Abstract
The purpose of this study was to measure the tension in the flexor digitorum profundus (FDP) tendon in zone II and the digit angle during joint manipulations that replicate rehabilitation protocols. Eight FDP tendons from eight human cadavers were used in this study. The dynamic tension in zone II of the tendon and metacarpophalangeal (MCP) joint angle were measured in various wrist and digit positions. Tension in the FDP tendon increased with MCP joint extension. There was no tension with the finger fully flexed and wrist extended (synergistic motion), but the tendon force reached 1.77 ± 0.43 N with the MCP joint hyperextended 45 degrees with the distal interphalangeal and proximal interphalangeal joints flexed. The combination of wrist extension and MCP joint hyperextension with the distal interphalangeal and proximal interphalangeal joints fully flexed, what the authors term ‘‘modified synergistic motion,’’ produced a modest tendon tension and may be a useful alternative configuration to normal synergistic motion in tendon rehabilitation.
Flexor tendon injury is common and often results in disability, especially in the zone II region of the hand.1–3 Early mobilization, either passive or active, starting within a few days of repair, has been shown to produce superior results to postoperative immobilization.4–7 Early mobilization after tendon repair decreases adhesion formation and improves repair site strength, permitting more complete recovery of tendon excursion and digital range of motion.6,8,9 Early mobilization is thought to inhibit or disrupt adhesion formation and also to promote intrinsic healing and synovial diffusion, to produce stronger tissue than with immobilization.8–11
Despite its benefits, however, the safety and effectiveness of current mobilization techniques remain major concerns. If early active motion is too aggressive, it may also have the detrimental effect of causing gap formation or suture rupture.12–14 At the other extreme, traditional passive motion programs may not be aggressive enough. The digit may move but the tendon may not glide if the passive forces applied to the tendon do not overcome the internal resistance to gliding with the tendon sheath.15–18 For the tendon to move without breaking, the force applied to the tendon during the therapy must be greater than the gliding resistance during finger motion and less than the suture breaking strength.
In an effort to provide the optimal level of tendon loading to promote tendon motion, various approaches to postoperative tendon management have been developed, including passive, active, and synergistic mobilization protocols.11,19–23 Passive motion protocols, which have been used for several decades, are considered safe and achieve better excursion than immobilization protocols.21,24–26 However, the question remains whether the relatively low tension applied to the tendon during passive motion can reliably produce an effective excursion.27–29 Horii et al.18 reported that, in a cadaver model of passive mobilization after flexor tendon repair, with the wrist immobilized in flexion, buckling within the tendon sheath limited tendon excursion. Active motion protocols can potentially reduce the problem of buckling, but increase the risk of gap formation and tendon rupture after tendon repair.11,12
The synergistic motion protocol (wrist flexion with finger extension and wrist extension with finger flexion) combines elements of active and passive motion.9,22,30–32 Tendon tension is applied to the proximal portion of the flexor digitorum profundus (FDP) tendon by active extension of the wrist, while the fingers are moved passively. Synergistic motion rehabilitation increases tendon excursion compared with rehabilitation with the wrist fixed in flexion, as would be the case in the classic Kleinert protocol.18,30,31 In studies using a canine model, Lieber et al.29,32 reported that synergistic motion combined low tendon force, similar to passive motion, and high tendon excursion. However, when Silva et al.27 compared passive and synergistic motion methods using an in vivo canine model, increased in vivo tendon excursion due to synergistic wrist motion did not significantly affect ex vivo flexion of the distal and interphalangeal joints or tendon displacement. In addition, while the force produced by synergistic wrist motion increases the tendon tension, the force is still small and may not be enough to overcome friction, especially in an injured sheath and with a repaired tendon.
The tendon forces associated with passive and active finger motion and with pinch and grasp activities have been measured in patients undergoing surgery for carpal tunnel syndrome.33,34 However, the effect of digit position on tendon loading has not been explored or discussed. Bright et al.34 reported that the FDP tendon tension was 1 N in the resting position and ranged from 10 to 25 N for full active finger range of motion. Schuind et al.33 investigated the FDP tendon force at the wrist level in vivo during the wrist open surgery. The average FDP tendon force values were up to 1 N for passive finger flexion, 3 N for passive wrist extension, and 20 N for active finger flexion. They did not report joint position, though, thus precluding comparison of theoretical predictions with the measured tendon force.
