Summary
Cortisol plays an important role in learning and memory. An inverted-U shaped function has been proposed to account for the positive and negative effects of cortisol on cognitive performance and memory in adults, such that too little or too much impair but moderate amounts facilitate performance. Whether such relationships between cortisol and mental function apply to early infancy, when cortisol secretion, learning, and memory undergo rapid developmental changes, is unknown. We compared relationships between learning/memory and cortisol in preterm and full-term infants and examined whether a greater risk for adrenal insufficiency associated with prematurity produces differential cortisol–memory relationships. Learning in three-month old (corrected for gestational age) preterm and full-term infants was evaluated using a conjugate reinforcement mobile task. Memory was tested by repeating the same task 24 h later. Salivary cortisol samples were collected before and 20 min after the presentation of the mobile. We found that preterm infants had lower cortisol levels and smaller cortisol responses than full-term infants. This is consistent with relative adrenal insufficiency reported in the neonatal period. Infants who showed increased cortisol levels from 0 to 20 min on Day 1 had significantly better memory, regardless of prematurity, than infants who showed decreased cortisol levels.
Keywords: Stress, Memory, Infancy, HPA-axis, Cortisol, Glucocorticoid receptors
1. Introduction
Cortisol secretion (i.e. glucocorticoids, GC) modulates neuronal activity in regions of the brain that play a role in learning and memory (e.g. hippocampus). In rodent studies, GC has been shown to affect long-term potentiation (LTP) in hippocampal neurons (e.g. Filipini et al., 1991; Diamond et al., 1992). This relationship is thought to be caused by the activation of two types of GC receptors: mineralcorticoid receptors (MR), which have a high affinity for GC, and glucocorticoid receptors (GR), which have a lower affinity for GC than MR. The relative balance between these receptors affects memory in adults (e.g. de Kloet et al., 1999). de Kloet et al. (1999) refer to the MR/ GR ratio to explain the relationship between GC and cognitive performance. They propose, for example, that memory performance is optimal when GC levels are mildly elevated (high MR/GR ratio) and less than optimal when GC levels are either too low or too high (low MR/GR ratio). In animal studies, memory facilitation occurs when MR are fully occupied and GR are partially occupied (e.g. when GCs are at a basal level or mildly elevated); under these conditions LTP is enhanced (Diamond et al., 1992). In contrast, memory impairment may occur when GR are highly saturated (e.g. when GC are highly elevated); under these conditions LTP is depressed (Pavlides et al., 1993).
Recent human studies (Lupien et al., 2002; Abercrombie et al., 2003) in adults support the cortisol-memory and MR/GR ratio hypothesis and reconcile previous studies reporting positive (e.g. Beckwith et al., 1986) and negative (e.g. Newcomer et al., 1994; Kirschbaum et al., 1996) effects of GC on memory. For example, Lupien et al. (2002) found that administration of metrapone, which inhibits cortisol secretion, increases the rate of forgetting in young adults, and that GC replacement reverses this effect. Abercrombie et al. (2003) recently reported on the dose-response facilitative and disruptive effects of acute elevations in GC on memory. These studies extend and replicate animal studies using pharmacological treatment in adrenalectomized rodents to demonstrate the mnemonic and amnesiac effects of GC (e.g. Diamond et al., 1992).
Whether cortisol-induced changes in memory apply to early infancy, when cortisol secretion, learning, and memory undergo rapid changes in development, is unknown (see Heffelfinger and Newcomer, 2001 for a review). It is well established that, in healthy full-term infants, and a tremendous surge in cognitive capacity characterizes the first months of life; in addition, there is some evidence of a developmental decline in GC secretion during this period. From 2 to 4 months, GC levels and secretion decline in response to mild challenges, such as routine physical examinations (Lewis and Ramsay, 1995; Gunnar et al., 1996). During this period, infants reliably demonstrate learning and memory (Rovee-Collier et al., 1980; Fagen and Rovee-Collier, 1983). For example, infants increase their kick rate when kicks produce movement in an overhead mobile (learning) and their kick rate remains high, even when tested after a delay (memory).
Pharmacological manipulation of GC is a gold standard for studying the relationship between cortisol and memory in adults. However, such a study is not feasible in human infants. We examined whether patterns of cortisol responses during a contingency learning task would affect memory at three months of age. We have previously shown that this task induces behavioural and autonomic responses reflective of stress in preterm infants at three months of age. We have also shown that preterm infants have lower cortisol levels than full-term infants (Haley et al., 2004). In the present study, we evaluated the relationship of cortisol to learning and memory in preterm and full-term infants. We hypothesized that increased cortisol secretion during learning would facilitate memory.
2. Methods
2.1. Participants
The participants of this study were 24 preterm infants born ≤32 weeks of gestational age and 18 full-term infants seen at 3 months of age (corrected for prematurity). Preterm infants were recruited through the Neonatal Intensive Care Unit (NICU) at the Children’s and Women’s Health Centre of British Columbia, Vancouver as part of an ongoing program of studies on the effects of premature birth and early pain exposure on regulatory processes and development (Grunau et al., 2004, 2005; Haley et al., 2004). As part of this larger study, chart reviews from birth to term were carried out (see Table 1). The term-born infants were recruited from two major metropolitan nurseries in Vancouver, the Children’s and Women’s Health centre of British Columbia and St Paul’s Hospital, Vancouver, both affiliated with the University of British Columbia. Infants with a major congenital anomaly, neurosensory impairment, or reported maternal drug use during pregnancy were excluded from the study. Mothers were primarily Caucasian (80%), married (80%), and college educated (education: M=15.9 years). Mothers were on average 33 years of age, with 1.7 children (range=1–4).
