Abstract
DNA looping is one of the key factors allowing proteins bound to different DNA sites to signal one another via direct contacts. We demonstrate that DNA looping can be generated in an arbitrary chosen site by sequence-directed targeting of double-stranded DNA with pseudocomplementary peptide-nucleic acids (pcPNAs). We designed pcPNAs to mask the DNA from cleavage by type IIs restriction enzyme PleI while not preventing the enzyme from binding to its primary DNA recognition site. Direct interaction between two protein molecules (one bound to the original recognition site and the other to a sequence-degenerated site) results in a totally new activity of PleI: it produces a nick near the degenerate site. The PNA-induced nicking efficiency varies with the distance between the two protein-binding sites in a phase with the DNA helical periodicity. Our findings imply a general approach for the fine-tuning of proteins bound to DNA sites well separated along the DNA chain.
Introduction
DNA looping plays a key role in the regulation of gene activity allowing direct contacts between proteins bound to sites well separated along the DNA chain (Lee and Schleif, 1989; Kahn and Crothers, 1993; Law et al., 1993; Sinden, 1994; Rippe et al., 1997; Schleif, 2000; DeSanctis et al., 2002; Ptashne and Gann, 2002). In some cases, DNA looping is thought to facilitate enzyme action (Friedhoff et al., 2001; Halford, 2001; Gormley et al., 2002). Normally, DNA looping is mediated by particular DNA-bending proteins targeted to specialized sites on doublestranded (ds)DNA (Sinden, 1994; Cherny et al., 1999). In this connection, a sequence-universal DNA-binding ligand, which induces looping in chosen sites of dsDNA, can be a valuable tool to directly modulate the activity of DNA-binding proteins.
Here we demonstrate that a promising ligand of this type is a new brand of peptide-nucleic acid (PNA) molecules, pseudocomplementary (pc)PNAs (Lohse et al., 1999; Izvolsky et al., 2000; Demidov et al., 2002). A pair of pcPNAs sequencespecifically invades the DNA duplex generating a hinge. Using type IIs restriction enzyme PleI as a test system, we checked the possibility of the pcPNA-mediated interaction between proteins bound to distantly located DNA sites. We show that the (ds)DNA looping induced at a chosen site by sequence-unrestricted invasion of pcPNAs modifies the recognition specificity of the restriction enzyme and converts it into a rare-cleaving site-specific DNA nickase.
Thus obtained 'artificial' nickase exhibits an ∼20-bp apparent recognition specificity cleaving dsDNA at a site with degenerated recognition sequence. The efficiency of nicking activity strongly depends on the location of the degenerate site with respect to the pcPNA-binding site displaying an explicit phasing effect with the periodicity of the DNA double helix. Given that a number of the DNA nicking-based biotechnological, diagnostic and molecular-biological assays, including site-directed mutagenesis (Shortle et al., 1982; Wang and Hays, 2001), strand-displacement amplification (Walker et al., 1992) and DNA labeling (Li et al., 2002), can significantly benefit from employment of DNA nickases targeted at unique predetermined sites, and that such enzymes are essentially absent at present (Roberts and Macelis, 2001), we expect our results to find practical applications.
Dimerization of many type IIs restriction enzymes bound to specific sequences is thought to be required for the activation of DNA cleavage (Bitinaite et al., 1998; Besnier and Kong, 2001; Higgins et al., 2001; Vanamee et al., 2001; Bath et al., 2002). We therefore assume that the pcPNA-generated flexible bend (or hinge) within dsDNA, which is clearly seen in atomic force microscopy (AFM) images, allows the long-range interaction between a pair of enzymes bound to their recognition sites on dsDNA: one with the correct recognition sequence; another one is a pseudosite with a degenerated recognition sequence. As a result, a single-strand break is introduced at a degenerate PleI recognition site. Consequently, pcPNA represents the first example of a sequence-unrestricted DNA-looping ligand, which modifies the activity of DNA-bound proteins over a distance.
