Abstract
A total of 301 adult questing Ixodes ricinus ticks were collected at 15 different locations along the south and east coasts of Sweden to determine the Borrelia genospecies diversity. Thirty-two ticks (11%) were found to be positive by nested PCR with Borrelia burgdorferi sensu lato-specific primers. Species determination was based on partial sequencing of the 16S rRNA gene and the flagellin gene. Five different Borrelia species were found. The nucleotide sequence of the Borrelia DNA found in two ticks differed extensively from the nucleotide sequences of the Borrelia DNA found in the other ticks, and analysis revealed that they were closely related to the relapsing fever borrelia species Borrelia miyamotoi. This is the first report of a B. miyamotoi-like borrelia in I. ricinus and in Europe. Moreover, the Borrelia DNA of two ticks (6%) clustered within the B. valaisiana complex. B. valaisiana has not previously been reported in Sweden. B. afzelii DNA was found in 14 ticks (44%), and B. garinii DNA was found in 10 ticks (31%). B. burgdorferi sensu stricto DNA was found in four ticks (13%). We conclude that all of the known human-pathogenic species (B. garinii, B. afzelii, and B. burgdorferi sensu stricto) and B. valaisiana found elsewhere in Europe are also present in the Swedish host-seeking tick population and that a B. miyamotoi-like Borrelia species seems to be present in I. ricinus ticks in Europe.
Ticks transmit many clinically important pathogens of the genus Borrelia. These spirochetes are responsible for two groups of human disease: Lyme borreliosis (LB) and relapsing fever (RF). LB is the most prevalent tick-borne zoonosis in Europe and North America and affects the skin, joints, heart, and nervous system (31). Borreliae that cause LB are transmitted by hard ticks of the genus Ixodes. In Europe the principal vector is Ixodes ricinus. The disease is caused by spirochetes of the Borrelia burgdorferi sensu lato complex, which consists of 10 different named genospecies. Three species, all found in Europe, are known to be pathogenic for humans: B. burgdorferi sensu strico, B. garinii (4), and B. afzelii (4, 9). Another two species, B. valaisiana (34) and B. lusitaniae (17), have been isolated from European ticks. The pathogenic capabilities of the last two species are still uncertain, although B. valaisiana DNA has been amplified by PCR from patients with LB (29). Two additional Borrelia species have been found in European patients with LB; B. bissettii sp. nov. (26) has been isolated from patients in Slovakia (25), and a novel Borrelia species has been isolated from a patient in The Netherlands (35). There has been an increasing interest in the clinical and diagnostic implications of the different Borrelia species, since an association between the clinical manifestations of LB and the infective species has been suggested (3, 9, 22, 33). The infective Borrelia species also influences the immune response (8, 30).
Tick-borne RF, with periodic febrile episodes as the main symptom, is caused by a genetically and ecologically different group of Borrelia species. RF is rarely seen in Europe but is reported in the most southern parts of Europe (1). Borrelia species that cause tick-borne RF are usually considered vector species specific and are mostly transmitted by soft ticks (family Argasidae) of the genus Ornithodoros (24). Two RF-associated Borrelia species are exceptions and are found in hard ticks: B. lonestari, which is transmitted by Amblyomma americanum in North America (5), and B. miyamotoi, which is isolated from Ixodes persulcatus in Japan (10).
Different methods are used for Borrelia species determination. PCR detection and subsequent sequencing of the 16S rRNA gene is considered a sensitive and reliable method (36). Sequencing of the flagellin gene gives additional taxonomic data (11).
Since information on the Borrelia genospecies present in the tick population is essential to our understanding of the epidemiology, clinical spectrum, diagnosis, and prevention of LB, we conducted this study to determine the diversity of B. burgdorferi sensu lato among ground host-seeking ticks in Sweden.
MATERIALS AND METHODS
Study area and tick collection.
