as levels ≥ 2.3 mmol/l or use of lipid-lowering therapy. Hyperlipidemia was defined as meeting criteria for lipid-lowering therapy. Participants and clinical research staff remained blinded to the actual lipid values until drug therapy was indicated.
Events classification
Copies of all ECGs from hospital admissions suspected to be CVD related were collected, and sequential changes indicating differing levels of ECG abnormality were coded according to Minnesota code criteria. Members of the Outcome Adjudication Committee, blinded to treatment assignment, adjudicated all CVD events based upon centrally scored ECG, clinical history, and cardiac enzyme determinations. Silent myocardial infarction was classified by comparing the scheduled annual ECG to the baseline if no prior hospital ECGs had been recorded.
Coronary revascularization included coronary artery bypass surgery or percutaneous angioplasty. Coronary artery disease by angiography was defined as ≥ 50% stenosis of any coronary artery in the absence of a clinical event and included some participants with inappropriate coronary lesions for revascularization or for whom revascularization failed. Classification of nonfatal stroke included a physician-diagnosed stroke in a patient hospitalized for > 24 h. Peripheral arterial disease was defined as a revascularization procedure or amputation for peripheral arterial disease. Cardiac arrhythmia was defined as a physician-diagnosed cardiac arrhythmia requiring a > 24-h hospitalization or a procedure such as insertion of a permanent pacemaker or implantable defibrillator. A diagnosis of congestive heart failure or unstable angina pectoris required physician diagnosis and a > 24-h hospitalization. CVD hospitalizations included cardiac arrhythmia, peripheral arterial disease, congestive heart failure, coronary artery disease by angiography, and unstable angina.
Statistical analysis
Participants were followed for an average of 3.2 years from the start of the study in June 1996 through 31 July 2001, a period 4 months longer than that reported previously (1). This period was chosen to maximize the available data that were collected during the masked phase of the DPP, as unmasking occurred in early August 2001. The report includes all adjudicated events that occurred before 31 July 2001 and were adjudicated by 7 May 2003.
The study design and analysis followed the intention-to-treat principle. CVD event rates among treatment groups were compared using Poisson regression. Fixed-effects models with the assumption of normally distributed errors (23) were used to assess mean differences over time in blood pressure, triglyceride levels, HDL cholesterol levels, and LDL particle size adjusted for the baseline value. Generalized estimating equations (23) were used to assess differences over time in the percentage of participants with categorically defined hypertension and dyslipidemia.
RESULTS
Baseline CVD risk factor characteristics for the cohort (mean age at entry was 51 years) have been previously reported (24,25). Prevalent CVD, hypertension, hypercholesterolemia, and hypertriglyceridemia at entry into DPP are shown by sex in Table 1. The low (< 5%) prevalence of CVD may reflect the exclusion of those who had a recent coronary event or reduced life expectancy, as well as a known tendency for selection of healthier participants into a clinical trial. Nevertheless, a substantial proportion of the participants were at risk for CVD based on the baseline prevalence of hypertension, elevated LDL cholesterol level, or elevated triglyceride levels.
Table 1.
Baseline CVD events and risk factors
| Overall | Men | Women | |
|---|---|---|---|
| n | 3,234 | 1,043 | 2,191 |
| Hypertension | 957 (29.6) | 387 (37.1) | 570 (26.0) |
| Elevated LDL (or taking medication) | 1,425 (44.1) | 509 (48.9) | 950 (43.4) |
| Elevated triglycerides (or taking medication) | 932 (28.8) | 302 (29.0) | 533 (24.4) |
| History of myocardial infarction | 32 (1.0) | 23 (2.2) | 9 (0.4) |
| History of stroke | 34 (1.1) | 18 (1.7) | 16 (0.7) |
| History of revascularization | 16 (0.5) | 13 (1.2) | 3 (0.1) |
| Minnesota code of myocardial infarction by ECG | 60 (2.0) | 34 (3.4) | 26 (1.3) |
Data are n (%).