We hypothesized that the tendon tension achieved by various hand therapy protocols might not be sufficient to overcome the forces likely to be experienced during tendon rehabilitation. The purpose of this study was therefore to compare the tendon tension in finger positions achieved during commonly used rehabilitation protocols after flexor tendon injury, by simultaneously measuring the FDP tendon tension in zone II and digit angle during simulation of these protocols.
MATERIALS AND METHODS
Subjects
Eight fresh frozen human forearms, including the elbow, were obtained from eight different cadavers. The specimens had a mean age of 79 years (range, 71–85 years). Eight FDP tendons from the second finger were used in this study. The sample size requirements were determined by a power calculation. Previous studies performed in our laboratory of tendon tension measured at the zone II level in a canine model have shown that the peak force generated for passive digit extension with the wrist extended was 16.4 N (SD 6.2 N), and 2.7 N (SD 0.4 N) with the wrist flexed. To detect such a difference with an alpha of 0.05 and a power of 0.8, for a two-sided comparison, required eight specimens per experimental group.
Procedure
The FDP tendon was approached through a mid-lateral incision in the finger between the A2 and A3 pulleys. The flexor tendon in zone II was exposed and the sheath was incised at the distal edge of the A2 pulley. Two marks were made on the FDP tendon at the level of the distal edge of the A2 pulley, one with the finger in full extension and the other with the finger in full flexion. The distance between these two markers was considered to be the normal range of tendon excursion in zone II.
The proximal phalanx was cut at the distal edge of the A2 pulley and the distal part of the finger was removed, along with the FDS tendon. A small cantilever beam transducer (BG-1000G; Kulite Semiconductor Products Inc., Leonia, NJ) was fixed to the remaining proximal phalanx with a screw. The sensitivity of this transducer was 0.01 N, with a range of 20 N. The FDP tendon was cut at the distal marker and the tendon end was connected to the transducer by a 2-0 braided polygalactic acid suture (Vicryl; Ethicon, Somerville, NJ). The distal mark on the FDP was kept at the level of the distal edge of the proximal pulley, representing the tendon position in full distal interphalangeal (DIP) joint and proximal interphalangeal (PIP) joint flexion (Figure 1).
FIGURE 1.

A small transducer was fixed to the remaining proximal phalanx with a screw. The FDP tendon was connected to this transducer transversely by a cable. Four external reflective markers were placed on the radial side of the second digit.
Metacarpophalangeal (MCP) joint position was measured with a two-dimensional motion analysis system (Motion Analysis Corporation, Santa Rosa, CA), using four spherical (5-mm diameter) retro-reflective markers placed on the radial side aspect of the second finger.35–39 Using rigid pins, two markers were attached to the remaining proximal phalanx and two markers were attached to the metacarpal (Figure 1). The angle of flexion-extension motion was recorded using the motion capture camera with the camera placed perpendicular to the long axis of the second finger and oriented so that all four retro-reflective markers stayed in focus during the testing.
The experimental design consisted of wrist and digit manipulation over the entire range of motion in one of the following ten configurations, with repeated testing: group 1 (DIP and PIP joint flexion): (a) wrist 60 degrees flexion, (b) wrist 30 degrees flexion, (c) wrist 0 degrees (d) wrist 30 degrees extension, and (e) wrist 60 degrees extension; group 2: (DIP and PIP joint extension): (a) wrist 60 degrees flexion, (b) wrist 30 degrees flexion, (c) wrist 0 degrees, (d) wrist 30 degrees extension, and (e) wrist 60 degrees extension.
The wrist position was maintained using a custom-built external fixator fitted on the ulnar aspect of the forearm, which allowed the wrist to be set at any selected angle of flexion and extension (Figure 1). The MCP joint was dynamically moved through its flexion/extension range of motion simulating the passive therapy commonly done after tendon surgery. Each cycle of manipulation took approximately 20 seconds. For each position, three repetitions were made. The first two repetitions were considered preconditioning; data were collected on the third repetition. Force and MCP joint angle data were collected by the computer at a frequency of 10 Hz.
After the measurement of tendon tension and MCP joint angle in DIP and PIP joint flexion, the FDP tendon was cut at the proximal marker to replicate the position of the FDP tendon during full extension of the DIP and PIP joints. The tendon was then reconnected to the transducer with the proximal marker set at the level of the distal edge of the proximal pulley and the procedures were repeated.