Results and Discussion
In the absence of pcPNAs, PleI introduces a double-strand break downstream of the 5′-GAGTC site (Higgins et al., 2001) (Figure 1A). As expected from previous studies (Izvolsky et al., 2000; Winters, 2000; Casey and Glazer, 2001; Nielsen, 2001; Pooga et al., 2001; Braasch and Corey, 2002; Demidov, 2002), binding of a pair of pcPNAs in the close proximity (3–5 bp) downstream of PleI recognition site prevents cleavage at the correct site (Figure 1B). Surprisingly, a novel sequencespecific activity of the PleI enzyme is observed in this case at a remote site with degenerated recognition sequence. Plasmid DNA constructs designed for our study contain the PleI recognition sequence 5′-GAGTC and a binding site for a pcPNAs pair Figure 1A. These constructs also contain the pseudosite with the degenerated PleI recognition sequence, 5′-TAATC, near the site of the sequence-specific PNA-induced activity of PleI. [We have fortuitously found this site while studying the site-directed interference of pcPNA–dsDNA complexes with the DNA-cleaving/nicking activity of type IIs restriction enzymes (manuscript in preparation)]. According to our notation, pDN-M stands for a construct with the distance between the PleI recognition site and the PNA-binding site of N bp and the distance between the PNA site and the 5′-end of the pseudo-site of M bp. The remote site is located either on the forward (top) or the reverse (bottom) DNA strand, denoted F or R, respectively.
Figure 1.
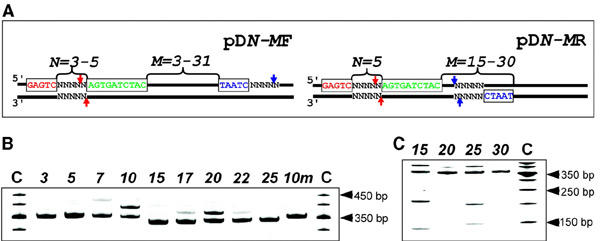
The effect of PNA binding on the activity of PleI. (A) DNA constructs are pUC19 derivatives with 31–52 bp inserts cloned into BamHI–HindIII sites. All plasmids contain a PleI recognition site (red), a site with the degenerate recognition sequence 5′-TAATC (blue), and a binding site for the pcPNA pair carrying 2,6-diaminopurine (D) and 2-thioracil (sU) with the sequences HLys-GsUDGDsUCDCsU-LysNH2 and HLys-DGsUGDsUCsUDC-LysNH2 (green). DNA constructs differ in the position of these sites with respect to each other (for notation see text). In the absence of pcPNAs, PleI cleaves dsDNA downstream of its recognition sequence as shown by red arrows. We observe a novel pcPNA-induced PleI activity indicated by blue arrows. PNA-induced activity of PleI for pD5-MF (B) and pD5-MR (C) constructs as resolved by gel-shift assay. Numbers at the top of the gel correspond to the distance in base pairs between the PNA-binding site and 5′-end of the degenerate recognition sequence 5′-TAATC. The 10m presents pD5-10F carrying a single mismatch 5′-GTGTC (mismatch is underlined) in the PleI recognition site. Lane C corresponds to molecular weight standards.
When the pDN-MF series of plasmids are targeted with pcPNAs and subjected to the PleI digestion, gel electrophoretic analysis reveals the accumulation of a product with a slower mobility than the original intact fragment (Figure 1B). This retardation effect is indicative of a structural perturbation, namely a nick (Kuhn et al., 2002), introduced within DNA fragments by the altered, pcPNA-induced Ple I activity. The sequence mapping proves that the (PNA + enzyme)-treated DNA fragments are sequencespecifically singly nicked on the forward strand (Figure 2). The exact location of the singlestrand break was determined to be 4 bp downstream from the 5′-TAATC site. The nicking efficiency clearly depends on the location of the degenerate DNA site with respect to the PNA-binding site. Plotting the yield of nicked DNA fragments against the distance between two sites reveals clear spatial periodicity of the nicking activity with peaks at 10 and 20 bp and troughs at 15 and 25 bp (Figure 3A).