Hypertension was present in 30% of participants at study entry and increased over time in both the placebo and metformin treatment groups (Fig. 1). In contrast, the intensive lifestyle modification group had no significant change in hypertension prevalence, accompanied by significantly decreased lower systolic and diastolic blood pressures, compared with that in the other treatment groups (P < 0.001; Table 2). The use of antihypertensive medications at baseline was 17% in all treatment groups. At 3 years, the point prevalence of antihypertensive pharmacologic therapy is significantly lower (by 27–28%) in the lifestyle group (23%) compared with that in the placebo (31%) and metformin (32%) groups (P < 0.001).
Figure 1.
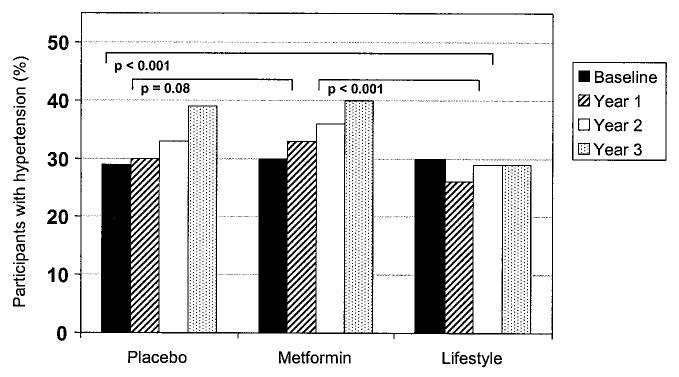
Categorical changes in hypertension over time by treatment assignment. P represents the pairwise comparison from generalized estimating equation models.
Table 2.
Blood pressure by treatment group regardless of pharmacologic therapy
| Placebo
|
Metformin
|
Intensive lifestyle
|
||||
|---|---|---|---|---|---|---|
| Blood pressure (mmHg) | Systolic | Diastolic | Systolic | Diastolic | Systolic | Diastolic |
| Baseline (means ± SD) | 123.5 ± 14.4 | 78.0 ± 9.2 | 124.0 ± 14.9 | 78.2 ± 9.5 | 123.7 ± 14.8 | 78.6 ± 9.2 |
| Change at year 1 | − 0.90 ± 0.4 | −0.89 ± 0.2 | −0.91 ± 0.4* | −1.26 ± 0.2* | −3.4 ± 0.4* | −3.6 ± 0.2* |
| Change at year 2 | −0.52 ± 0.4 | −1.07 ± 0.2 | −0.94 ± 0.4* | −1.06 ± 0.2* | −3.4 ± 0.4* | −3.33 ± 0.2* |
| Change at year 3 | −0.57 ± 0.5 | −1.88 ± 0.3 | −0.29 ± 0.5* | −1.59 ± 0.3* | −3.27 ± 0.5* | −3.82 ± 0.3* |
Data are means ± SE unless otherwise noted.
P < 0.001 vs. placebo for changes in mean over time.
Total cholesterol and LDL cholesterol levels at study entry did not differ significantly among the groups, at 5.3 mmol/l (203 mg/dl) and 3.2 mmol/l (124 mg/dl), respectively. Over time, there were no statistical differences among the placebo, metformin, and intensive lifestyle groups in the overall mean percent change from baseline in either total cholesterol level (− 1.2% vs. − 0.9% vs. − 2.3%, respectively) or LDL cholesterol level (− 1.3% vs. − 0.3% vs. − 0.7%, respectively).
Triglyceride levels fell in all groups, but fell significantly more in the intensive lifestyle group (− 0.296 mmol/l [− 25.4 mg/dl]) than in the placebo (− 0.13 mmol/l [− 11.9 mg/dl]) and metformin (− 0.08 mmol/l [− 7.4 mg/dl]) groups (Fig. 2A). The HDL cholesterol level significantly increased in the lifestyle group (− 0.026 mmol/l [− 1.0 mg/dl]) compared with the metformin (− 0.008 mmol/l or [− 0.3 mg/dl]) and placebo (− 0.002 mmol/l [− 0.1 mg/dl]) groups (Fig. 2B). Furthermore, intensive lifestyle favorably altered the LDL phenotype with a reduction in the prevalence of phenotype B representing a smaller, denser, more atherogenic LDL particle (P < 0.001 compared with both placebo and metformin) (Fig. 2C).