Data Analysis
At MCP angles of 90, 75, 60, 45, 30, and 15 degrees flexion and 0, 15, 30, and 45 degrees extension, the tendon force was analyzed using two-factor analysis of variance followed by a Tukey-Kramer multiple comparisons procedure to compare individual tendon tension measurement models, assuming unequal variances. In all cases, the statistical tests were two-sided and the threshold of statistical significance was set at p < 0.05.
RESULTS
The mean ± SD tendon excursion in zone II (i.e., the distance between the two tendon markers) was 20.9 ± 2.6 mm. The average dynamic tendon tension data for eight specimens during flexion-extension motion of the digit in each wrist and elbow position is shown in Figures 2 and 3, and the force data at 15-degree intervals are shown in Table 1. The highest passive forces were achieved with the wrist, DIP, and PIP joints in maximum extension (17.64 ± 3.29 N), while the lowest passive forces were achieved with the wrist, DIP, and PIP joints flexed (0 N) (Table 1).
FIGURE 2.
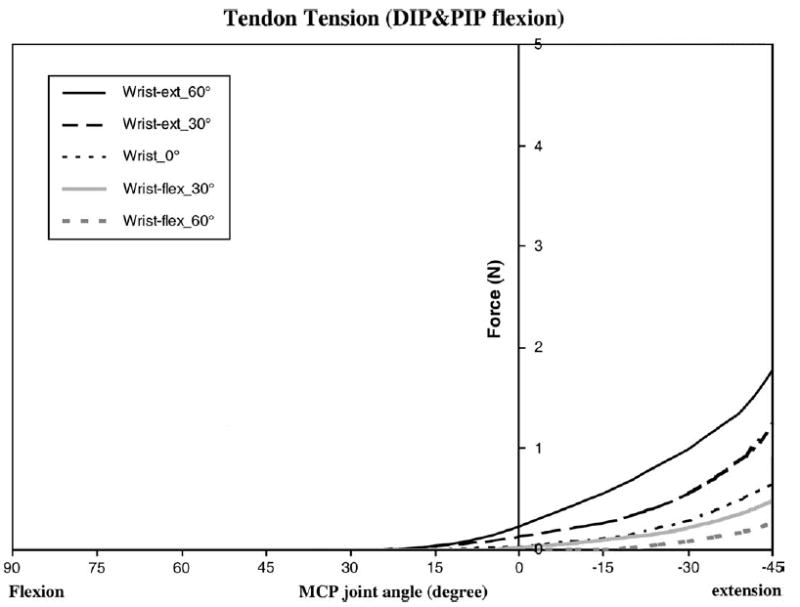
Average dynamic tendon tension data for the eight specimens in zone II with DIP and PIP joints in full flexion.
FIGURE 3.
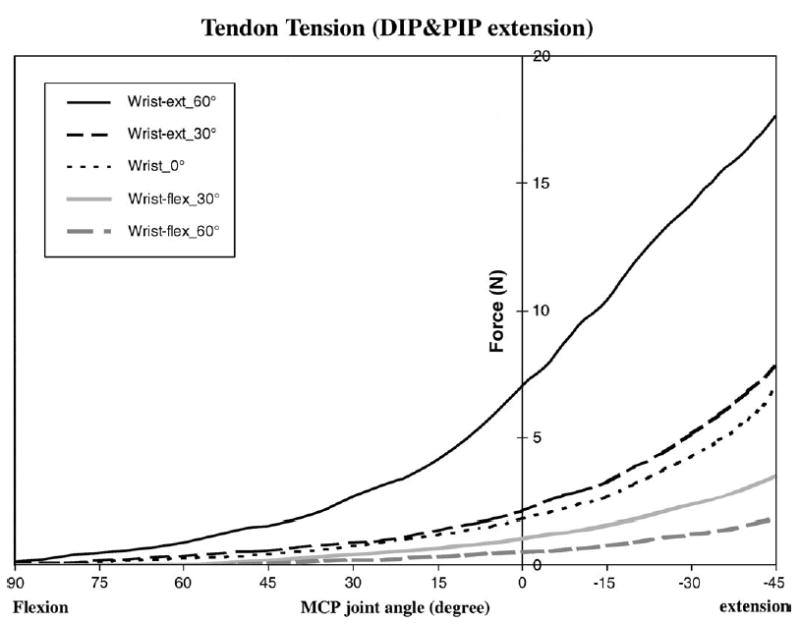
Average dynamic tendon tension data for the eight specimens in zone II with DIP and PIP joints in full extension.
TABLE 1.
