Abstract
The localized transcription of several muscle genes at the motor endplate is controlled by the Ets transcription factor GABP. To evaluate directly its contribution to the formation of the neuromuscular junction, we generated transgenic mice expressing a general Ets dominant-negative mutant specifically in skeletal muscle. Quantitative RT–PCR analysis demonstrated that the expression of genes containing an Ets-binding site was severely affected in the mutant mice. Conversely, the expression of other synaptic genes, including MuSK and Rapsyn, was unchanged. In these animals, muscles expressing the mutant transcription factor developed normally, but examination of the post-synaptic morphology revealed marked alterations of both the primary gutters and secondary folds of the neuromuscular junction. Our results demonstrate that Ets transcription factors are crucial for the normal formation of the neuromuscular junction. They further show that Ets-independent mechanisms control the synaptic expression of a distinct set of synaptic genes.
Introduction
The current model for the neuronal control of synaptic gene transcription in skeletal muscle involves two distinct mechanisms. On the one hand, neurally evoked electrical activity in muscle fibres represses transcription of synaptic genes in extra-synaptic areas. On the other, nerve-derived signals specifically activate synaptic gene expression in sub-synaptic myonuclei (for a review see Schaeffer et al., 2001).
We have previously identified a DNA regulatory element, termed the N-box, which is required for targeting the transcription of the acetylcholine receptor (AChR) δ and AChR ε subunit-encoding genes to sub-synaptic nuclei (Koike et al., 1995; Duclert et al., 1996). Interestingly, the utrophin and acetylcholinesterase (AChE) genes have subsequently been shown to contain a functional N-box (Chan et al., 1999; Gramolini et al., 1999; Khurana et al., 1999). Further studies have shown that an N-box is required for neuregulinstimulated transcription of the utrophin, AChR δ and AChR ε promoters in cultured muscle cells (Fromm and Burden, 1998; Sapru et al., 1998; Schaeffer et al., 1998; Khurana et al., 1999) and of the utrophin promoter in intact skeletal muscle (Gramolini et al., 1999). In these experiments, the contribution of the Ets-related transcription factor GABPα/β was also demonstrated because of its ability to bind the N-box and to transcriptionally activate the utrophin, AChR δ and AChR ε genes in response to ARIA. Recently, Briguet and Ruegg (2000) highlighted the importance of GABP in vivo by showing its requirement for the formation of agrin-induced ectopic synapses.
Given that the N-box is required for regulating the expression of genes encoding critical components of the motor endplate, we have evaluated the contribution of N-box-dependent transcription to the formation of the neuromuscular junction (NMJ) using an in vivo model system. Since some synaptic genes, such as AChR α, do not possess an N-box but contain the GGAA core Ets-binding sequence, it was also important to test whether N-box-independent mechanisms can contribute to the expression of synaptic genes. To address these questions, we have generated transgenic mice expressing an Ets dominant-negative mutant in skeletal muscle. Analysis of the mutant animals revealed that blocking the Ets-binding sites specifically reduces the expression of Ets-binding site containing synaptic genes, thereby resulting in alterations in NMJ morphology and ultrastructure. Additionally, our results demonstrate the existence of Ets-independent pathways that regulate the expression of a distinct set of synaptic genes.
Results and Discussion
Transgenic line
The DNA-binding domain (DBD) of Ets-2 on its own acts as a classical trans-dominant-negative mutant. The absence of the natural DBD flanking sequences strengthens the binding of the mutant to DNA (Langer et al., 1992). The Ets-2 DBD has already been shown to efficiently block activation of the N-box (Sapru et al., 1998) and, when fused to β-galactosidase, retains the Ets dominant-negative activity (Langer et al., 1992). This fusion protein was used to make the transgene, since this made it possible to detect muscle fibers expressing the trans-dominant-negative mutant. To drive expression of the fusion protein, the myosin light chain 1F (MLC1F) promoter was chosen (Kelly et al., 1997). This promoter possesses several key properties. First, it produces some of the highest expression levels in transgenic mouse skeletal muscle (Rao et al., 1996) and is specific for skeletal muscle. Furthermore, the transgene is active in the myotome by day 9.5 of embryonic development and in forming limb muscles by day 11.5 (Grieshammer et al., 1992 and references therein). Finally, expression of the transgene is 100-fold lower in slow versus fast muscle fibers and occurs in a proximo-distal gradient, thus limiting expression in vital muscles such as the diaphragm (Donoghue et al., 1991).