Figure 2.
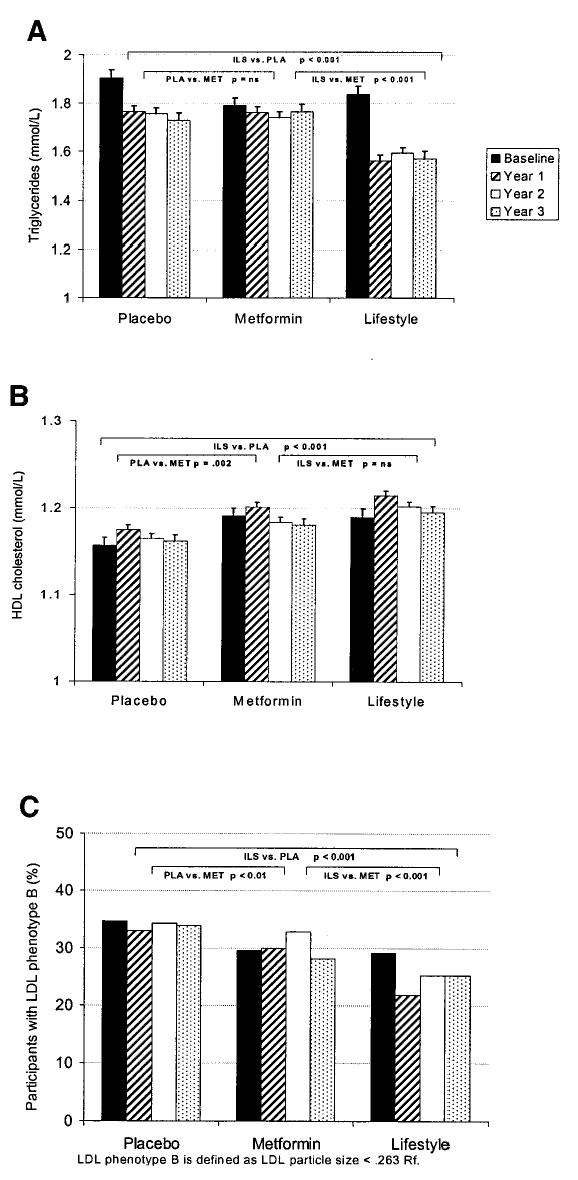
Mean lipid levels by treatment assignment and year. A: Mean triglyceride levels. B: Mean HDL cholesterol levels. C: Percent with LDL phenotype B. P represents the pairwise comparisons of the treatment groups from repeated-measures models (A and B) and a general estimating equation model (C).
At study entry, only 5.2% of participants reported taking pharmacologic therapy for dyslipidemia. Fewer intensive lifestyle participants (12%) required drug therapy for either elevated triglyceride or LDL cholesterol levels by ATP II guidelines at year 3 compared with placebo (16%) or metformin (16%) (Fig. 3).
Figure 3.
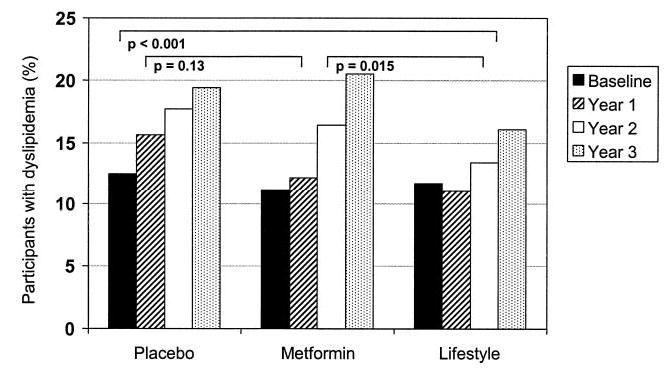
Prevalence of hyperlipidemia by treatment assignment. Hyperlipidemia was defined by either treatment with cholesterol or triglyceride-lowering pharmacologic therapy or by laboratory values meeting ATP II criteria for institution of drug terapy. P represents the pairwise comparison of treatment groups from generalized estimating equation models.