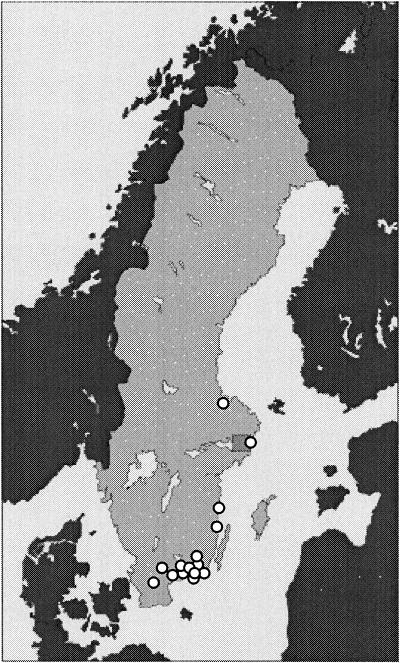
During the summer of 1999 questing adult ticks were collected by flagging at 15 different locations with mixed vegetation along the south and east coasts of Sweden. A total of 301 adult unfed I. ricinus ticks were collected. Twenty-one ticks were collected at two locations in the province of Skåne, 233 ticks were collected at nine different locations in the province of Blekinge (108 ticks were collected from a single location), 24 ticks were collected at two locations in the province of Kalmar, 16 ticks were collected in the proximity of Stockholm, and 7 ticks were collected farther north at a location in the proximity of Gävle (Fig. 1). Of the ticks collected, 165 (55%) were male and 136 (45%) were female. The ticks were placed into coded tubes and stored at −20°C until September 2000.
FIG. 1.
Map of Sweden showing the locations of tick collection.
DNA extraction.
The ticks were processed individually. Each tick was washed in 70% ethanol and cut in half sagittally on a glass slide with a drop of phosphate-buffered saline. One half was saved for future use, and the other half was crushed and transferred to a test tube (Eppendorf; 1.5 ml) for DNA extraction. A QIAamp tissue kit (Qiagen) was used for DNA extraction according to the protocol of the manufacturer, with a few modifications. Samples were incubated overnight with proteinase K solution and eluted twice with 100 μl of AE buffer in order to increase the DNA yield. Purified DNA was stored at −20°C.
PCR amplification.
For detection of Borrelia-infected ticks, the 16S rRNA sequence was amplified by a nested PCR. Primers16S-F and 16S-R were used in the first amplification (19). The master mixture contained 0.5 μM each primer, 10 mM Tris-HCl, 1.5 mM MgCl2, 50 mM KCl (pH 8.3), 0.2 μM each deoxynucleoside triphosphate, and 1.25 U of Taq DNA polymerase. The reaction volume was set to 50 μl containing 5 μl of sample, and the amplification was conducted by using a program of 94°C for 2 min, followed by 35 cycles of 94°C for 45 s, 55°C for 60 s, 72°C for 90 s and finally 72°C for 7 min in a Perkin-Elmer 9600 thermocycler. Primers LD-1 and LD-2 (18) were used in the second reaction. The master mixture described above was used, except that 0.2 μM each primer was added and 2 μl of the PCR product from the first PCR was used as the template in the reaction. The volume was set to 50 μl, and amplification was conducted by using a program of 94°C for 2 min, followed by 40 cycles of 94°C for 45 s, 56°C for 60 s, 72°C for 60 s and finally 72°C for 7 min. The products were visualized by electrophoresis in a 1.5% agarose gel stained with ethidium bromide. A negative control and a positive control were included in all PCR runs. Contamination was minimized by performing the different steps in separate rooms. Gloves and filter tips were always used.
Nucleotide sequence analysis.
Partial sequencing of the 16S rRNA gene was performed for all positive samples. The products were extracted with a QIAquick gel extraction kit (Qiagen) according to the protocol of the manufacturer and sequenced by the BIG DYE method (Applied Biosystems) by direct sequencing by PCR. Each strand was analyzed with an ABI 3100 instrument (Applied Biosystems) by the Biomolecular Resource Facility at Lund University. Primers 16S-F and 569r (5′-TACGCCCAATAATCCCGAACAAC-3′) were used. The flagellin gene in four of the samples was partially sequenced by use of primer flaC (5′-ATTGAAAT AGAGCAACTTACAGA-3′) and primer flaL4 (5′-TTATCTAAGCAATGACAAAACATAT-3′). The DNA sequences were compared with the Borrelia gene sequences registered in the GenBank database. BioEdit software (T. A. Hall, Nucleic Acids. Symp. Ser. 41:95-98, 1999) was used for analysis of the results.
Nucleotide sequence accession numbers.