Of the seven lines of transgenic animals obtained, five expressed the transgene, according to positive LacZ staining. All the lines exhibited the same proximo-distal expression pattern, with the transgene being mainly expressed in the hindlimb fast muscles (vastus lateralis, tibialis anterior and extensor digitorum longus) after birth (Figure 1). Because the vastus lateralis showed the strongest expression, we focused our gene expression and morphological studies on this muscle. The extent of LacZ staining was variable among the positive lines. Hence, the line with the highest average LacZ staining, reflecting stronger transgene expression, was chosen for further analyses.
Figure 1.
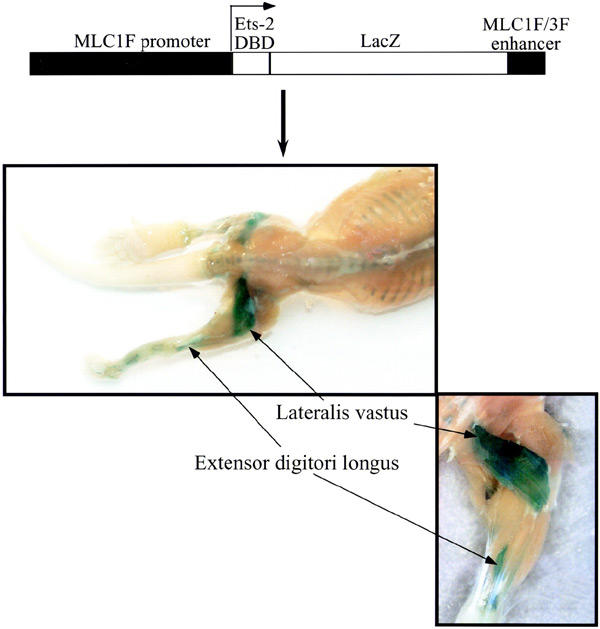
Transgene construction and expression pattern. Transgene expression was detected mainly in the vastus lateralis and extensor digitori longus muscles by in toto LacZ staining of 1-week-old transgenic animals.
Although the MLC1F promoter drives expression of the Ets mutant in the myotome and throughout the development of fast hindlimb, we did not detect any morphological abnormalities in muscles expressing the mutant post-natally. Ets transcription factors are thus dispensable for the events leading to the formation of differentiated limb muscles from the myotome.
Synaptic gene expression
The expression of various components of the post-synaptic apparatus of the NMJ was determined by quantitative real-time RT–PCR on mRNAs isolated from the vastus lateralis of 3-week-old animals. The results obtained with the LacZ-positive transgenic mice were normalized to β-actin, and then compared with those obtained with their LacZ-negative litter mates (Figure 2). Three gene products tested depend on an N-box and GABP for their synaptic expression, i.e. the AChR ε subunit, acetylcholinesterase and utrophin A mRNAs (Duclert et al., 1996; Chan et al., 1999; Gramolini et al., 1999). The promoter of the β2 laminin gene contains Ets-binding sites, but their role has yet to be examined (Brandenberger et al., 1996). Two of the gene products tested do not contain a consensus N-box in their promoters: the AChR α and utrophin B mRNAs, although the AChR α subunit promoter contains putative Ets-binding sites (Merlie and Kornhauser, 1989; Burton et al., 1999). Finally, the murine promoters of rapsyn and MuSK have not been characterized.
Figure 2.