Only 89 CVD events were confirmed through 31 July 2001 by adjudication over an average of 3 years of follow-up per participant. Neither the cumulative incidence of CVD (percentage of participants suffering an event) nor the event rate (number of events per 1,000 patient years exposed) was different among groups. CVD-related deaths occurred in only four participants in the placebo group, one in the metformin group, and two in the intensive lifestyle group. Nonfatal CVD events occurred in 18 placebo participants (1.7%) with an incidence of 7.3 events per 1,000 patient-years. No statistically significant differences were seen in the metformin group (1.5%, 5.2/1,000 patient-years) or the intensive lifestyle group (2.2%, 9.7/1,000 patient-years) compared with placebo. The small non-significant excess of events in the intensive lifestyle group consisted of CVD hospitalizations and revascularization procedures. Exercise tolerance testing was performed for safety at the time of study entry in those participants randomly assigned to the intensive lifestyle intervention who either had a history of preexisting CVD or were considered to be at high risk for CVD. This increased ascertainment of CVD is supported by five events observed in the first 6 months of the study among the intensive lifestyle group, most of which were revascularization procedures. In addition, study personnel saw participants in the intensive lifestyle group far more often as part of their core curriculum. With the initiation of a physical activity program, case managers were cognizant of the potential exacerbation of CVD. It is possible that the increased activity associated with the intervention differentially unmasked preexisting CVD in this group.
CONCLUSIONS
An estimated 10 million persons in the U.S. meet the DPP eligibility criteria for fasting glucose, age, and BMI (26). IGT, although not usually considered a clinical disease in its own right, is recognized as a risk factor for the future development of both diabetes and cardiovascular disease (27). Stern (28) hypothesized that both stem from a “common soil” of risk factors with variable expression of pathophysiologic changes over time. Metaregression analysis demonstrates a continuous and positive relationship between 2-h postglucose levels and the subsequent development of CVD (29). When adjusted for covariates such as BMI, blood pressure, and cholesterol, IGT increased the hazard rate for CVD mortality by 34% (30). The National Health and Nutrition Examination Survey (NHANES) II data showed a 42% increased relative risk of all causes of mortality and a 19% increased risk for CVD death among the subset with IGT (31). In NHANES III, however, a cross-sectional association between IGT and nonfatal myocardial infarction and stroke was entirely attributable to traditional CVD risk factors (32). These data highlight the need for a randomized controlled clinical trial such as DPP to better assess causality.
CVD risk factors are typically present in individuals with IGT (33–35). Increased abdominal obesity together with hyperinsulinemia, hypertension, elevated triglyceride levels, and lower HDL cholesterol levels are observed in patients with IGT and constitute the core characteristics of metabolic syndrome as defined by ATP III (36). A recent report from NHANES suggests an overall metabolic syndrome prevalence in the U.S. of ~ 22% or ~ 47 million people (37).
The DPP cohort entered the study with prevalence of hypertension of 30%, hypertriglyceridemia of 29%, and hypercholesterolemia of 44%. Annual assessment of these outcomes demonstrated progressive increases in prevalence of hypertension and dyslipidemia in the placebo and metformin groups with attenuation by intensive lifestyle intervention. Aggressive blood pressure management was mandated by protocol, and all treatment groups demonstrated absolute reductions in systolic and diastolic blood pressures; however, intensive lifestyle intervention achieved this with only 5% additionally requiring antihypertensive therapy, compared with 14–15% in the placebo and metformin participants.
Annual assessment of lipids was performed and placed into risk categories according to the ATP II standards applicable at the time. Although mean levels of total cholesterol and LDL cholesterol changed very little during the course of the trial and did not differ among treatment groups, the need for LDL-lowering pharmacologic therapy was significantly less in the intensive lifestyle group compared with that in either placebo or metformin groups (both P < 0.001). Triglyceride levels fell during intensive lifestyle intervention compared with the other treatments, and again participants in this group required less pharmacologic intervention (12% of participants) compared with placebo and metformin (16 and 20.1% with P < 0.03, respectively). The deterioration in lipid levels and blood pressure demonstrated by those in the placebo group reflect the high-risk status of our population with IGT and the lack of efficacy of metformin in modulating that risk.