Table 1.
Demographic information of sample by group (preterm vs. full-term) and direction (increasers vs. decreasers).
| Cortisol change x group
|
||||
|---|---|---|---|---|
| Increasers (n=14)
|
Decreasers (n=25)
|
|||
| Preterm (n=9) | Full-term (n=5) | Preterm (n=13) | Full-term (n=12) | |
| Gestational age (weeks) | 28 (2.89) | 40 (.89) | 29 (2.71) | 40 (1.24) |
| Corrected age (weeks) | 13 (0.88) | 13 (1.35) | 12 (.78) | 13 (1.03) |
| Sex (male/female) | 5/4 | 2/3 | 6/7 | 10/2 |
| Weight (grams) | 1346 (603.02) | 3412 (594.59) | 1260 (400.27) | 3580 (353.05) |
| Days on mechanical ventilation | 17.78 (24.21)
Median: 3.00 |
– | 11.00 (21.18)
Median: 4.00 |
– |
| Number of skin- breaking procedures | 142 (105.45)
Median: 95.00 |
– | 119 (93.57)
Median: 84.00 |
– |
2.2. Procedures
This study was approved by the Clinical Research Ethics Board, University of British Columbia, and the Children’s and Women’s Health Centre of British Columbia Research Review Committee. Infants were tested at home on two consecutive days (24 h±120 min apart), at a time selected by their caregiver, when the infant was likely to be awake and alert. Upon arrival, informed consent was collected from the parent, and then a basal saliva sample was collected from the infant. A second saliva sample was collected 20 min after the introduction of the mobile. Saliva samples were obtained using a cotton dental roll, which was then placed into a needleless syringe. No stimulant was used. Infants were tested using a conjugate reinforcement mobile task and the procedures developed by Rovee-Collier (Rovee and Rovee, 1969; Rovee-Collier et al., 1980). To measure the baseline kicking rate, the infant was placed in a crib, a ribbon was attached to the infant’s foot, and the infant was exposed to a mobile (baseline of 3 min). Following the baseline, the other end of the ribbon was attached to a hook on the mobile stand. The infant’s foot kicks caused the mobile to move (learning phase of 9 min). Following the learning phase, the ribbon was detached from the hook (extinction phase of 3 min), so that the infant’s kicking was no longer reinforced. Following the extinction phase, the mobile was immediately removed from the infant’s view by a research assistant, and the infant was removed from the crib by the caregiver.
During this procedure, the experimenters and the caregiver stayed out of the infant’s view to avoid distracting the infant. The only exception to this was when the infant became slightly fussy or upset. In this situation, based on the procedures of Rovee-Collier et al. (1980), the research assistant made attempts to comfort the infant. If these attempts were ineffective, the infant was removed from the crib by the caregiver, and the data from this subject was removed from data analyses.
A video camera was set up to capture the infant’s facial and bodily movements during the study. Video signals from the camera were recorded on an analogue VHS tape and were later used for coding. Events were cued on the video tape using inaudible tones, which signalled the start of each experimental phase for later coding.
2.3. Behavioural coding
Computerized behavioural coding was carried out using Noldus’ Observer software (version 5.0). Coding of videotapes was carried out with the scorer blinded to the group status and all other information about the infants.
2.4. Learning
To assess learning, the frequency of kicks was scored during baseline, learning, and extinction phases of the procedure during Sessions 1 and 2. A kick was defined as a vertical or horizontal movement of the foot that retraced its original path in a smooth, continuous motion (Rovee and Rovee, 1969). We used two approaches to measuring learning and memory. First, we scored learning based on the methods of Gekoski et al., 1984. Using this approach, we evaluated relative rates of kicking for Days 1 and 2 by dividing the average number of kicks for each 3 min block of learning by the average number of kicks at baseline on Day 1, which was then averaged across the three blocks. In this way, we computed two scores: a relative learning ratio for Day 1=kicking rate during learning on Day 1 relative to kicking rate during baseline on Day 1, and a relative learning ratio for Day 2=kicking rate during training on Day 2 relative to kicking rate during baseline on Day 1. We considered the relative learning ratio on Day 2 to reflect learning and memory.
We also used a categorical approach for scoring learning and memory (Rovee-Collier et al., 1980). In Rovee-Collier’s approach, learning is defined as a kick rate of 1.5 times greater than baseline in any two consecutive minutes of reinforcement during any session of training. In this approach, short-term memory is defined as relative kicking during the final period of non-reinforcement (extinction) at the end of each session (i.e. immediate retention test), which we refer to as the immediate retention ratio and which we calculated for Days 1 and 2.