Tendon Tension
| MCP | Flexion | Extension | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Wrist | 90° | 75° | 60° | 45° | 30° | 15° | 0° | 15° | 30° | 45° |
| DIP and PIP flexion | ||||||||||
| 60° (flex) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.08 ± 0.01 | 0.26 ± 0.06 |
| 30° (flex) | 0 | 0 | 0 | 0 | 0 | 0 | 0.02 ± 0.03 | 0.09 ± 0.04 A | 0.22 ± 0.05 A | 0.48 ± 0.12 A |
| 0° | 0 | 0 | 0 | 0 | 0 | 0 | 0.03 ± 0.05 | 0.11 ± 0.06 A | 0.28 ± 0.08 A | 0.64 ± 0.22 B |
| 30° (ext) | 0 | 0 | 0 | 0 | 0 | 0.03 ± 0.03 | 0.14 ± 0.04 C | 0.26 ± 0.08 C | 0.56± 0.17 C | 1.22 ± 0.32 C |
| 60° (ext) | 0 | 0 | 0 | 0 | 0 | 0.04 ± 0.03 D | 0.23 ± 0.07 D | 0.55 ± 0.11 D | 0.99 ± 0.20 D | 1.77 ± 0.43 D |
| DIP and PIP extension | ||||||||||
| 60° (flex) | 0 | 0 | 0 | 0.07 ± 0.07 | 0.21 ± 0.11 | 0.34 ± 0.11 | 0.54 ± 0.08 | 0.77 ± 0.21 | 1.22 ± 0.39 | 1.83 ± 0.69 |
| 30° (flex) | 0 | 0 | 0.02 ± 0.03 | 0.18 ± 0.12 | 0.40 ± 0.14 A | 0.67 ± 0.20 A | 1.06 ± 0.30 A | 1.58 ± 0.35 A | 2.38 ± 0.54 A | 3.44 ± 0.90 A |
| 0° | 0.04 ± 0.07 | 0.11 ± 0.14 | 0.25 ± 0.25 B | 0.44 ± 0.21 B | 0.74 ± 0.28 B | 1.19 ± 0.39 B | 1.79 ± 0.42 B | 2.68 ± 0.48 B | 4.23 ± 0.81 B | 6.95 ± 1.46 B |
| 30° (ext) | 0.05 ± 0.10 | 0.17 ± 0.11 A | 0.36 ± 0.11 C | 0.58 ± 0.14 C | 0.87 ± 0.16 C | 1.34 ± 0.33 B | 2.12 ± 0.68 C | 3.25 ± 0.90 C | 5.18 ± 1.30 C | 7.84 ± 1.86 C |
| 60° (ext) | 0.13 ± 0.06 D | 0.49 ± 0.14 D | 0.88 ± 0.22 D | 1.52 ± 0.48 D | 2.67 ± 0.80 D | 4.17 ± 1.06 D | 7.06 ± 2.03 D | 10.43 ± 2.81 D | 14.17 ± 2.76 D | 17.64 ± 3.29 D |
Data are presented as mean ± SD.
Statistical significant difference for each wrist positions is denoted by different letters (p < 0.05). flex = flexion; ext = extension.
The wrist position had a significant effect on tendon tension for any finger position. When the wrist was flexed 60 degrees, the tendon tension was significantly lower compared with when the wrist was extended ( p < 0.05) (Table 1). With the DIP and PIP joints extended, the peak tendon forces were 1.83 ± 0.69 N for 60-degree wrist flexion and 17.64 ± 3.29 N for 60-degree wrist extension. Similarly, with the DIP and PIP joints flexed, the peak tendon forces were 0.26 ± 0.06 N for 60-degree wrist flexion and 1.77 ± 0.43 N for 60-degree wrist extension.
The MCP joint position also had a large effect on tendon tension. Generally, the tendon tension in the zone II region was lower with MCP joint flexion and higher with MCP joint extension (Table 1). For the models of DIP and PIP joint flexion and DIP and PIP joint extension, the peak tendon force was significantly higher at 45 degrees MCP extension compared with 90 degrees MCP flexion, for a given wrist position ( p < 0.05).
DISCUSSION
The safe zone in which therapy operates is bounded by the strength of the repaired tendon, which represents the maximum force that can be applied, and the resistance of the digit to motion, which represents the minimum force that must be overcome. Although the safe zone for postoperative therapy lies between these two limits, there is little evidence to suggest that greater loading, once motion is achieved, produces better results. Thus, the ideal force would be closer to the lower bound than to the upper, as it would provide the greatest margin of safety against tendon rupture. However, there are little data from which the surgeon or therapist can estimate the forces that are being applied to the repaired tendon during postoperative hand therapy.