Reductions in serum triglyceride levels were accompanied by concomitant increases in HDL cholesterol levels and LDL cholesterol size in the intensive lifestyle group, sustained over the course of the study. LDL density, assessed by Rf, was comparable among treatment groups at study entry with mean values at the threshold for the small dense LDL phenotype. Intensive lifestyle intervention caused a prompt increase in LDL size with mean values well into the large buoyant range and a significant (P < 0.001) reduction in the percentage of patients with the proatherogenic phenotype B.
The few CVD events in the DPP did not provide adequate statistical power to test for a significant impact of lifestyle interventions. The ongoing additional 5 years of follow-up in the DPP Outcomes Study will permit additional study of the impact of DPP interventions on CVD events.
In summary, intensive lifestyle intervention reduced known risk factors for CVD including hypertension, high triglyceride levels, low HDL levels, and small dense LDL. Such risk factor modification in other trials (38–41) resulted in substantial reductions in both fatal and nonfatal CVD events, suggesting that prolonged observation of our cohort may ultimately demonstrate a beneficial CVD effect of lifestyle change.
Acknowledgments
Funding was provided by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases, the Office of Research on Minority Health, the National Institute of Child Health and Human Development, the Office of Women’s Health, and the National Institute on Aging. In addition, the Indian Health Service, the Centers for Disease Control and Prevention, the American Diabetes Association, Bristol-Myers Squibb, and Parke-Davis contributed support. The General Clinical Research Center Program, National Center for Research Resources, supported many of the clinical centers. Support to the clinical centers and the Coordinating Center was provided by the National Institute of Diabetes and Digestive and Kidney Diseases through a Cooperative Agreement, except for the Southwestern American Indian Centers, which were supported directly by the National Institute of Diabetes and Digestive and Kidney Diseases and the Indian Health Service.
LifeScan, Health O Meter, Hoechst Marion Roussel, Lipha Pharmaceuticals, Merck-Medco Managed Care, Merck, Nike Sports Marketing, Slim Fast Foods, and Quaker Oats donated materials, equipment, or medicines for concomitant conditions. McKesson BioServices, Matthews Media Group, and the Henry M. Jackson Foundation provided support services under subcontract with the Coordinating Center.
We thank the thousands of volunteers in this program for their devotion to the goal of diabetes prevention.
Footnotes
A table elsewhere in this issue shows conventional and Système International (SI) units and conversion factors for many substances.
See accompanying editorial, p. 971.
References
- 1.Edelstein SL, Knowler WC, Bain RP, Andres R, Barrett-Connor EL, Dowse GK, Haffner SM, Pettitt DJ, Sorkin JD, Muller DC, Collins VR, Hamman RF. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes. 1997;46:701–710. doi: 10.2337/diab.46.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuller JH, Shipley MJ, Rose G, Jarrett RJ, Keen H. Coronary heart disease risk and impaired glucose tolerance: the Whitehall Study. Lancet. 1980;8183:1373–1376. doi: 10.1016/s0140-6736(80)92651-3. [DOI] [PubMed] [Google Scholar]
- 4.Donahue RP, Abbott RD, Reed DM, Yano K. Post-challenge glucose concentration and coronary heart disease in men of Japanese ancestry: the Honolulu Heart Study. Diabetes. 1987;36:689–692. doi: 10.2337/diab.36.6.689. [DOI] [PubMed] [Google Scholar]
- 5.Wilson PWF, Cupples A, Kannel WB. Is hyperglycemia associated with cardiovascular disease? The Framingham Study. Am Heart J. 1991;121:586–590. doi: 10.1016/0002-8703(91)90729-2. [DOI] [PubMed] [Google Scholar]