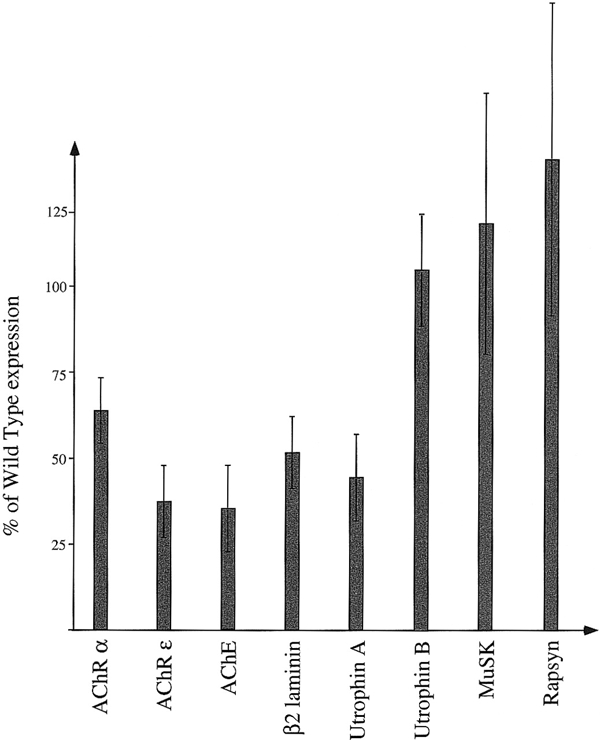
Relative expression of synaptic genes in transgenic muscles. The expression levels of the various genes tested are given as percentages of their expression levels in wild-type muscle. Error bars correspond to the standard deviation. Analysis of variance was performed using a one-way ANOVA (P < 0.01 for AChR α and ε, AChE, utrophin A and β2 laminin. Variations of MuSK, Rapsyn and utrophin B expression were not found to be significant). AChR α and ε, acetylcholine receptor subunit gene α and ε; AChE, acetylcholinesterase.
In agreement with our hypothesis, expression of the AChR α and AChR ε, AChE, utrophin A, and β2 laminin mRNAs were reduced in mutant muscles (respectively, 64, 37, 35, 45 and 52% relative to the expression levels in control animals; see Figure 2), thus showing that, in vivo, the expression of these genes is under the control of Ets transcription factors. In comparison to genes containing a consensus N-box, the effect of the mutant factor on AChR α gene expression was less pronounced. This could reflect the presence of weak Ets-binding sites in the AChR α promoter.
The levels of utrophin B, rapsyn and MuSK mRNAs were not significantly affected in muscles of mutant animals. To exclude the possibility that extra-synaptic variations of gene expression could interfere with our results, extra-synaptic regions of the vastus lateralis from mutant and control animals were dissected, but no variation could be observed for any of the genes tested (data not shown). In addition, the synaptic/extra-synaptic ratio for the MuSK RNA is higher than for the AChR α RNA, thus showing that in the vastus lateralis, MuSK expression is even more sharply compartmentalized than is AChR expression (data not shown).
The lack of effect of the mutant on the expression of MuSK, rapsyn or utrophin B suggests that the expression of some synaptic genes is controlled by a mechanism that does not involve Ets transcription factors. Interestingly, the transcription factor Sp1 has already been shown to mediate the transcriptional activation of the AChR δ and AChR ε promoters by neuregulins in P-19 teratocarcinoma cells (Alroy et al., 1999). This mechanism does not have to be strictly transcriptional, and could, for example, involve selective RNA stabilization and degradation, in synaptic versus extrasynaptic zones, respectively (for further discussion, see Newey et al., 2001). However, it is also possible that Ets transcription factors participate in MuSK and rapsyn expression, but that other factors also significantly stimulate their expression at the NMJ, thus minimizing the effect of the Ets mutant. Consistent with this is the fact that although MuSK expression is stimulated by neuregulins/erbB (Ip et al., 2000; Moore et al., 2001), this stimulation is only 3-fold in chick primary myotubes whereas AChR α subunit gene expression is activated 30-fold in the same cells (Altiok et al., 1995; Ip et al., 2000). It is thus conceivable that the contribution of neuregulins to MuSK expression is proportionally less important than in the case of AChR genes, thus rendering it undetectable by our approach. Finally, we cannot exclude the possibility that the perturbation of the NMJ by the Ets mutant induces a compensatory mechanism in order to preserve MuSK expression.