The 16S rRNA sequences of the borreliae from the following Borrelia strains determined in this study have been deposited in GenBank and given the indicated accession numbers: Ri11, AY083470; St1, AY083471; To60, AY083472; To72, AY083473; Ku10, AY083474; To76, AY083475; Al10, AY083476; As15, AY083477; Ga2, AY083478; Os8, AY083479; Ri13, AY083480; St12, AY083481; St13, AY083482; St8, AY083483; Tr2, AY083484; Ri9, AY083485; Al12, AY083486; Al16, AY083487; As18, AY083488; As7, AY083489; Ha3, AY083490; Ha5, AY083491; Os10, AY083492; Os2, AY083493; Osk2, AY083494; Osk3, AY083495; Sa5, AY083496; To106, AY083497; To16, AY083498; To89, AY083499; Na34, AY083500; and St4, AY083501. The flagellin gene sequences of the borreliae from the following Borrelia strains determined in this study have been deposited in GenBank and given the indicated accession numbers: St4, AY083502; Na34, AY083503; To76, AY083504; and Ku10, AY083505.
RESULTS
Borrelia PCR.
Of the 301 ticks, 32 (11%) were positive by use of the B. burgdorferi sensu lato-specific primers in the nested PCR. The PCR products of two of the tick samples yielded atypical bands by gel electrophoresis, but the two samples were still considered positive. There were no differences in the prevalences of Borrelia spp. between male and female ticks.
Borrelia nucleotide sequence analysis.
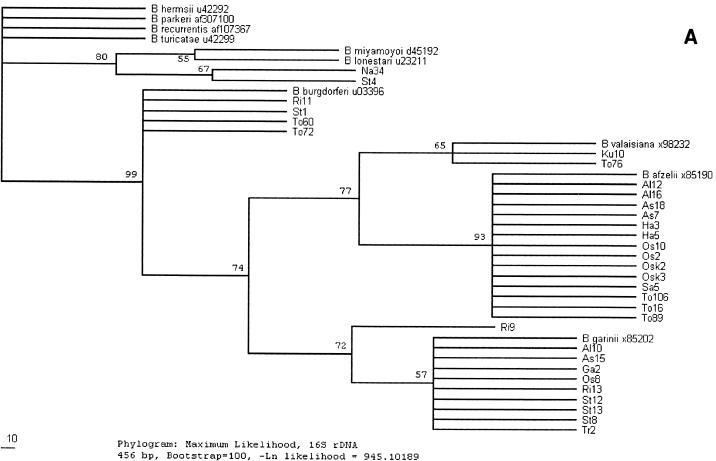
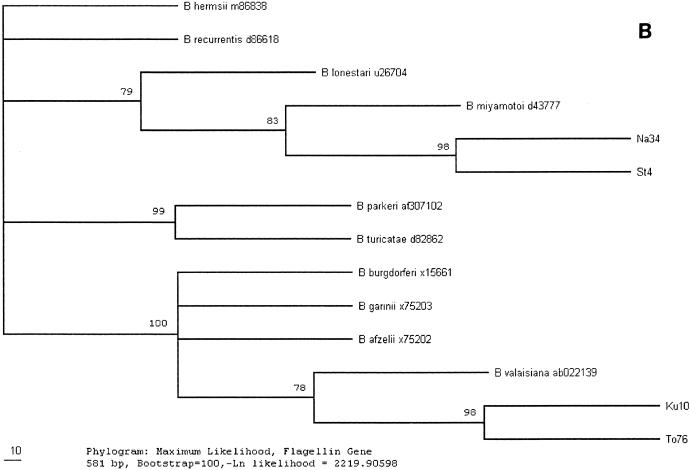
Species determination was made by DNA nucleotide sequence analysis, and the sequences were compared with those for previously reported strains available in the GenBank database. Partial sequencing of the 16S rRNA gene, with a minimum of 496 bp analyzed, was performed for all 32 Borrelia 16S rRNA gene-positive ticks, and the results are summarized in Table 1. The flagellin gene was partially sequenced (a minimum of 608 bp was analyzed) in order to confirm and specify the findings for four of the Borrelia-positive ticks (Na34, St4, To76, and Ku10). Five different Borrelia species were identified in the ticks examined. DNA from two of the ticks (Na34 and St4, collected in Blekinge and Stockholm, respectively) yielded atypical PCR bands by gel electrophoresis (the strongest band was about 1,500 bp, and no LD primer-specific band was seen). The sequences of the 16S rRNA and flagellin genes obtained from the two ticks were identical but differed extensively from the sequences of the genes from the other ticks. Analysis indicated that they are closely related, but not identical, to the previously reported sequences of the genes from B. miyamotoi strains (Table 1). B. afzelii DNA was found in 14 ticks (44%), B. garinii DNA was found in 10 ticks (31%), and both species were widely distributed geographically. B. burgdorferi sensu stricto DNA was found in four ticks (13%) collected in Blekinge and Stockholm. The DNA of the 16S rRNA and flagellin gene sequenced in two ticks (6%), collected in Blekinge (from two different locations), belonged to the B. valaisiana genomic group. A phylogenetic tree was constructed from the DNA sequence data obtained (Fig. 2A and B).