We also computed two measures of long-term memory: A baseline ratio, which is calculated by dividing the infant’s kick rate during the long-term test (i.e. first 3 min of Session 2) by that same infant’s kick rate during the first 3 min at the outset of Session 1 (i.e. baseline). Although the baseline ratio indicates whether or not an infant remembered, it is not informative about the degree of retention. This information is provided by the retention ratio, which is calculated by dividing an infant’s kick rate during the long-term test by that infant’s kick rate during the final period of non-reinforcement at the end of training on Day 1. A retention ratio of 1.00 indicates perfect retention, while a ratio of less than 1.00 indicates that some forgetting has occurred.
To evaluate inter-rater reliability, 30% of the sample was recoded by a second blinded coder. For kicking, average intra class correlation coefficients were 0.86 (baseline), 0.86 (learning), and 0.83 (extinction). Alpha values were the same as the intra class correlation coefficients.
2.5. Salivary cortisol
The saliva was expressed into a vial and stored at −20 °C until assayed using the Salametrics High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit for quantitative determination of salivary cortisol (Salimetrics LLC, Philadelphia, PA) in Weinberg’s laboratory at the University of British Columbia. Intra and inter assay coefficients of variation were 2.92 and 3.41%, respectively. Cortisol data were examined for outliers, defined as any value more than ±3 SD from the mean (Gunnar et al., 1989). One infant had an outlier value for post cortisol on Day 2. This value was winsorized using Tukey’s method (1977), which involves replacing the outlier value with the closest value within the 3 SD range, which is then used for data analyses. Time of testing on Day 1 was not related to basal, F(1,36)=0.71, p=0.41, or response, F(1,36)=0.78, p=0.38, cortisol values and was not considered in the analyses.
2.6. Statistical analyses
Repeated measures analysis of variance (ANOVA) was conducted to examine the effects of cortisol change (increase vs. decrease on Day 1) and group (preterm vs. full-term) with day (Days 1 and 2) and sample (pre and post) as the repeated measures on cortisol. Planned contrasts were conducted to examine significant interactions. Data were examined for assumption of sphericity and a greenhouse correction was used if sphericity was violated. One-way ANOVAs were conducted to examine significant main effects. A second repeated measures ANOVA was conducted to examine the effects of cortisol change (increase vs. decrease on Day 1) and group (preterm vs. full-term) with day (Days 1 and 2) as the repeated measures on learning. In addition to looking at the effects of direction of cortisol change on learning and memory, a multiple regression analysis was conducted to examine whether the quantity of cortisol change on Day 1 was related to memory (i.e. learning on Day 2). Bivariate correlations were computed to examine continuous relationships among absolute cortisol concentrations and learning on Days 1 and 2. In addition, three univariate ANOVAs were conducted to examine the effects of cortisol change (increase vs. decrease on Day 1) and group (preterm vs. full-term) on the immediate retention and long-term retention scores (baseline ratio and retention ratio).
3. Results
3.1. Cortisol change and magnitude on Day 1
Pre-test to post-test cortisol levels increased in 14 (nine preterm and five full-term) infants and decreased in 25 (13 preterm and 12 full-term) infants. Three infants showed no change in cortisol values (±0.01 μg/dl), and these were not considered in the main analyses, as there were not enough of them to examine as a separate group. We did include these infants in our analyses using multiple regression and correlations. Chi-square analyses indicated that the proportion of infants who increased or decreased cortisol levels was not related to prematurity status. Preterm infants had lower cortisol levels as compared to full-term infants overall, F(1,34)=5.77, p=0.022 (see Fig. 1). In addition, the magnitude of change in cortisol was significantly greater for full-term than for preterm infants, regardless of whether cortisol levels increased or decreased, F(1,35)=6.18, p= 0.018 (see Fig. 2). In addition, decreasers had significantly higher pre-test cortisol levels than increasers, p=0.001, but this effect was limited to Day 1. As indicated in Fig. 2, cortisol levels did not change significantly on Day 2 for either increasers or decreasers, indicating a decline in reactivity or habituation from Days 1 to 2.
Figure 1.
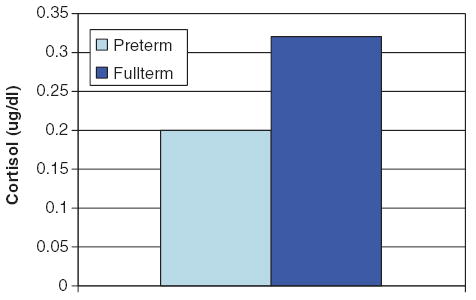
Mean cortisol in preterm and full-term infants.
Figure 2.
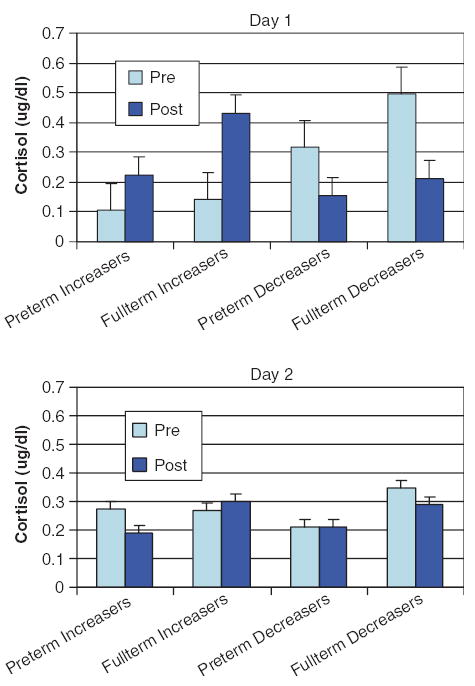
Means of pre and post cortisol levels on Days 1 and 2 by group and direction of cortisol change.