Previous studies in cadaveric models16,40 have shown that the gliding resistance measured during excursion of the FDP tendon within the A2 pulley for uninjured flexor tendons is about 0.3 N, but that after tendon repair, the peak gliding resistance increases significantly, to about 1.1 N. The force pulling proximally produced by the therapy must overcome at least this increased resistance to produce tendon excursion. The force needed to produce initial gap formation after tendon repair is reported to be about 7 N.40 Therefore, at least theoretically, the safe and effective zone for therapy should be between 1.1 and 7 N. However, the actual force applied to patients is also dependent upon factors such as joint stiffness, tendon elasticity, resistance from the synovial sheath and pulleys, and tendon edema, all of which will alter the force needed to induce tendon motion.
In our study, we investigated the effect of various combinations of wrist and finger position, to determine the tendon tension. We found that the effect of MCP joint position was significant, and we consider this an important finding of this study. Put in terms of commonly used hand therapy maneuvers, for example, there was no tension with the finger fully flexed with the wrist extended (synergistic motion), but the tendon force reached 1.77 N with the wrist extended, the MCP joint hyperextended 45 degrees, and the DIP and PIP joints flexed (hook fist position) (Table 1). This increased force is in the ‘‘safe zone’’ described above.
Based on our study, we have designed the following ‘‘modified synergistic’’ therapy protocol, which is the combination of synergistic motion and MCP joint hyperextension with DIP and PIP joint flexion (Figure 4).
FIGURE 4.
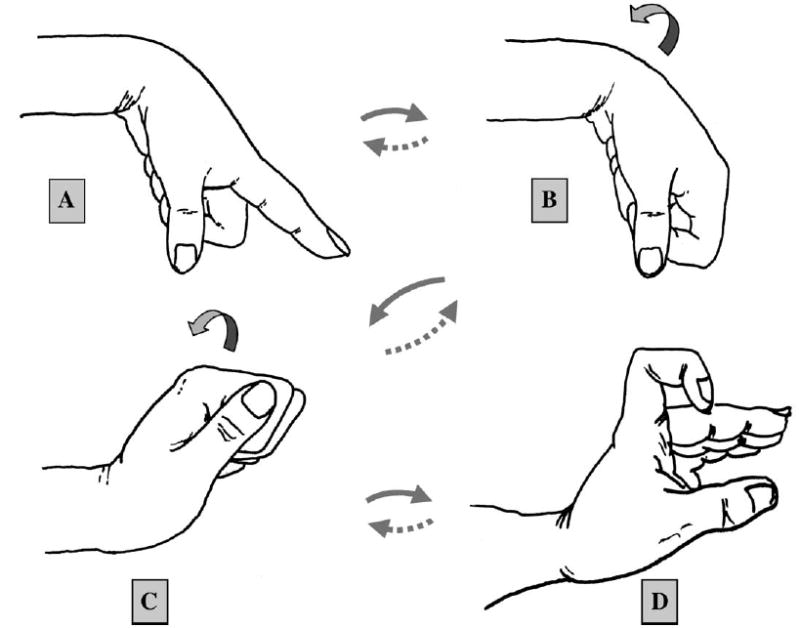
Modified synergistic motion protocol. Step A: Passive extension of the MCP joint with DIP and PIP joints fully extended and wrist flexed 60 degrees. This pulls the tendon distally. Step B: Passive full flexion of the finger with wrist flexed 60 degrees. Step C: Gradual extension of the wrist to 60 degrees with the finger fully flexed. Step D: Gradual and gentle extension of the MCP joint to 45 degrees while maintaining flexion of the DIP and PIP joints. This ‘‘modified synergistic’’ position applies additional proximal pull on the tendon. The sequence is the continued in reverse order to return to the starting position (A→B→C→D→C→B→A). The traditional passive therapy involves steps A→B ( passive finger flexion/extension with the wrist fixed in flexion) and the classical synergistic therapy ( passive finger extension with wrist flexion followed by finger flexion with wrist extension) is described by steps A→C.