TABLE 1.
Analysis of signature nucleotide positions of the partial 16S rRNA in the Borrelia strains studied
| Borrelia DNA source(s)a | Nucleotide at the following 16S rRNA positionb:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 77 | 126 | 170 | 175 | 178 | 181 | 252 | 273 | 471 | |
| B. burgdorferi sensu strico B31 (U03396), Ri11, St1, To60, To72 | C | T | G | G | A | T | A | A | T |
| B. afzelii DK1 (X85190), As18, Osk2, Osk3, To16, To89, To106, As7, Ha3, Os2, Al12, Al16, Sa5 | C | T | G | A | A | T | G | G | C |
| B. garinii DK27 (X85202), Os8, St8, St12, St13, Ga2, As15, Ri13, Tr2, Al10 | C | C | A | A | A | T | A | G | T |
| Ri9 | C | T | A | A | A | T | A | G | T |
| B. valaisiana VS116 (X98232), To76, Ku10 | T | T | G | A | A | T | G | G | T |
| B. miyamotoi HT31 (D45192) | T | C | A | A | A | A | G | T | C |
| Na34, St4 | C | C | G | A | G | G | G | T | T |
| B. parkeri (AF307100) | C | T | G | A | G | A | A | T | T |
| B. recurrentis (AF107367) | C | T | G | G | G | A | A | T | T |
| B. turicatae (U42299) | C | T | G | A | G | A | A | T | T |
| B. lonestari (U23211) | T | C | A | A | G | T | A | T | T |
| B. hermsii (U42292) | C | T | G | A | G | A | A | T | T |
GenBank accession numbers are shown in parentheses. The nucleotides of B. burgdorferi sensu stricto B31, B. afzelii DK1, B. garinii DK27, B. valaisiana VS116, B. miyamotoi HT31, B. parkeri, B. recurrentis, B. turicatae, B. lonestari, and B. hermsii and their GenBank accession numbers are included for comparison purposes.
B. burgdorferi B31 numbering; insertion of a C residue in position 213 has been ignored.
FIG. 2.
(A) Phylogenetic tree, based on a comparison of the 16S rRNA sequences (456 bp) of Borrelia species, obtained by the maximum-likelihood method. B. hermsii, B. parkeri, B. recurrentis, B. turicatiae, B. miyamotoi, B. lonestari, B. burgdorferi, B. valaisiana, B. afzelii, and B. garinii and their GenBank accession numbers are included for comparison purposes. (B) Phylogenetic tree, based on a comparison of the flagellin gene sequences (581 bp) of Borrelia species, obtained by the maximum-likelihood method.
DISCUSSION
The genetic diversity of Borrelia species in the tick population has not previously been studied in Sweden. In this study five different Borrelia species were identified. We obtained Borrelia DNA from two I. ricinus ticks that were not closely related to any of the B. burgdorferi sensu lato genospecies. Phylogenetic analysis of the flagellin and 16S rRNA sequences indicated that they were closely related to the B. miyamotoi genospecies, previously isolated only in Japan from I. persulcatus ticks (10). B. miyamotoi has not yet been shown to cause disease in humans and is phylogenetically most closely related to B. lonestari among the relapsing fever borreliae (11, 27). B. lonestari is transmitted by the hard A. americanum tick (classified within the Metastriata subfamily) (5) and can cause an erythema migrans-like rash (15). B. lonestari is also closely related to the agent of bovine borreliosis, Borrelia theileri, which is transmitted by hard ticks from the Metastriata subfamily (28). Phylogenetic analysis distinguishes two separate clusters within the group of RF borreliae that are transmitted by ixodid ticks: the Metastriata tick-transmitted species B. lonestari and B. theileri cluster together, and the B. miyamotoi-like borreliae transmitted by Ixodes spp. form a separate cluster. The present study is the first report of B. miyamotoi-like Borrelia species in Europe and in I. ricinus ticks. We found B. miyamotoi-like DNA in two ticks collected 400 km apart, which indicates that the findings were not isolated but, rather, that the species is geographically widespread. The monophyletic cluster of B. miyamotoi-like borreliae thus seems to be distributed on two continents and transmitted by two subspecies of Ixodes ticks. Although genetically classified within the RF borreliae, B. miyamotoi seems to have ecological characteristics of a borrelia of the B. burgdorferi sensu lato complex. Further studies are needed to characterize the biological properties of this Borrelia species. Isolation and more records are needed to confirm its presence in the European tick population.