Neuromuscular junction morphology and ultrastructure
To determine whether perturbations in the expression of Ets-binding site-containing genes affect the formation of NMJs, we examined the morphology of wild-type and mutant junctions by confocal microscopy, using rhodamine-labelled α-bungarotoxin. At 1 week of post-natal development, NMJs have not reached their mature shape, and AChRs form a simple patch under the nerve terminal. In comparison with wild-type tissue at this stage of development, AChR patches in mutant animals are slightly smaller (Figure 3A). At 3 weeks of post-natal development, the NMJs have matured and the primary gutters and secondary folds have formed. At this stage, the morphology of NMJs from mutant animals is clearly different from that of wild-type NMJs (Figure 3B). In muscles from mutant mice, two distinct categories of NMJs can be observed. The first represents 30% of all synapses and is characterized by atrophic NMJs whose primary gutters form a single ring. Conversely, the second category represents 70% of the junctions and in these the primary gutters are more ramified than in the wild-type muscles, thus forming more AChR-free sub-domains. In addition, the primary gutters appear less sharply defined than those seen in normal NMJs. The overall surface of mutant NMJs is only slightly smaller than in wild-type mice, consistent with the notion that the size of the NMJ is mainly determined by the nerve terminal (Sanes and Lichtman, 2001). However, the average surface occupied by AChRs (i.e. the surface of the primary gutters) is twofold smaller at mutant NMJs; this is consistent with the reduction of AChR ε expression. The number of AChR-free sub-domains per NMJ also gives an idea of the organization of the primary gutters. In mutant muscles, the average number of such domains is three times higher than in wild-type muscles.
Figure 3.
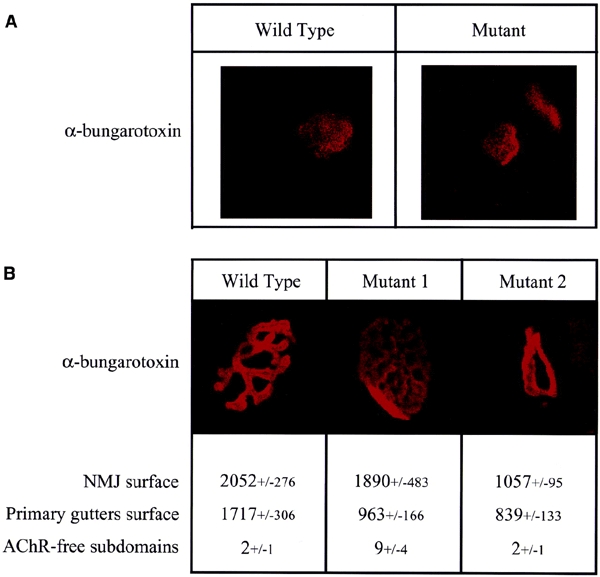
Comparison of the wild-type and mutant NMJ morphologies. NMJs were labelled with rhodaminated α-bungarotoxin and observed by confocal microscopy. (A) One week post-natal NMJ. (B) Three week post-natal NMJ. The NMJ surface corresponds to the surface occupied by the NMJ. Surfaces are expressed in arbitary units. The primary gutter surface corresponds to the surface labelled by, and thus occupied by, the AChR. AChR-free domains correspond to the number of closed surfaces, comprised in the NMJs and not labelled by the α-bungarotoxin. The +/− values correspond to the standard deviation. Mutants 1 and 2 illustrate the two morphological types of mutant NMJ.
Ultrastructural analysis of thin sections from the NMJs from mice expressing the Ets dominant-negative mutant disclosed severe perturbations in the post-synaptic apparatus (Figure 4). Compared with the postsynaptic membranes of NMJs in control animals (Figure 4A) those of the mutant NMJs showed disorganized secondary folds (arrows in Figure 4B) in some cases. At other junctions, the folds were scarce or even lacking (Figure 4C).
Figure 4.