Figure 2.
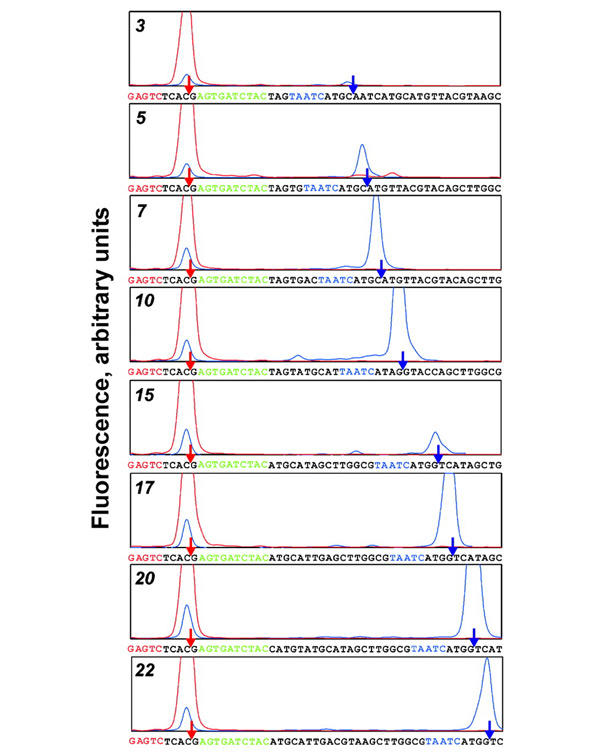
Sequence mapping of the PNA-induced PleI-generated nicks. PCR-derived DNA fragments carrying a Cy5 label on the 5′-end of the forward strand were obtained using the pD5-MF-type plasmids as templates (M is indicated on the left side of the graph). Denaturing gel electrophoresis traces of the samples digested by PleI in the presence of PNA (blue) are compared with the PleI cleavage pattern without PNA (red) for each DNA construct. The exact position of the break (indicated by the arrows) is determined by alignment with the sequencing ladders. The color scheme is the same as used in Figure 1A.
Figure 3.
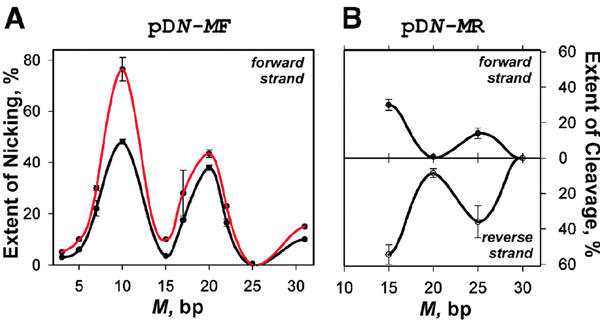
PNA-mediated phased activity of PleI. Extent of the forward strand nicking for the pD5-MF set (A) or the forward (upper panel; nicking only) and reverse (lower panel; nicking and cutting) strands cleavage for pD5-MR set (B) is plotted against the distance between the PNA-binding site and the 5′-end of the degenerate recognition sequence 5′-TAATC. The extent of the reaction was assessed following 40 min (black) and 80 min (red) incubation with PleI using the protocol described in Methods. Bar deviations in graphs correspond to the standard errors.
The modified, PNA-induced activity of the PleI enzyme is also observed when a degenerate site is positioned on the reverse strand (the pDN-MR series of plasmids). But now the gel electrophoretic pattern reveals that in addition to a major nicked product (slower migrating band), two DNA fragments (faster migrating bands) are also generated (Figure 1C). We identified these products as a fragment with a nick introduced on the reverse strand 4 bp downstream from the pseudosite and two fragments originated from a double-strand cleavage near the degenerate site. Note that in the case of the two-site substrates, the cleavage efficiency of type IIs restriction enzymes may depend on the relative orientation of their binding/recognition sites (Bath et al., 2002). Again, the PNA-modulated activity of PleI depends on the position of the degenerate site with respect to the PNA site in a periodic manner with maxima (and minima) separated by 10 bp for both strands (Figure 3B).