The primers used in this study were not designed to find any borreliae other than those from the B. burgdorferi sensu lato complex. The tick templates in which the B. miyamotoi-like DNA was found reacted with the primers, but the PCR products were of a different size. The published data do not enable analysis of how the 16S rRNA-specific primers correspond to the 16S rRNA sequence of B. miyamotoi. The 3′ ends of the LD-1 and LD-2 primers differ at 3 and 2 bp, respectively, from the sequence of the B. miyamotoi type strain (strain HT31).
Of the B. burgdorferi sensu lato species found, B. afzelii and B. garinii dominated, with 44 and 31% of the Borrelia isolates being of these two species, respectively, which is consistent with clinical findings in Sweden (22) and with reports from Europe (13). B. burgdorferi sensu stricto has just recently been found in Swedish LB patients by PCR (23) and has previously been reported from Ixodes ticks collected from migrating birds arriving in Sweden (21). B. valaisiana has been reported from a number of European countries (13), but it has never before been reported in Sweden.
We found a Borrelia prevalence of 11% in the Swedish host-seeking tick population by use of the PCR technique. Previous studies have used immunofluorescence analysis (6, 12), phase-contrast microscopy (7, 14, 20, 32), or dark-field microscopy (2); and prevalences ranging between 7 and 29% have been recorded in adult ticks from locations in southern and central Sweden. Significant spatial and seasonal variations in the prevalence of Borrelia-infected ticks have been observed previously (16, 20); and the year, the location of sampling, and the method used for analysis may account for the differences between the reported prevalences.
We conclude that all of the known pathogenic species (B. garinii, B. afzelii, and B. burgdorferi sensu strico) and B. valaisiana found elsewhere in Europe are also present in the Swedish host-seeking tick population. Of particular interest is our finding of B. miyamotoi-like Borrelia in Europe.
Acknowledgments
This study was supported by grants from the County Council of Blekinge and GlaxoSmithKline.
We thank I. Eilasson for continued support and cooperation. We also thank J. Bunikis for assistance with the analysis and valuable comments and K. Ornstein, R. Eitrem, and S. Bergström for critically reading the manuscript.
REFERENCES
- 1.Anda, P., W. Sánchez-Yebra, M. del Mar Vitutia, E. Rérez Pastrana, I. Rodríguez, N. S. Miller, P. B. Backenson, and J. L. Benach. 1996. A new Borrelia species isolated from patients with relapsing fever in Spain. Lancet 348:162-165. [DOI] [PubMed] [Google Scholar]
- 2.Åsbrink, E., B. Hederstedt, and A. Hovmark. 1984. The spirochetal etiology of erythema chronicum migrans afzelius. Acta Dermatol. Venerol. 64:291-295. [PubMed] [Google Scholar]
- 3.Balmelli, T., and J. C. Piffaretti. 1995. Association between different clinical manifestations of Lyme disease and different species of Borrelia burgdorferi sensu lato. Res. Microbiol. 146:329-340. [DOI] [PubMed] [Google Scholar]
- 4.Baranton, G., D. Postic, I. Saint Girons, P. Boerlin, J. C. Piffaretti, M. Assous, and P. A. Grimont. 1992. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS 461 associated with Lyme borreliosis. Int. J. Syst. Bacteriol. 42:378-383. [DOI] [PubMed] [Google Scholar]
- 5.Barbour, A. G., G. O. Maupin, G. J. Teltow, C. J. Carter, and J. Piesman. 1996. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J. Infect. Dis. 173:403-409. [DOI] [PubMed] [Google Scholar]
- 6.Berglund, J., and R. Eitrem. 1993. Tick-borne borreliosis of the archipelago of southern Sweden. Scand. J. Infect. Dis. 25:67-72. [PubMed] [Google Scholar]