3.1.1. Outliers in learning and memory
Three infants had relative learning ratio and baseline ratio scores greater than 2 SD units above the mean when measured during training on Day 2. Upon further inspection of the data, we observed that all three subjects kicked only once or twice during baseline on Day 1. Despite low spontaneous rates of baseline kicking, however, all three subjects showed evidence of learning on Day 1 and reached the conventional learning criterion (i.e. values=1.67, 4.33, and 16.00 relative learning ratio). Interestingly, two of the infants (preterm and full-term) showed moderate increases in cortisol, while the third infant (full-term) showed cortisol decreases. To test our main hypothesis, subsequent analyses involving relative learning ratio and baseline ratio scores were conducted with and without these subjects.
3.1.2. Cortisol change x group on learning and memory
As illustrated in Fig. 3, there were no differences in mean relative learning ratios on Day 1 between preterm and full-term infants or between cortisol increasers and decreasers. However, increasers showed a three-fold improvement in mean relative learning ratio from Days 1 to 2, F(2,35)=4.50, p=0.041. After rerunning this analysis without the three highest scoring subjects, there was no longer a significant effect of cortisol change.
Figure 3.
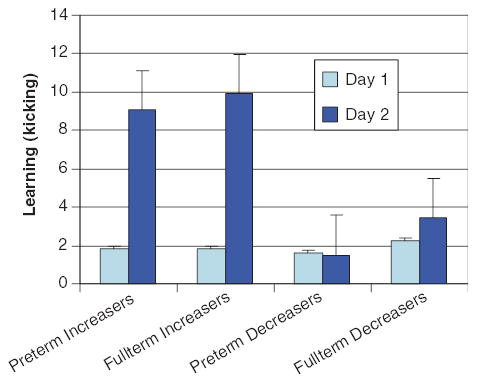
Means of relative learning ratios on Days 1 and 2 by group and direction of cortisol change.
3.2. Relationships between cortisol level and learning
To evaluate whether cortisol concentration rather than cortisol change was related to cognitive performance, bivariate correlations were conducted separately for preterm and full-term infants (see Table 2). Positive relationships were found between cortisol levels (basal and response) and relative learning ratios for Days 1 and 2 in preterm infants. However, in full-term infants, only a trend for a negative relationship was found between cortisol levels and relative learning ratios. As an exploratory analysis, we examined the relationship between continuous measures of cortisol change and relative learning ratio on Day 2, which revealed an interesting although not statistically significant quadratic relationship between cortisol and memory, R2=0.41, p=0.13.
Table 2.
Bivariate correlations between salivary cortisol levels and relative learning on Days 1 and 2 for preterm and full-term infants.
| Day 1
|
Day 2
|
|||
|---|---|---|---|---|
| Pre-test | Post- test | Pre-test | Post- test | |
| Preterm infants (n = 24) | ||||
| Day 1 learning | 0.05 | 0.37+ | 0.34 | 0.35 |
| Day 2 learning | 0.05 | 0.38+ | 0.55* | 0.38+ |
| Full-term infants (n = 16) | ||||
| Day 1 learning | −0.02 | −0.14 | −0.23 | −0.27 |
| Day 2 learning | −0.21 | −0.26 | −0.32 | −0.50+ |
p<0.1,
p<0.01.
3.3. Categorical approach to learning and short-term memory
As indicated in Table 3, there were no differences in mean kicking rates at baseline and extinction on Days 1 or 2. Among preterm and full-term groups, 75 and 50% of the infants, respectively, reached the learning criterion. On average, infants who reached the learning criterion did so in the first 3 min block of the learning task (M=1.94 relative learning ratio, SD=0.38). Among infants who reached the learning criterion, there were no differences in group or cortisol direction on mean immediate retention ratio on Day 1 (kicking rate during extinction on Day 1 divided by baseline kicking rate). However, we did find that full-term infant had higher immediate retention ratio scores on Day 2 (kicking rate during extinction on Day 2 divided by kicking rate during the outset of Day 2) than preterm infants (see Table 3).
Table 3.
Mean kick rates/minute during baseline Day 1, extinction Day 1, baseline Day 2, and the immediate retention ratio, baseline ratio, and retention ratio by preterm status.
| Preterm (n=24) | Full-term (n=16) | p-value | |
|---|---|---|---|
| Kick rates (minute) | |||
| Mean Day 1 Baseline (SE) | 10.98 (1.64) | 15.15 (3.09) | ns |
| Mean Day 1 Extinction (SE) | 15.71 (2.11) | 17.85 (3.72) | ns |
| Mean Day 2 Baseline (SE) | 12.23 (1.69) | 17.54 (3.45) | ns |
| Infants to reach learning criterion | 75% (n=16) | 50% (n=8) | ns |
| aShort-term memory | |||
| Mean immediate retention ratio Day 1 (SE) | 2.60 (0.92) | 2.70 (0.99) | ns |
| Mean immediate retention ratio Day 2 (SE) | 1.13 (0.20) | 3.21 (1.71) | * |
| aLong-term memory | |||
| Mean baseline ratio (SE) | 4.04 (2.58) | 5.00 (3.00) | ns |
| Mean retention ratio (SE) | 3.97 (2.62) | 1.36 (.27) | ns |
When analysed separately for infants who reached the learning criterion, preterm infants (M=0.93; SE=0.16) had lower immediate retention ratios than full-term infants (M=5.55; SE=7.95), F=5.03, p<0.05; however, no other group differences were found.