Step A is the passive extension of the MCP joint with DIP and PIP joint fully extended, with the wrist flexed 60 degrees. During this motion the tendon is pulled distally. This motion is a common component of passive, synergistic, and active motion protocols. Step B is the passive full flexion of the finger with wrist flexed 60 degrees. Again, this position is common to the passive, synergistic, and active motion protocols. While exerting little tendon force in a proximal direction, it at least mobilizes the joints and soft tissues. Step C is the gradual extension of the wrist to 60 degrees with the finger fully flexed. This is one of the two classic synergistic positions. Step D is the gradual and gentle extension of the MCP joint to 45 degrees while maintaining flexion of the DIP and PIP joints. This ‘‘modified synergistic’’ position applies additional proximal pull on the tendon.
The sequence is then continued in reversed order to return to the starting position (A→B→C→D→C→B→A). We believe this modified synergistic therapy protocol may be an effective and safe therapy after tendon repair. For comparison, the traditional passive therapy involves steps A→B ( passive finger flexion/extension with the wrist fixed in flexion) and the classical synergistic therapy ( passive finger extension with wrist flexion followed by finger flexion with wrist extension) is described by steps A→C.
The main limitation of our study was that this was a cadaver study, so that the effects of physiologic forces, such as muscle tone and muscle contraction, were not considered. However, these forces are relatively small. Evans et al.41,42 reported the estimate of force in the relaxed position of the FDP tendon was 0.8 N. The maximum force that we measured during finger motion was of similar magnitude to the in vivo human study of Schuind et al.33 Of note, neither our study nor that of Schuind et al.’s looked at the forces generated by motion of repaired tendons. It is likely that because of swelling of the repaired tendon, adhesions, and postoperative joint stiffness, such forces would be greater, and could approach the limit of tendon repair strength. In addition, of course, is that because this was a cadaver study, we could not investigate the effect of this protocol on tendon healing. Further animal and clinical studies are needed to clarify the effect of modified synergistic motion in vivo.
Footnotes
This study was supported by National Institutes of Health (NIAMS) Grant AR44391.
References
- 1.Lane JM, Black J, Bora FW., Jr Gliding function following flexor-tendon injury. A biomechanical study of rat tendon function. J Bone Joint Surg Am. 1976;58:985–90. [PubMed] [Google Scholar]
- 2.Jansen CW, Watson MG. Measurement of range of motion of the finger after flexor tendon repair in zone ii of the hand. J Hand Surg [Am] 1993;18:411–7. doi: 10.1016/0363-5023(93)90083-f. [DOI] [PubMed] [Google Scholar]
- 3.Peck FH, Bucher CA, Watson JS, Roe A. A comparative study of two methods of controlled mobilization of flexor tendon repairs in zone 2. J Hand Surg [Br] 1998;23:41–5. doi: 10.1016/s0266-7681(98)80216-7. [DOI] [PubMed] [Google Scholar]
- 4.Halikis MN, Manske PR, Kubota H, Aoki M. Effect of immobilization, immediate mobilization, and delayed mobilization on the resistance to digital flexion using a tendon injury model. J Hand Surg [Am] 1997;22:464–72. doi: 10.1016/S0363-5023(97)80014-7. [DOI] [PubMed] [Google Scholar]
- 5.Woo SL, Gelberman RH, Cobb NG, Amiel D, Lothringer K, Akeson WH. The importance of controlled passive mobilization on flexor tendon healing. A biomechanical study. Acta Orthop Scand. 1981;52:615–22. doi: 10.3109/17453678108992156. [DOI] [PubMed] [Google Scholar]
- 6.Strickland JW, Glogovac SV. Digital function following flexor tendon repair in zone ii: A comparison of immobilization and controlled passive motion techniques. J Hand Surg [Am] 1980;5A:537–43. doi: 10.1016/s0363-5023(80)80101-8. [DOI] [PubMed] [Google Scholar]