- 7.Bergström, S., B. Olsén, N. Burman, L. Gothefors, T. G. Jaenson, M. Jonsson, and H. A. Mejlon. 1992. Molecular characterization of Borrelia burgdorferi isolated from Ixodes ricinus in northern Sweden. Scand. J. Infect. Dis. 24:181-188. [DOI] [PubMed] [Google Scholar]
- 8.Bunikis, J., B. Olsén, G. Westman, and S. Bergström. 1995. Variable serum immunoglobulin responses against different Borrelia burgdorferi sensu lato species in a population at risk for and patients with Lyme disease. J. Clin. Microbiol. 33:1473-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canica, M. M., F. Nato, L. du Merle, J. C. Mazie, G. Baranton, and D. Postic. 1993. Monoclonal antibodies for identification of Borrelia afzelii sp. nov. associated with late cutaneous manifestations of Lyme borreliosis. Scand. J. Infect. Dis. 25:441-448. [DOI] [PubMed] [Google Scholar]
- 10.Fukunaga, M., Y. Takahashi, Y. Tsuruta, O. Matsushita, D. Ralph, M. McClelland, and M. Nakao. 1995. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Int. J. Syst. Bacteriol. 45:804-810. [DOI] [PubMed] [Google Scholar]
- 11.Fukunaga, M., K. Okada, M. Nakao, T. Konishi, and Y. Sato. 1996. Phylogenetic analysis of Borrelia species based on flagellin gene sequences and its application for molecular typing of Lyme disease borreliae. Int. J. Syst. Bacteriol. 46:898-905. [DOI] [PubMed] [Google Scholar]
- 12.Gustavsson, R., T. G. Jaenson, A. Gardulf, H. Mejlon, and B. Svenungsson. 1995. Prevalence of Borrelia burgdorferi sensu lato infection in Ixodes ricinus in Sweden. Scand. J. Infect. Dis. 27:597-601. [DOI] [PubMed] [Google Scholar]
- 13.Hubalek, Z., and J. Halouzka. 1997. Distribution of Borrelia burgdorferi sensu lato genomic groups in Europe, a review. Eur. J. Epidemiol. 13:951-957. [DOI] [PubMed] [Google Scholar]
- 14.Jaenson, T. G., and L. Tälleklint. 1992. Incompetence of roe deer as reservoirs of the Lyme borreliosis spirochete. J. Med. Entomol. 29:813-817. [DOI] [PubMed] [Google Scholar]
- 15.James, A. M., D. Liveris, G. P. Wormser, I. Schwartz, M. A. Montecalvo, and B. J. Johnson. 2001. Borrelia lonestari infection after a bite by an Amblyomma americanum tick. J. Infect. Dis. 183:1810-1814. [DOI] [PubMed] [Google Scholar]
- 16.Kirstein, F., S. Rijpkema, M. Molkenboer, and J. S. Gray. 1997. Local variations in the distribution and prevalence of Borrelia burgdorferi sensu lato genomospecies in Ixodes ricinus ticks. Appl. Environ. Microbiol. 63:1102-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Fleche, A., D. Postic, K. Girardet, O. Peter, and G. Baranton. 1997. Characterization of Borrelia lusitaniae sp. nov. by 16S ribosomal DNA sequence analysis. Int. J. Syst. Bacteriol. 47:921-925. [DOI] [PubMed] [Google Scholar]
- 18.Marconi, R. T., and C. F. Garon. 1992. Development of polymerase chain reaction primer sets for diagnosis of Lyme disease and for species-specific identification of Lyme disease isolates by 16S rRNA signature nucleotide analysis. J. Clin. Microbiol. 30:2830-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marconi, R. T., D. Liveris, and I. Schwartz. 1995. Identification of novel insertion elements, restriction fragment length polymorphism patterns, and discontinuous 23S rRNA in Lyme disease spirochetes: phylogenetic analyses of rRNA genes and their intergenic spacers in Borrelia japonica sp. nov. and genomic group 21038 (Borrelia andersonii sp. nov.) isolates. J. Clin. Microbiol. 33:2427-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mejlon, H., and T. G. T. Jaenson. 1993. Seasonal prevalence of Borrelia burgdorferi in Ixodes ricinus in different vegetation type in Sweden. Scand. J. Infect. Dis. 25:449-456. [DOI] [PubMed] [Google Scholar]