Several infants showed low baseline rates of kicking on Day 1 and high rates of kicking on Day 2, which has been observed previously (e.g. Rovee and Fagen, 1976). This increases the value of the mean ratio as compared to the value of the ratio of the means.
3.4. Categorical approach to long-term memory
In terms of long-term retention, we examined the baseline ratio at the outset of Day 2 (baseline kicking on Day 2 relative to baseline kicking on Day 1). Consistent with our initial analyses of relative learning ratios on Day 2, we found a significant trend that infant cortisol increasers showed greater recognition of the mobile (M=13.74 baseline ratio, SD=24.00) as compared to infant cortisol decreasers on Day 2 (M=2.34 baseline ratio, SD=3.76), F(1,23)=3.87, p=0.064. After removing the three highest scoring subjects, we found only a statistical trend for a group x cortisol direction interaction, p= 0.08. In terms of the degree of retention, however, we found no effects of cortisol direction on the mean retention ratio scores.
To further investigate the relationship between the quantity of cortisol increases and memory, we divided cortisol increasers into three groups: Low (0.01 μg/dl–0.10 μg/dl), Moderate (0.11–0.30 μg/dl), and High (0.31–0.61 μg/dl) in relation to the baseline ratio measured immediately at the outset of Day 2. As indicated in Fig. 4, we found that regardless of preterm status infants who secreted moderate amounts of cortisol (preterm, n=3, and full-term, n=2) showed greater evidence of long-term recognition memory than those who secreted low (preterm, n=4, and full-term, n=1) or high (preterm, n=1, and full-term, n=2) amounts of cortisol, F(1,32)= 5.67, p<0.005.
Figure 4.
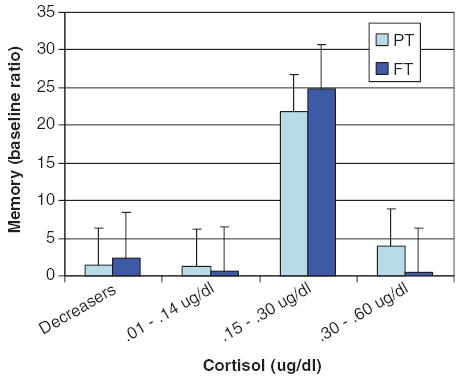
Recognition memory (baseline ratio) as a function of preterm status and amount of cortisol secreted during Day 1 learning task.
4. Discussion
The major finding of this study is that cortisol secretion during a learning task is related to memory in infants. Infants who showed a cortisol increase from pre-testing to post-testing on Day 1 had better performance and greater evidence of memory on Day 2 than infants whose cortisol decreased. Overall, these findings are consistent with animal studies (e.g. Roozendaal et al., 1996) and recent human studies in adults (e.g. Buchanan and Lovallo, 2001; Abercrombie et al., 2003), suggesting that secretion of cortisol facilitates memory. In addition, we found that moderate levels of cortisol were related to greater memory recognition than low or high secretions of cortisol, suggesting that too little or too much cortisol impair, while moderate amounts of cortisol facilitate memory.
Memory facilitation in humans has primarily been demonstrated in studies that have manipulated cortisol levels through the administration of exogenous agents (e.g. Roozendaal et al., 1996; Buchanan and Lovallo, 2001; Lupien, 2002; Abercrombie et al., 2003) or by psychosocial stressors (e.g. Wolf et al., 2001). In contrast, the current study is the first to examine whether cortisol secretion resulting from a learning task per se is related to memory in infants. A critical feature of this study’s design is that cortisol is not manipulated prior to or after learning, but rather is measured in response to the learning task. This approach may provide a more direct test of the hypothesis that cortisol elevations in response to a cognitive task play an important role in the formation of memory than exogenous treatment designs. It is also interesting to note that we observed a two to three-fold change in cortisol levels among increasers and decreasers. This is similar to cortisol changes reported in adult studies using pharmacological agents or social stressors. Given that 40% of our infants showed an increase in cortisol during the learning task, the study of cortisol-memory relationships in early infancy appears feasible.
We used a continuous (Gekoski et al., 1984) as well as a categorical approach (Rovee-Collier et al., 1980) to evaluate learning and memory in relation to changes in cortisol. We found consistent results using each of these methodologies. With the categorical approach, we observed that cortisol increasers showed greater evidence of long-term recognition than cortisol decreasers. Unlike the continuous approach, the categorical approach allowed us to examine the degree of retention. There were, however, no effects of cortisol on this measure of memory. In short, secretion of cortisol appears to influence whether infants remember seeing the mobile (indicative of the baseline ratio) but not related to the degree to which they remember seeing the mobile (retention ratio).