- 6.Jackson CA, Yudkin JS, Forrest RD. A comparison of the relationships of the glucose tolerance test and the glycated hemoglobin assay with diabetic vascular disease in the community: the Islington Diabetes Survey. Diabetes Res Clin Pract. 1992;17:111–1123. doi: 10.1016/0168-8227(92)90156-l. [DOI] [PubMed] [Google Scholar]
- 7.Pyorala K, Savolainen E, Lehtovirta E, Punsar S, Siltanen P. Glucose tolerance and coronary heart disease: Helsinki Policemen Study. J Chron Dis. 1979;32:729–745. doi: 10.1016/0021-9681(79)90052-3. [DOI] [PubMed] [Google Scholar]
- 8.Ohlson LO, Svardsudd K, Welin L, Eriksson H, Wilhelmsen L, Tibblin G, Larsson B. Fasting blood glucose and risk of coronary heart disease, stroke and all cause mortality: a 17 year follow up study of men born in 1913. Diabet Med. 1986;3:33–37. doi: 10.1111/j.1464-5491.1986.tb00702.x. [DOI] [PubMed] [Google Scholar]
- 9.Gerstein HC. Glucose: a continuous risk factor for cardiovascular disease. Diabet Med. 1997;14:S25–S31. doi: 10.1002/(sici)1096-9136(199708)14:3+<s25::aid-dia441>3.3.co;2-t. [DOI] [PubMed] [Google Scholar]
- 10.Welborn TA, Wearne K. Coronary heart disease incidence and cardiovascular mortality in Busselton with reference to glucose and insulin concentrations. Diabetes Care. 1979;2:154–160. doi: 10.2337/diacare.2.2.154. [DOI] [PubMed] [Google Scholar]
- 11.Howard G, O’Leary DH, Zaccaro D, Haffner S, Rewers M, Hamman R, Selby JV, Saad MF, Savage P, Bergman R. Insulin sensitivity and atherosclerosis. Circulation. 1996;93:1809–1817. doi: 10.1161/01.cir.93.10.1809. [DOI] [PubMed] [Google Scholar]
- 12.Mykkanen L, Haffner SM, Ronnemaa T, Bergman RN, Laakso M. Low insulin sensitivity is associated with clustering of cardiovascular disease risk factors. Am J Epidemiol. 1997;146:315–321. doi: 10.1093/oxfordjournals.aje.a009272. [DOI] [PubMed] [Google Scholar]
- 13.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program: design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22:623–634. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program: Baseline characteristics of the randomized cohort. Diabetes Care. 2000;23:1619–1629. doi: 10.2337/diacare.23.11.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP): Description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crow RS, Prineas RJ, Hannan PJ, Grandits G, Blackburn H. Prognostic associations of Minnesota Code serial electrocardiographic change classification with coronary heart disease mortality in the Multiple Risk Factor Intervention Trial. Am J Cardiol. 1997;80:138–144. doi: 10.1016/s0002-9149(97)00307-x. [DOI] [PubMed] [Google Scholar]
- 17.Prineas RJ, Crow R, Blackburn H: The Minnesota Code Manual of Electrocardiographic Findings Littleton, MA, John Wright-PSG, 1982
- 18.Warnick GR. Enzymatic methods for quantification of lipoprotein lipids. Methods Enzymol. 1986;129:101–123. doi: 10.1016/0076-6879(86)29064-3. [DOI] [PubMed] [Google Scholar]
- 19.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantification of high density lipoprotein cholesterol. Clin Chem. 1982;28:1279–1288. [PubMed] [Google Scholar]
- 20.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without the use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 21.Hainline A Jr, Karon J, Lippel K (Eds.): Manual of Laboratory Operations. 2nd ed. Bethesda, MD, Lipid Research Clinics Program, Lipid and Lipoprotein Analysis, U.S. Department of Health and Human Service, National Institutes of Health, 1983
- 22.National Cholesterol Education Program. Summary of the second report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel II) JAMA. 1993;269:3015–3023. [PubMed] [Google Scholar]
- 23.Diggle PJ, Liang K-Y, Zeger SL: Analysis of Longitudinal Data. New York, Oxford University Press, 1994