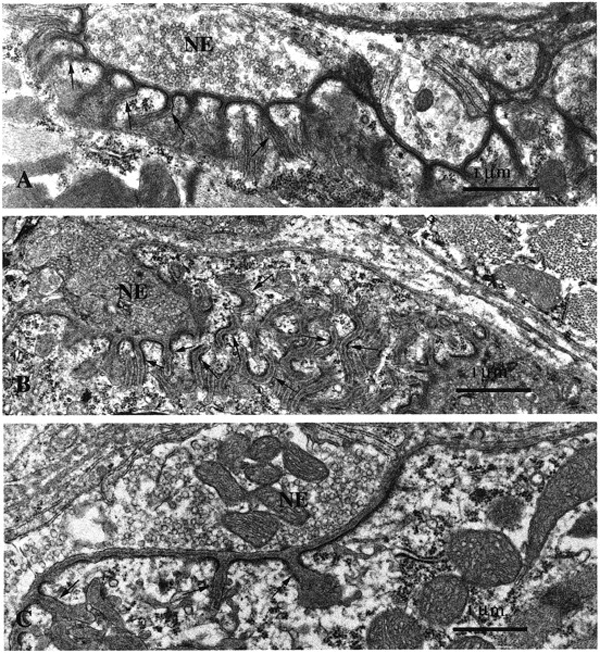
Comparative ultrastructural analysis of mutant versus wild-type NMJs. In agreement with confocal studies, the ultrastructural analysis of NMJs of mutant mice on thin sections disclosed severe perturbations in the post-synaptic apparatus. Compared with control animals (A) the post-synaptic membranes of the NMJs in vastus lateralis muscles showed disorganized secondary folds [arrows in (B)]. In other junctions, the folds were scarce or even lacking (C). Nerve endings (NE) looked normal in all muscles. Magnification = 20 000× in (A–C).
The morphological and ultrastructural alterations of the NMJ by the mutant Ets factor are reminiscent of what is observed in laminin β2- or utrophin-deficient mice (Noakes et al., 1995; Grady et al., 1997). In both cases the formation of synaptic secondary folds is affected.
EM studies also identified two types of mutant NMJs, which possibly correlate with the classes observed by confocal microscopy, but the difficulty in finding a large number of synapses at the EM level precluded the statistical analyses required to directly validate this hypothesis.
Altogether, our results show that, in vivo, Ets transcription factors are essential for synapse-specific expression of several synaptic genes and for the proper formation of NMJs. The altered NMJ morphology of muscle fibers expressing the Ets-dominant-negative mutant is most likely a direct result of the reduction in the expression of key components of the NMJ, i.e. the AChR α and AChR ε subunits, acetylcholinesterase, utrophin A and laminin β2, which are known to be involved in the control of neurotransmission, ultrastructure and nerve sprouting, respectively.
Point mutations in the N-box of the AChR ε subunit promoter have been identified in human patients suffering from congenital myasthenia, and are known to reduce expression of the AChR ε gene in these patients (Nichols et al., 1999; Ohno et al., 1999). The transgenic animals generated in the present study could therefore be useful to design protocols for Ets-independent synaptic gene activation.
Methods
Dominant-negative mutant construction.
The Ets-2 DBD (encoding amino acids 336–501) was amplified by RT–PCR and cloned upstream and in-frame with the lacZ gene, in the p1F-nlac-E plasmid kindly provided by R. Kelly and M. Buckingham (Kelly et al., 1997).
Transgenic animal production.
The purified MLC1F promoter/Ets-2 DBD-LacZ/Enhancer fragment was injected into fertilized oocytes of C57BL6/SJL hybrids. To identify the transgenic animals, genomic DNA was isolated from tail biopsies and tested by PCR to detect the presence of the LacZ gene.
Synaptic gene mRNA level analysis.