In addition, our findings suggest that the observed cortisol–memory relationships were related to the effects of cortisol on memory consolidation, rather than to the effects on encoding or retrieval. Specifically, learning on Day 1 and cortisol levels on Day 2 were not different among cortisol increasers and decreasers. The first finding suggests that learning differences on Day 1 were not attributed to the ability to secrete cortisol. Conversely, the finding suggests that cortisol secretion on Day 1 was not driven by the activity of kicking, which was used to measure learning and might have confounded the relationship between changes in cortisol and learning. Second, relationships between cortisol on Day 1 and learning on Day 2 were not due to changes in cortisol on Day 2. Interestingly, we found that the magnitude of the cortisol response was greater on Day 1 (regardless of cortisol direction) than on Day 2. This decline in cortisol reactivity to the mobile on the second day may reflect a familiarity or habituation effect registered at the physiological level, whereby infant cortisol responses habituate to repeated exposure. For example, newborn infants showed diminished cortisol reactivity to a physical examination when repeated 24 h after a previous examination (Gunnar et al., 1989). Taken together, these results support the interpretation that changes in cortisol during a learning task affect memory consolidation.
The first few months of life has been thought to be characterized by a decline in cortisol reactivity and an increase in cognitive capacity. It has been suggested that a decline in cortisol reactivity in infants parallels their increased capacity for discriminating between familiar and novel events (Lewis & Ramsay, 1995). Given our finding that infants with high or low cortisol responses showed impaired memory as compared to infants with moderate cortisol responses (see Fig. 4), it would appear that the capacity to mount a moderate cortisol response is adaptive and not inconsistent with this developmental decline in reactivity. Whether the value of a moderate amount of cortisol varies or is fixed in a developmental context remains to be determined. Developmental context may also be characterized by activation of other components of the stress system involved in learning and memory (e.g., autonomic activity). Further work is needed to evaluate the role of multiple components of the stress system in relation to novelty learning and memory.
Several of the subjects in the current study showed an interesting pattern of learning and memory involving a significant rise in kicking rate from Days 1 to 2, which has been observed previously (e.g. Rovee and Fagen, 1976). Interestingly, two of the three subjects that showed the clearest example of this pattern had moderate increases in cortisol and contributed significantly to our results. Further work is needed to evaluate whether this pattern of learning reflects a greater capacity for information processing independently of the mobile task and whether cortisol secretion would play a similar role in other aspects of learning and memory.
Explanations for the relationships observed between changes in cortisol and memory can be considered in terms of the effects of GC on MR/GR (Lupien and McEwen, 1997; de Kloet et al., 1999). First, our finding that moderate elevations of cortisol during a learning task facilitate memory supports and extends prior studies showing that moderate elevations of cortisol associated with a high MR/GR ratio (i.e. full activation of MR and only some GR) provide optimal conditions for enhancing LTP (e.g. Filipini et al., 1991; Diamond et al., 1992). In addition, previous work with animals and humans shows the facilitative effects of cortisol on memory consolidation (see Lupien and Lepage, 2001 for a review). We found evidence for a quadratic relationship between the amount of increase in cortisol and memory. That is, infants who had the lowest or greatest cortisol elevations performed more poorly than infants who had moderate increases in cortisol. This pattern is consistent with recent work showing an inverted-U shaped function between cortisol and memory in humans (e.g. Abercrombie et al., 2003). That is, pharmacologically induced elevations in cortisol were related to 48-h memory recall, with moderate elevations in cortisol facilitating memory and extreme elevations impairing memory. The effects of cortisol on memory consolidation are thought to be primarily mediated by GR activation (de Kloet et al., 1999; Lupien and Lepage, 2001). In contrast, MR activation is associated with behavioural reactivity and encoding (Orizl and de Kloet, 1992) and may be a prerequisite for memory. Our finding that infants who had a cortisol increase during learning showed better memory (i.e. recognition of the mobile) than infants who had a cortisol decrease may be caused primarily by cortisol activation of GR. Thus, the MR/GR ratio hypothesis was supported by our data.
Basal cortisol values on Day 1 differed between increasers and decreasers. For example, infants who showed a cortisol decrease had significantly higher basal cortisol levels than infants who showed a cortisol increase. This basal/response pattern raises the issue of the law of initial values, in which an elevated baseline value has more opportunity to change to a lower value. Lewis and Ramsay (1995) postulated that situational factors (e.g. coming to the new environment of the lab) may contribute to high cortisol basal levels. In the current study, however, all infants were seen in the home. Elevated basal cortisol values may, therefore, reflect individual differences.
We also examined whether relationships between cortisol concentrations and cognitive performance would differ between preterm and full-infants. When we examined correlations between cortisol response levels and learning, full-term babies showed a trend towards a negative association between cortisol and learning on Day 2 (i.e. higher cortisol was associated with poorer learning), which is consistent with prior work reporting on the negative effects of GC on cognitive performance in older infants (Gunnar and Nelson, 1994). For preterm infants, however, higher cortisol response to the task on Day 1 was associated with better learning on Day 2.