- 24.The Diabetes Prevention Program Research Group. Hypertension, insulin and proinsulin in participants with impaired glucose tolerance: the Diabetes Prevention Program. Hypertension. 2002;40:679–686. doi: 10.1161/01.hyp.0000035706.28494.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Diabetes Prevention Program Research Group. Association of demographic, anthropometric and metabolic factors with baseline cardiovascular disease (CVD) risk factors in the Diabetes Prevention Program (Abstract) Diabetes. 2003;52:A168. [Google Scholar]
- 26.Trends in the prevalence and incidence of self-reported diabetes mellitus—United States, 1980–1994. MMWR Morb Mortal Wkly Rep. 1997;46:1014–1018. [PubMed] [Google Scholar]
- 27.Unwin N, Shaw J, Zimmet P, Alberti KGMM. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. 2002;19:708–723. doi: 10.1046/j.1464-5491.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 28.Stern MP. Diabetes and cardiovascular disease: the common soil hypothesis. Diabetes. 1995;44:369–374. doi: 10.2337/diab.44.4.369. [DOI] [PubMed] [Google Scholar]
- 29.Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events: a meta-regression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–240. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 30.DECODE Study Group. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hr diagnostic criteria. Arch Intern Med. 2001;161:397–405. doi: 10.1001/archinte.161.3.397. [DOI] [PubMed] [Google Scholar]
- 31.Saydah SH, Loria CM, Eberhardt MS, Brancati FL. Subclinical states of glucose intolerance and risk of death in the U.S. Diabetes Care. 2001;24:447–453. doi: 10.2337/diacare.24.3.447. [DOI] [PubMed] [Google Scholar]
- 32.Qureshi AI, Giles WH, Croft JB. Impaired glucose tolerance and the likelihood of nonfatal stroke and myocardial infarction: the Third National Health and Nutrition Examination Survey. Stroke. 1998;29:1329–1332. doi: 10.1161/01.str.29.7.1329. [DOI] [PubMed] [Google Scholar]
- 33.Laakso M, Lehto S. Epidemiology of risk factors for cardiovascular disease in diabetes and impaired glucose tolerance. Atherosclerosis. 1998;137:S65–S73. doi: 10.1016/s0021-9150(97)00314-6. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez BL, Curb JD, Burchfiel CM, Huang B, Sharp DS, Lu GY, Fujimoto W, Yano K. Impaired glucose tolerance, diabetes, and cardiovascular disease risk factor profiles in the elderly: the Honolulu Heart Program. Diabetes Care. 1996;19:587–590. doi: 10.2337/diacare.19.6.587. [DOI] [PubMed] [Google Scholar]
- 35.Liao D, Shofer JB, Boyko EJ, McNeely MJ, Leonetti DL, Kahn SE, Fujimoto WY. Abnormal glucose tolerance and increased risk for cardiovascular disease in Japanese Americans with normal fasting glucose. Diabetes Care. 2001;24:39–44. doi: 10.2337/diacare.24.1.39. [DOI] [PubMed] [Google Scholar]
- 36.National Institutes of Health: Third reportof the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III). Bethesda, MD, National Institute of Health, 2001 (NIH publ. no. 01-3670)
- 37.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the Third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 38.Goldberg RB, Mellies MJ, Sacks FM, Moye LA, Howard BV, Howard WJ, Davis BR, Cole TG, Pfeffer MA, Braunwald E. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analysis in the Cholesterol and Recurrent Events Trial. Circulation. 1998;98:2513–2519. doi: 10.1161/01.cir.98.23.2513. [DOI] [PubMed] [Google Scholar]
- 39.Haffner SM, Alexander CM, Cook TJ, Boccuzzi SJ, Musliner TA, Pedersen TR, Kjekshus J, Pyorala K. Reduced coronary events in simvastatin treated patients with coronary heart disease and diabetes or impaired fasting glucose levels: subgroup analyses in the Scandinavian Simvastatin Survival Study. Arch Intern Med. 1999;159:2661–2667. doi: 10.1001/archinte.159.22.2661. [DOI] [PubMed] [Google Scholar]
- 40.Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253–259. [PubMed] [Google Scholar]
- 41.Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, Wilt TJ, Wittes J. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N Engl J Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]