Quantitative real-time PCR analysis. Total RNA of muscles was extracted using the Qiagen RNeasy midi kit (Qiagen, France) with DNase treatments. First-strand cDNA was synthesized from 1 μg of total RNA using the Taqman Reverse Transcript kit (Perkin Elmer, Applied Biosystems). Hybridization primer/probe assays for real-time PCR detection were developed with the ABIprism Primer Express software (Applied Biosystems). The sequences of primers and probes were as follows (from 5′ to 3′, the number in brackets indicates the initial and final position of the primers): β-actin (DDBJ/EMBL/GenBank accession number X03765): for [344–363], rev [428–446], probe [398–422]; AChR-α (M17640) for [606–626], rev [652–672], probe [628–651]; AChR-ε (X55718) for [787–807], rev [832–854], probe [813–830]; Musk (X86444) for [656–677], rev[723–744], probe [687–706]; Rapsyn (X15788) for [827–846], rev [897–918], probe [864–883]; laminin β2 (NM008483) for [712–733], rev [817–838], probe [762–788]. All probes were 3′ labelled with TAMRA as quencher dye and were 5′ labelled with FAM as reporter dye (Eurogentec, Sereing, Belgium). TaqMan PCR was perfomed using TaqMan PCR Master mix (Applied Biosystems).
All mRNAs were quantitated relative to β-actin mRNA using the comparative C† (threshold cycle) method (Jones et al., 1999). To ensure that the ΔΔC† calculation was valid, the amplification efficiency was examined and found to be identical for all the genes measured. Taqman probes designed for acetylcholinesterase and utrophin did not fulfill this condition and were thus not quantified by this method.
Quantitative PCR analysis. Acetylcholinesterase, utrophin A and utrophin B transcript levels were analyzed by quantitative PCR as previously described (Gramolini et al., 1999). All measurements were taken during the linear phase of amplification.
The quantification experiments were performed on 3-week-old animals. For each gene, the results obtained with a negative control were compared with those obtained with at least two positive littermates. The experiments were repeated five times. Analysis of variance was performed using a one-way ANOVA.
Synaptic and extra-synaptic RNA purification and analysis. RNAs were purified from synaptic and extra-synaptic regions of lateralis vastus muscles to compare the sharpness of MuSK mRNA compartmentalization to that of AChR subunit mRNAs. For this purpose, NMJs were stained with the Karnovsky and Roots reaction (Karnovsky and Roots, 1964), and the synaptic and extrasynaptic regions of individual fibres were microdissected. Total RNA was then extracted from the isolated synaptic and extra-synaptic regions using the Qiagen RNeasy mini kit (Qiagen, France). The AChR α, ε and MuSK RNAs were quantified using quantitative real-time RT–PCR and the results were normalized to β actin. This allowed a 150- to 1000-fold enrichment of the various mRNAs in synaptic, compared with extra-synaptic, preparations. The AChR α mRNA clearly had a lower synaptic/extra-synaptic ratio than the MuSK and AChR ε mRNAs.
NMJ morphological analysis.
Dissected lateralis vastus muscles were either processed for electron microscopy as previously described (Agbulut et al., 2001), or labelled with rhodamine-labelled α-bungarotoxin (Molecular Probes) and observed under a confocal microscope (LSM 510, Zeiss, Germany) as previously described (Altiok et al., 1995). The topological data were calculated from the confocal images using NIH image.
At least 20 NMJs from each mutant individual in a litter were analysed. For the confocal analysis, 50 NMJs from a 3-week-old control animal and 150 NMJs from three 3-week-old mutant animals were analyzed. The morphometric results were pooled and their statistical significance was determined with a one-way ANOVA (P < 0.01).
To ascertain that the phenotype was not due to the perturbation of the expression of a critical gene by the insertion of the transgene in the genome, the expression and NMJ morphology were also measured on diaphragm muscles from transgenic animals. In such animals, the diaphragm muscle contains the transgene but does not express it, and synaptic gene expression and NMJ morphology are normal (data not shown).
Acknowledgments
We thank Margaret Buckingham and Robert Kelly for the transgene promoter, Nathalie Duclert, Marie Vandromme and Marie-Aline Ludosky for expert technical assistance. This work was supported by the Association Française contre les Myopathies (AFM), the Centre National de la Recherche Scientifique, the Collège de France, the EEC. A. de K.d'E. is Research Associate of the National Fund for Scientific Research (Belgium).