One finding that may help explain why relationships between cortisol and learning differed between preterm and full-term infants is that absolute cortisol levels were significantly lower in preterm infants. Clinical studies have reported adrenal insufficiency in the neonatal period in some preterm infants born at very low gestational ages (e.g. Watterberg and Scott, 1995; Bolt et al., 2002; Ng et al., 2004; see Watterberg, 2004 for a review). Whether the cortisol difference between preterm and full-term infants reflects delayed maturation in normal development or prolonged alterations in the early programming of the hypothalamic-pituitary-adrenal (HPA) axis are unclear. In a previous study of 116 babies tested at the age of three months, we found that preterm infants showed significantly lower levels of cortisol than term-born infants, suggesting that the low levels seen in the neonatal period may persist into early infancy (Haley et al., 2004). The developmental status of full-term infants may also help explain why relationships between cortisol and learning differ between preterm and full-term infants. There is a developmental decline in cortisol levels between 2 and 4 months in full-term infants (Lewis and Ramsay, 1995). Accordingly, very preterm infants with abnormally low cortisol concentrations may contribute to the upward segment whereas full-term infants who are developing normally lower cortisol concentrations may contribute to the downward segment of the inverted-U shaped function underlying the relationship between cortisol and cognitive performance.
If the lower cortisol levels we found in preterm infants persist beyond infancy, we speculate that our findings could contribute to a risk for future learning difficulties. It has long been observed that infants born very prematurely show difficulties in academic learning (e.g. Saigal et al., 2000; Grunau et al., 2002) that persist through adolescence (e.g. Hack et al., 2002). The etiology of these learning difficulties is unknown. Recently, we found that stress and pain during the neonatal period are associated with alterations in HPA activation in subsequent challenges (Grunau et al., 2004, 2005). Together with these data, our current findings begin to elucidate the complex linkages between cortisol secretion, learning, and memory in preterm infants.
The mechanisms underlying memory remain highly speculative. This is the first study to show that cortisol facilitates memory in infants. This work supports and extends prior research in animals and humans showing the enormous impact of stress hormones on learning and memory. In addition, this study breaks new ground in understanding GC-memory relationships in the context of early development and opens an exciting line of research focused on the psycho-neuroendocrinology of memory consolidation in infancy.
Acknowledgments
This work was primarily funded by the National Institute for Child Health and Human Development grant HD39783 (REG), with additional funding from the Canadian Institutes for Health Research grant MOP42469 (REG), and the Human Early Learning Partnership grants 20R41341 (JW) and 2OR31739 (REG) through the B.C. Ministry for Children and Families. Dr. Grunau is supported by a Senior Scholar Award from the Michael Smith Foundation for Health Research. We would like to express our appreciation to Dr Rovee-Collier for providing the test materials and for consultation on the procedures. We are grateful to the families who participated and to our research staff for their invaluable dedication to the project.
References
- Abercrombie HC, Kalin NH, Thurow ME, Rosenkranz MA, Davidson RJ. Cortisol variation in humans affects memory for emotionally laden and neutral information. Behav Neurosci. 2003;117:505–516. doi: 10.1037/0735-7044.117.3.505. [DOI] [PubMed] [Google Scholar]
- Beckwith BE, Petros TV, Scaglione C, Nelson J. Dose-dependent effects of hydrocortisone on memory in human males. Physiol Behav. 1986;36:283–286. doi: 10.1016/0031-9384(86)90017-x. [DOI] [PubMed] [Google Scholar]
- Bolt RJ, Van Weissenbruch MM, Popp-Snijders C, Sweep FG, Lafeber HN, Delemarre-van de Waal HA. Maturity of the adrenal cortex in very preterm infants is related to gestational age. Pediatr Res. 2002;52:405–410. doi: 10.1203/00006450-200209000-00017. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26:307–317. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- de Kloet RE, Oitzl MS, Joëls M. Stress and cognition: Are corticosteroids good or bad guys? Trends Neurosci. 1999;10:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- Fagen JW, Rovee-Collier C. Memory retrieval: A time locked process in infancy. Science. 1983;222:1349–1351. doi: 10.1126/science.6658456. [DOI] [PubMed] [Google Scholar]
- Filipini D, Gijsbers K, Birmingham MK, Dubrovsky B. Effects of adrenal steroids and their reduced metabolites on hippocampal long-term potentiation. J Steroid Biochem Mol Bio. 1991;40:87–92. doi: 10.1016/0960-0760(91)90171-z. [DOI] [PubMed] [Google Scholar]
- Gekoski MJ, Fagen JW, Pearlman MA. Early learning and memory in the preterm infant. Inf Behav Dev. 1984;7:267–275. [Google Scholar]
- Grunau RE, Whitfield MF, Davis C. Pattern of learning disabilities in children with extremely low birth weight and broadly average intelligence. Arch Pediatr Adolesc Med. 2002;156:615–620. doi: 10.1001/archpedi.156.6.615. [DOI] [PubMed] [Google Scholar]