- 7.Strickland JW. Flexor tendon repair. Hand Clin. 1985;1:55–68. [PubMed] [Google Scholar]
- 8.Feehan LM, Beauchene JG. Early tensile properties of healing chicken flexor tendons: Early controlled passive motion versus postoperative immobilization. J Hand Surg [Am] 1990;15:63–8. doi: 10.1016/s0363-5023(09)91107-8. [DOI] [PubMed] [Google Scholar]
- 9.Hitchcock TF, Light TR, Bunch WH, et al. The effect of immediate constrained digital motion on the strength of flexor tendon repairs in chickens. J Hand Surg [Am] 1987;12:590–5. doi: 10.1016/s0363-5023(87)80213-7. [DOI] [PubMed] [Google Scholar]
- 10.Baktir A, Turk CY, Kabak S, Sahin V, Kardas Y. Flexor tendon repair in zone 2 followed by early active mobilization. J Hand Surg [Br] 1996;21:624–8. doi: 10.1016/s0266-7681(96)80145-8. [DOI] [PubMed] [Google Scholar]
- 11.Small JO, Brennen MD, Colville J. Early active mobilisation following flexor tendon repair in zone 2. J Hand Surg [Br] 1989;14B:383–91. doi: 10.1016/0266-7681_89_90152-6. [DOI] [PubMed] [Google Scholar]
- 12.Elliot D, Moiemen NS, Flemming AF, Harris SB, Foster AJ. The rupture rate of acute flexor tendon repairs mobilized by the controlled active motion regimen. J Hand Surg [Br] 1994;19:607–12. doi: 10.1016/0266-7681(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 13.Silfverskiold KL, May EJ. Gap formation after flexor tendon repair in zone ii. Results with a new controlled motion programme. Scand J Plast Reconstr Surg Hand Surg. 1993;27:263–8. [PubMed] [Google Scholar]
- 14.Singer M, Maloon S. Flexor tendon injuries: the results of primary repair. J Hand Surg [Br] 1988;13B:269–72. doi: 10.1016/0266-7681_88_90083-6. [DOI] [PubMed] [Google Scholar]
- 15.Zhao C, Amadio PC, Zobitz ME, An KN. Gliding characteristics of tendon repair in canine flexor digitorum profundus tendons. J Orthop Res. 2001;19:580–6. doi: 10.1016/S0736-0266(00)00055-3. [DOI] [PubMed] [Google Scholar]
- 16.Zhao C, Amadio PC, Zobitz ME, Momose T, Couvreur P, An KN. Gliding resistance after repair of partially lacerated human flexor digitorum profundus tendon in vitro. Clin Biomech. 2001;16:696–701. doi: 10.1016/s0268-0033(01)00056-0. [DOI] [PubMed] [Google Scholar]
- 17.Zhao C, Amadio PC, Momose T, Couvreur P, Zobitz ME, An KN. The effect of suture technique on adhesion formation after flexor tendon repair for partial lacerations in a canine model. J Trauma. 2001;51:917–21. doi: 10.1097/00005373-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Horii E, Lin GT, Cooney WP, Linscheid RL, An KN. Comparative flexor tendon excursion after passive mobilization: an in vitro study. J Hand Surg [Am] 1992;17A:559–66. doi: 10.1016/0363-5023(92)90371-u. [DOI] [PubMed] [Google Scholar]
- 19.Duran RJ, Houser RG. Controlled Passive Motion Following Flexor Tendon Repair in Zones 2 and 3. St. Louis: CV Mosby, 1975, pp 105–14.
- 20.Gratton P. Early active mobilization after flexor tendon repairs. J Hand Ther. 1993;6:285–9. doi: 10.1016/s0894-1130(12)80329-2. [DOI] [PubMed] [Google Scholar]
- 21.Cannon NM, Strickland JW. Therapy following flexor tendon surgery. Hand Clin. 1985;1:147–65. [PubMed] [Google Scholar]
- 22.Cooney WP, Lin GT, An KN. Improved tendon excursion following flexor tendon repair. J Hand Ther. 1989;2:102–6. [Google Scholar]
- 23.Cullen KW, Tolhurst P, Lang D, Page RE. Flexor tendon repair in zone 2 followed by controlled active mobilisation. J Hand Surg [Br] 1989;14:392–5. doi: 10.1016/0266-7681_89_90153-8. [DOI] [PubMed] [Google Scholar]
- 24.Kleinert HE, Kutz JE, Atasoy E, Stormo A. Primary repair of flexor tendons. Orthop Clin North Am. 1973;4:865–76. [PubMed] [Google Scholar]
- 25.Dunn MJ, Johnson C. Static scapholunate dissociation: a new reconstruction technique using a volar and dorsal approach in a cadaver model. J Hand Surg [Am] 2001;26:749–54. doi: 10.1053/jhsu.2001.26025. [DOI] [PubMed] [Google Scholar]