References
- Agbulut O., Li Z., Perie S., Ludosky M.A., Paulin D., Cartaud J. and Butler-Browne G. (2001) Lack of desmin results in abortive muscle regeneration and modifications in synaptic structure. Cell Motil. Cytoskel., 49, 51–66. [DOI] [PubMed] [Google Scholar]
- Alroy I., Soussan L., Seger R. and Yarden Y. (1999) Neu differentiation factor stimulates phosphorylation and activation of the Sp1 transcription factor. Mol. Cell. Biol., 19, 1961–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altiok N., Bessereau J.L. and Changeux J.P. (1995) ErbB3 and ErbB2/neu mediate the effect of heregulin on acetylcholine receptor gene expression in muscle: differential expression at the endplate. EMBO J., 14, 4258–4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenberger R., Kammerer R.A., Engel J. and Chiquet M. (1996) Native chick laminin-4 containing the β2 chain (s-laminin) promotes motor axon growth. J. Cell Biol., 135, 1583–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briguet A. and Ruegg M.A. (2000) The Ets transcription factor GABP is required for postsynaptic differentiation in vivo. J. Neurosci., 20, 5989–5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton E.A., Tinsley J.M., Holzfeind P.J., Rodrigues N.R. and Davies K.E. (1999) A second promoter provides an alternative target for therapeutic up-regulation of utrophin in Duchenne muscular dystrophy. Proc. Natl Acad. Sci. USA, 96, 14025–14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R.Y., Boudreau-Lariviere C., Angus L.M., Mankal F.A. and Jasmin B.J. (1999) An intronic enhancer containing an N-box motif is required for synapse- and tissuespecific expression of the acetylcholinesterase gene in skeletal muscle fibers. Proc. Natl Acad. Sci. USA, 96, 4627–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue M.J., Merlie J.P., Rosenthal N. and Sanes J.R. (1991) Rostrocaudal gradient of transgene expression in adult skeletal muscle. Proc. Natl Acad. Sci. USA, 88, 5847–5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclert A., Savatier N., Schaeffer L. and Changeux J.P. (1996) Identification of an element crucial for the subsynaptic expression of the acetylcholine receptor ε subunit gene. J. Biol. Chem., 271, 17433–17438. [DOI] [PubMed] [Google Scholar]
- Fromm L. and Burden S.J. (1998) Synapsespecific and neuregulin-induced transcription require an ets site that binds GABPα/GABPβ. Genes Dev., 12, 3074–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady R.M., Merlie J.P. and Sanes J.R. (1997) Subtle neuromuscular defects in utrophin-deficient mice. J. Cell Biol., 136, 871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramolini A.O., Angus L.M., Schaeffer L., Burton E.A., Tinsley J.M., Davies K.E., Changeux J.P. and Jasmin B.J. (1999) Induction of utrophin gene expression by heregulin in skeletal muscle cells: role of the N-box motif and GA binding protein. Proc. Natl Acad. Sci. USA, 96, 3223–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieshammer U., Sassoon D. and Rosenthal N. (1992) A transgene target for positional regulators marks early rostrocaudal specification of myogenic lineages. Cell, 69, 79–93. [DOI] [PubMed] [Google Scholar]
- Ip F.C.F., Glass D.G., Gies D.R., Cheung J., Lai K.O., Fu A.K.Y., Yancopoulos G.D. and Ip N.Y. (2000) Cloning and characterization of musclespecific kinase in chicken. Mol. Cell. Neurosci., 16, 661–673. [DOI] [PubMed] [Google Scholar]
- Jones G., Moore C., Hashemolhosseini S. and Brenner H.R. (1999) Constitutively active MuSK is clustered in the absence of agrin and induces ectopic postsynaptic-like membranes in skeletal muscle fibers. J. Neurosci., 19, 3376–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky M.J. and Roots L. (1964) A 'direct colouring' thiocholine method for cholinesterases. J. Histochem. Cytochem., 12, 219–221. [DOI] [PubMed] [Google Scholar]