- Grunau RE, Weinberg J, Whitfield MF. Neonatal procedureal pain and preterm infant cortisol response to novelty at 8 months. Pediatrics. 2004;114:e77–e84. doi: 10.1542/peds.114.1.e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau RE, Holsti L, Haley DW, Oberlander T, Weinberg J, Solimano A, Whitfield MF, Fitzgerald C, Yu W. Neonatal procedural pain exposure predicts lower cortisol and behavioral reactivity in preterm infants in the NICU. Pain. 2005;113:293–300. doi: 10.1016/j.pain.2004.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Nelson CA. Event-related potential in year-old infants: Effects of social context variables and infant temperament. Ch Dev. 1994;63:290–303. [PubMed] [Google Scholar]
- Gunnar MR, Connors J, Isensee J. Lack of stability in neonatal adrenocortical reactivity because of rapid habituation of the adrenocortical response. Dev Psychobi. 1989;22:221–233. doi: 10.1002/dev.420220304. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Brodersen L, Krueger K. Dampening of adrenocortical responses during infancy: Normative changes and individual differences. Ch Dev. 1996;67:877–889. [PubMed] [Google Scholar]
- Hack M, Flannery DJ, Schluchter M, Cartar L, Borawski E, Klein N. Outcomes in young adulthood for very-low-birth-weight infants. N Eng J Med. 2002;346:149–157. doi: 10.1056/NEJMoa010856. [DOI] [PubMed] [Google Scholar]
- Haley DW, Grunau RE, Weinberg J, Whitfield MF. Parenting stress and infant cortisol responses in preterm and full-term infants at 3 months. Ped Res. 2004;55 (4):80A. [Google Scholar]
- Heffelfinger AK, Newcomer JW. Glucorticoid effects on memory function over the life span. Dev Psychop. 2001;13:491–513. doi: 10.1017/s0954579401003054. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH. Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sci. 1996;58:1475–1483. doi: 10.1016/0024-3205(96)00118-x. [DOI] [PubMed] [Google Scholar]
- Lewis M, Ramsay DS. Developmental change in infants responses to stress. Ch Dev. 1995;66:657–670. doi: 10.1111/j.1467-8624.1995.tb00896.x. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Lepage M. Stress, memory, and the hippocampus: Can’t live with it, can’t live without it. Behav Br Res. 2001;127:137–158. doi: 10.1016/s0166-4328(01)00361-8. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: Integration of animal and human models. Br Res Rev. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Wilkinson CW, Brière S, Ménard C, Ng Ying Kin NMK, Nair NPV. The modulatory effects of corticosteroids on cognition: Studies in young human populations. Psychoneuroendocrinology. 2002;27:401–416. doi: 10.1016/s0306-4530(01)00061-0. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Craft S, Hershey T, Askins K, Bardgett ME. Glucocorticoid-induced impairment in declarative memory performance in adult humans. J Neurosci. 1994;14:2047–2053. doi: 10.1523/JNEUROSCI.14-04-02047.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng PC, Lee CH, Lam CW, Ma KC, Fok TF, Chan IH, Wong E. Transient adrenocortical insufficiency of prematurity and systemic hypotension in very low birthweight infants. Arch Dis Ch Fet Neon Ed. 2004;89:F119–F126. doi: 10.1136/adc.2002.021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oitzl MS, de Kloet ER. Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behav Neurosci. 1992;106:62–71. doi: 10.1037//0735-7044.106.1.62. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Watanabe Y, McEwen BS. Effects of glucocorticoids on hippocampal long-term potentiation. Hippocampus. 1993;3:183–192. doi: 10.1002/hipo.450030210. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Bohus B, McGaugh JL. Dose-dependent suppression of adrenocortical activity with metyrapone: Effects on emotion and memory. Psychoneuroendocrinology. 1996;21:681–693. doi: 10.1016/s0306-4530(96)00028-5. [DOI] [PubMed] [Google Scholar]
- Rovee CK, Fagen JW. Extended conditioning and 24-hour retention in infants. J Exp Ch Psych. 1976;21:1–11. doi: 10.1016/0022-0965(76)90052-7. [DOI] [PubMed] [Google Scholar]
- Rovee CK, Rovee DT. Conjugate reinforcement of infant exploratory behavior. J Exp Ch Psych. 1969;8:33–39. doi: 10.1016/0022-0965(69)90025-3. [DOI] [PubMed] [Google Scholar]
- Rovee-Collier CK, Sullivan MW, Enright M, Lucas D, Fagen JW. Reactivation of infant memory. Science. 1980;208:1159–1161. doi: 10.1126/science.7375924. [DOI] [PubMed] [Google Scholar]
- Saigal S, Hoult LA, Streiner DL, Stoskopf BL, Rosenbaum PL. School difficulties at adolescence in a regional cohort of children who were extremely low birth weight. Pediatrics. 2000;105:325–331. doi: 10.1542/peds.105.2.325. [DOI] [PubMed] [Google Scholar]
- Tukey, J.W., 1997. Exploratory data analysis. Addison-Wesley, Don Mills, Ontario.
- Watterberg KL. Adrenocortical function and dysfunction in the fetus and neonate. Semin Neonat. 2004;9:13–21. doi: 10.1016/j.siny.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Watterberg KL, Scott SM. Evidence of early adrenal insufficiency in babies who develop bronchopulmonary dysplasia. Pediatrics. 1995;95:120–125. [PubMed] [Google Scholar]
- Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology. 2001;26:711–720. doi: 10.1016/s0306-4530(01)00025-7. [DOI] [PubMed] [Google Scholar]