- 21.Olsén, B., T. G. Jaenson, and S. Bergström. 1995. Prevalence of Borrelia burgdorferi sensu lato-infected ticks on migrating birds. Appl. Environ. Microbiol. 61:3082-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ornstein, K., J. Berglund, I. Nilsson, R. Norrby, and S. Bergström. 2001. Characterization of Lyme borreliosis isolates from patients with erythema migrans and neuroborreliosis in southern Sweden. J. Clin. Microbiol. 39:1294-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ornstein, K., J. Berglund, S. Bergström, R. Norrby, and A. G. Barbour. 2002. Three major Lyme Borrelia genospecies (Borrelia burgdorferi sensu stricto, B. afzelii and B. garinii) identified by PCR in cerebrospinal fluid from patients with neuroborreliosis in Sweden. Scand. J. Infect. Dis. 34:341-346. [DOI] [PubMed] [Google Scholar]
- 24.Parola, P., and D. Raoult. 2001. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin. Infect. Dis. 32:897-928. [DOI] [PubMed] [Google Scholar]
- 25.Picken, R. N., Y. Cheng, F. Strle, and M. M. Picken. 1996. Patient isolates of Borrelia burgdorferi sensu lato with genotypic and phenotypic similarities of strain 25015. J. Infect. Dis. 174:1112-1115. [DOI] [PubMed] [Google Scholar]
- 26.Postic, D., N. M. Ras, R. S. Lane, M. Hendson, and G. Baranton. 1998. Expanded diversity among Californian Borrelia isolates and description of Borrelia bissettii sp. nov. (formerly Borrelia group DN127). J. Clin. Microbiol. 36:3497-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ras, N. M., B. Lascola, D. Postic, S. J. Cutler, F. Rodhain, G. Baranton, and D. Raoult. 1996. Phylogenesis of relapsing fever Borrelia spp. Int. J. Syst. Bacteriol. 46:859-865. [DOI] [PubMed] [Google Scholar]
- 28.Rich, S. M., P. M. Armstrong, R. D. Smith, and S. R. Telford III. 2001. Lone star tick-infecting borreliae are most closely related to the agent of bovine borreliosis. J. Clin. Microbiol. 39:494-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rijpkema, S. G., D. J. Tazelaar, M. Molkenboer, G. T. Noordhoek, G. Plantinga, L. M. Schouls, and J. F. Schellekens. 1997. Detection of Borrelia afzelii, Borrelia burgdorferi sensu stricto, Borrelia garinii and group VS116 by PCR in skin biopsies of patients with erythema migrans and acrodermitis chronica atrophicans. Clin. Microbiol. Infect. 3:109-116. [DOI] [PubMed] [Google Scholar]
- 30.Robertson, J., E. Guy, N. Andrews, B. Wilske, P. Anda, M. Granström, U. Hauser, Y. Moosmann, V. Sambri, J. Schellekens, G. Stanek, and J. Gray. 2000. A European multicenter study of immunoblotting in serodiagnosis of Lyme borreliosis. J. Clin. Microbiol. 38:2097-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steere, A. 2001. Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 32.Tälleklint, L., and T. G. Jaenson. 1993. Maintenance by hares of European Borrelia burgdorferi in ecosystems without rodents. J. Med. Entomol. 30:273-276. [DOI] [PubMed] [Google Scholar]
- 33.van Dam, A. P., H. Kuiper, K. Vos, A. Widjojokusumo, B. M. de Jongh, L. Spanjaard, A. C. Ramselaar, M. D. Kramer, and J. Dankert. 1993. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin. Infect. Dis. 17:708-717. [DOI] [PubMed] [Google Scholar]
- 34.Wang, G., A. P. van Dam, A. Le Fleche, D. Postic, O. Peter, G. Baranton, R. de Boer, L. Spanjaard, and J. Dankert. 1997. Genetic and phenotypic analysis of Borrelia valaisiana sp. nov. (Borrelia genomic groups VS116 and M19). Int. J. Syst. Bacteriol. 47:926-932. [DOI] [PubMed] [Google Scholar]
- 35.Wang, G., A. P. van Dam, and J. Dankert. 1999. Phenotypic and genetic characterization of a novel Borrelia burgdorferi sensu lato isolate from a patient with Lyme borreliosis. J. Clin. Microbiol. 37:3025-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang, G., A. P. van Dam, I. Schwartz, and J. Dankert. 1999. Molecular typing of Borrelia burgdorferi sensu lato: taxonomic, epidemiological, and clinical implications. Clin. Microbiol. Rev. 12:633-653. [DOI] [PMC free article] [PubMed] [Google Scholar]