- 26.Werntz JR, Chesher SP, Breidenbach WC, Kleinert HE, Bissonnette MA. A new dynamic splint for postoperative treatment of flexor tendon injury. J Hand Surg [Am] 1989;14:559–66. doi: 10.1016/s0363-5023(89)80025-5. [DOI] [PubMed] [Google Scholar]
- 27.Silva MJ, Brodt MD, Boyer MI, et al. Effects of increased in vivo excursion on digital range of motion and tendon strength following flexor tendon repair. J Orthop Res. 1999;17:777–83. doi: 10.1002/jor.1100170524. [DOI] [PubMed] [Google Scholar]
- 28.Ketchum LD, Thompson D, Pocock G, Wallingford D. A clinical study of forces generated by the intrinsic muscles of the index finger and the extrinsic flexor and extensor muscles of the hand. J Hand Surg [Am] 1978;3:571–8. doi: 10.1016/s0363-5023(78)80008-2. [DOI] [PubMed] [Google Scholar]
- 29.Lieber RL, Amiel D, Kaufman KR, Whitney J, Gelberman RH. Relationship between joint motion and flexor tendon force in the canine forelimb. J Hand Surg [Am] 1996;21:957–62. doi: 10.1016/S0363-5023(96)80299-1. [DOI] [PubMed] [Google Scholar]
- 30.Zhao C, Amadio PC, Momose T, Couvreur P, Zobitz ME, An KN. Effect of synergistic wrist motion on adhesion formation after repair of partial flexor digitorum profundus tendon lacerations in a canine model in vivo. J Bone Joint Surg Am. 2002;84:78–84. [PubMed] [Google Scholar]
- 31.Zhao C, Amadio PC, Zobitz ME, Momose T, Couvreur P, An KN. Effect of synergistic motion on flexor digitorum profundus tendon excursion. Clin Orthop Relat Res. 2002:223–30. doi: 10.1097/00003086-200203000-00033. [DOI] [PubMed] [Google Scholar]
- 32.Lieber RL, Silva MJ, Amiel D, Gelberman RH. Wrist and digital joint motion produce unique flexor tendon force and excursion in the canine forelimb. J Biomech. 1999;32:175–81. doi: 10.1016/s0021-9290(98)00154-7. [DOI] [PubMed] [Google Scholar]
- 33.Schuind F, Garcia-Elias M, Cooney WP, III, An KN. Flexor tendon forces: in vivo measurements. J Hand Surg [Am] 1992;17:291–8. doi: 10.1016/0363-5023(92)90408-h. [DOI] [PubMed] [Google Scholar]
- 34.Bright DS, Urbaniak J. Direct measures of flexor tendon tension during active and passive digit motion and its application to flexor tendon surgery. Trans Orthop Res Soc. 1976;1:240. [Google Scholar]
- 35.Chiu HY, Su FC, Wang ST, Hsu HY. The motion analysis system and goniometry of the finger joints. J Hand Surg [Br] 1998;23:788–91. doi: 10.1016/s0266-7681(98)80098-3. [DOI] [PubMed] [Google Scholar]
- 36.Gupta A, Rash GS, Somia NN, Wachowiak MP, Jones J, Desoky A. The motion path of the digits. J Hand Surg [Am] 1998;23:1038–42. doi: 10.1016/S0363-5023(98)80012-9. [DOI] [PubMed] [Google Scholar]
- 37.Rash GS, Belliappa PP, Wachowiak MP, Somia NN, Gupta A. A demonstration of validity of 3-d video motion analysis method for measuring finger flexion and extension. J Biomech. 1999;32:1337–41. doi: 10.1016/s0021-9290(99)00140-2. [DOI] [PubMed] [Google Scholar]
- 38.Somia N, Rash GS, Wachowiak M, Gupta A. The initiation and sequence of digital joint motion. A three-dimensional motion analysis. J Hand Surg [Br] 1998;23B:792–5. doi: 10.1016/s0266-7681(98)80099-5. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Lee SW, Braido P. Determining finger segmental centers of rotation in flexion-extension based on surface marker measurement. J Biomech. 2003;36:1097–102. doi: 10.1016/s0021-9290(03)00112-x. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka T, Amadio PC, Zhao C, Zobitz ME, Yang C, An KN. Gliding characteristics and gap formation for locking and grasping tendon repairs: a biomechanical study in a human cadaver model. J Hand Surg [Am] 2004;29:6–14. doi: 10.1016/j.jhsa.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 41.Evans RB, Thompson DE. The application of force to the healing tendon. J Hand Ther. 1993;6:266–84. doi: 10.1016/s0894-1130(12)80328-0. [DOI] [PubMed] [Google Scholar]
- 42.Evans RB, Thompson DE. Immediate active short arc motion following tendon repair. In: Hunter J (ed). Tendon and Nerve Surgery in the Hand. St. Louis: CV Mosby, 1997, pp 324–93.