Introduction of a single mismatch in the correct recognition site of PleI in pD5-10F (site 5′-GTGTC; mismatch is underlined), impairs completely the PNA-induced nicking activity (Figure 1B, lane 10m). In addition, we assayed the effect of sequence of the degenerate site on the PNA-induced PleI nicking activity by substituting 5′-TAATC in pD5-9F with similar sequences. Table 1 shows that the PNA-induced activity depends on the degenerated sequence as follows: 5′-TAATC=AAGTC > TAGTC >> GAGTT and is completely lost for 5′-TAACT and 5′-ACTGA. Evidently, the degenerate sequence also affects the efficiency of cleavage of the reverse strand leading to doublestrand breaks for 5′-AAGTC and 5′-TAGTC.
Table 1.
PNA-induced activity of PleI depends on the sequence of pseudo-sitea
| Degenerate sequence | Fraction (%) of DNA fragmentsb |
||
| Intact |
Nickedc |
Cutd |
|
| AAGTC | 14 | 33 | 53 |
| TAATC | 17 | 83 | − |
| TAGTC | 34 | 48 | 18 |
| GAGTT | 76 | 24 | − |
| TAACT | 100 | − | − |
| ACTGA | 100 | − | − |
aDNA constructs were obtained by substituting pseudosite in pD5-9F with sequences similar to 5′-GAGTC (correct PleI recognition sequence). Changes are underlined.
bStandard error ≤7% (in triplicate experiments).
cNick is located 4 nt downstream of degenerate sequence on the forward strand.
dBoth strands are cleaved at the degenerate site.
Thus, both protein recognition sites are required for induction of a new activity of the restriction enzyme. To explain our results we assumed that the presence of pcPNAs close to the recognition site of the PleI enzyme allowed the stable binding of the restriction enzyme to dsDNA; however, cleavage at the correct DNA site was impaired. Phasing of the PNA-induced modulation of PleI activity with the distance along DNA substrate could be explained by the general (for many type IIs restriction enzymes) requirement to form a dimer of enzymes for activation of DNA cleavage, as had been postulated previously (Bitinaite et al., 1998; Besnier and Kong, 2001; Higgins et al., 2001; Vanamee et al., 2001). Such dimerization can be facilitated by the pcPNA-induced pliant bending of dsDNA, which is clearly seen in AFM images (Figure 4A). In the presence of PNAs, a significant fraction of dsDNA molecules with sharp bends of various angles is observed (Figure 4B). Consistently with high rigidity of dsDNA (Hagerman, 1988; Rees et al., 1993), the 345-bp DNA fragments we studied do not show any kinks without PNA.
Figure 4.
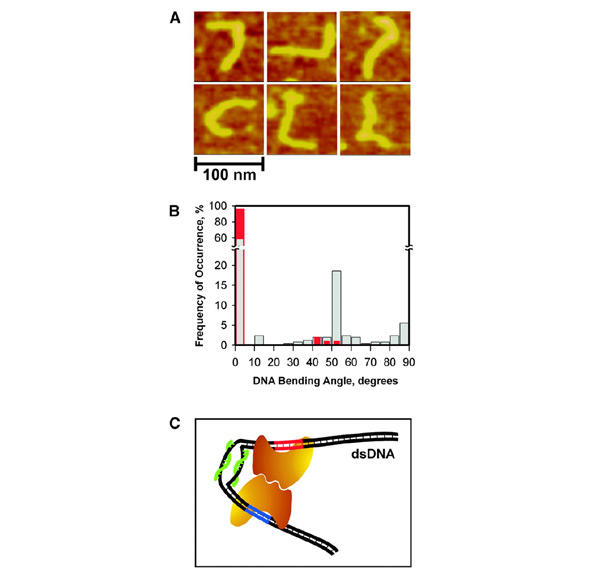
The PNA-induced dsDNA bending. (A) AFM images of 345-bp dsDNA fragments (pD5-10F) complexed with pcPNAs 125 bp apart from DNA terminus (the shortest arm). The double-duplex invasion of pcPNAs (Lohse et al., 1999; Izvolsky et al., 2000; Demidov et al., 2002) produces a hinge-like distortion within the DNA duplex seen here as sharp bends of various angles. (B) Histograms of the DNA bending angles measured from AFM images of PNA–DNA complexes (grey bars) and control with 'naked' DNA (red bars). (C) Model for the PNA-assisted dimerization of two enzyme molecules (the bean-like shapes), one bound to its primary recognition site on dsDNA (red) and the other to the site with degenerate recognition sequence (blue). This cartoon schematically illustrates our concept of the directed, smallsize (∼30–60 bp) DNA looping based on the local dynamic bending of dsDNA due to the double-duplex invasion of a pcPNA pair (green).