- Kelly R.G., Zammit P.S., Schneider A., Alonso S., Biben C. and Buckingham M.E. (1997) Embryonic and fetal myogenic programs act through separate enhancers at the MLC1F/3F locus. Dev. Biol., 187, 183–199. [DOI] [PubMed] [Google Scholar]
- Khurana T.S., Rosmarin A.G., Shang J., Krag T.O., Das S. and Gammeltoft S. (1999) Activation of utrophin promoter by heregulin via the ets-related transcription factor complex GA-binding protein α/β. Mol. Biol. Cell, 10, 2075–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike S., Schaeffer L. and Changeux J.P. (1995) Identification of a DNA element determining synaptic expression of the mouse acetylcholine receptor δ subunit gene. Proc. Natl Acad. Sci. USA, 92, 10624–10628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S.J., Bortner D.M., Roussel M.F., Sherr C.J. and Ostrowski M.C. (1992) Mitogenic signaling by colonystimulating factor 1 and ras is suppressed by the ets-2 DNA-binding domain and restored by myc overexpression. Mol. Cell. Biol., 12, 5355–5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlie J.P. and Kornhauser J.M. (1989) Neural regulation of gene expression by an acetylcholine receptor promoter in muscle of transgenic mice. Neuron, 2, 1295–1300. [DOI] [PubMed] [Google Scholar]
- Moore C., Leu M., Müller U. and Brenner H.R. (2001) Induction of multiple signaling loops by Musk during neuromuscular synapse formation. Proc. Natl Acad. Sci. USA, 98, 14655–14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newey S.E., Gramolini A.O., Wu J., Holzfeind P., Jasmin B.J., Davies K.E. and Blake D.J. (2001) A novel mechanism for modulating synaptic gene expression: differential localization of α-dystrobrevin transcripts in skeletal muscle. Mol. Cell. Neurosci., 17, 127–140. [DOI] [PubMed] [Google Scholar]
- Nichols P., Croxen R., Vincent A., Rutter R., Hutchinson M., Newsom-Davis J. and Beeson D. (1999) Mutation of the acetylcholine receptor εsubunit promoter in congenital myasthenic syndrome. Ann. Neurol., 45, 439–443. [PubMed] [Google Scholar]
- Noakes P.G., Gautam M., Mudd J., Sanes J.R. and Merlie J.P. (1995) Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin β2. Nature, 374, 258–262. [DOI] [PubMed] [Google Scholar]
- Ohno K., Anlar B. and Engel A.G. (1999) Congenital myasthenic syndrome caused by a mutation in the Ets-binding site of the promoter region of the acetylcholine receptor ε subunit gene. Neuromusc. Disord., 9, 131–135. [DOI] [PubMed] [Google Scholar]
- Rao M.V., Donoghue M.J., Merlie J.P. and Sanes J.R. (1996) Distinct regulatory elements control musclespecific, fiber-type-selective, and axially graded expression of a myosin light-chain gene in transgenic mice. Mol. Cell. Biol., 16, 3909–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J.R. and Lichtman J.W. (2001) Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat. Rev. Neurosci., 2, 791–805. [DOI] [PubMed] [Google Scholar]
- Sapru M.K., Florance S.K., Kirk C. and Goldman D. (1998) Identification of a neuregulin and protein-tyrosine phosphatase response element in the nicotinic acetylcholine receptor e subunit gene: regulatory role of an Rts transcription factor. Proc. Natl Acad. Sci. USA, 95, 1289–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer L., Duclert N., Huchet-Dymanus M. and Changeux J.P. (1998) Implication of a multisubunit Ets-related transcription factor in synaptic expression of the nicotinic acetylcholine receptor. EMBO J., 17, 3078–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer L., de Kerchove d'Exaerde A. and Changeux J.P. (2001) Targeting transcription to the neuromuscular synapse. Neuron, 31, 15–22. [DOI] [PubMed] [Google Scholar]