Indeed, the formation of a flexible bend (i.e. a hinge; presumably with torsion constraints) within the otherwise stiff, rod-like DNA duplex permits, via DNA looping, the long-range interaction/dimerization of two enzymes bound to their adjacent target sites, as shown schematically in Figure 4C. One of the enzyme molecules binds strongly to the correct recognition site forming a long-lived complex: although the PleI enzymatic activity is impaired by neighboring pcPNAs, binding of the protein is not affected. The other enzyme molecule binds transiently to a slightly sequence-degenerated site, 5′-TAATC or alike, located in close proximity (≥30 bp) in space. A protein dimer formed in such a way is capable of inducing the single- or doublestrand breaks at a degenerate site. Because of torsion constraints in the DNA hinge and insufficient structural flexibility of protein–DNA complexes, the proper interaction of proteins is only possible when two corresponding molecules are correctly arranged on the double helix to align their dimerization interfaces. This requirement manifests itself in nearly 10-bp phasing of the enzyme cleavage activity reflecting the dsDNA helical periodicity (Lee and Schleif, 1989; Law et al., 1993; Müller et al., 1996).
To conclude, we have proved for the first time that pcPNAs can serve as a tool for tailoring the activity of DNA-binding proteins by the regulated DNA looping. Since pcPNAs are sequence-unrestricted dsDNA binders (Lohse et al., 1999; Izvolsky et al., 2000; Demidov et al., 2002), we expect them to induce DNA looping at any designated site. Using this approach, we quantitatively conferred a unique super-rare (∼20-bp specific) nicking activity on the common DNA frequent cutter, the 5-bp specific restriction enzyme Ple I. Although several strategies have been developed for converting common restriction endonucleases into nicking enzymes by either modifying the proteins themselves (Stahl et al., 1996; Besnier and Kong, 2001; Xu et al., 2001) or using chemical modifications of their recognition sites (Walker et al., 1992), none of these approaches has provided super-rare DNA nickases. Note that the previously designed artificial DNA-bending ligands are based on the triplex recognition principle limited to oligopurine sequences (and alike) only (Liberles and Dervan, 1996). Therefore, the DNA-looping ability of PNA oligomers opens totally new opportunities for regulation of gene activity by modifying the action of DNA-binding proteins, whose activity is affected by interaction with other proteins.
Methods
Enzymatic analysis.
The PvuII digest of target plasmids yielding the ∼350-bp DNA fragments with the PNA-binding site was incubated in the presence of 5 μM pcPNAs 1495/1496 (Lohse et al., 1999) at 55°C for 2 h. Unbound PNA was removed by gel filtration through Sephadex G-50. The sample then was subjected to Ple I (New England Biolabs) digestion for 40 min at 37°C followed by 20 min at 65°C to inactivate the enzyme and facilitate dissociation of pcPNAs. The products were resolved on 8% polyacrylamide gels containing 7 M urea to enhance the retardation of the nicked fragment. Quantitative data were obtained in triplicate experiments.
The exact location of a nick was determined by sequence mapping. Fluorescently labeled DNA fragments (∼160 bp) were generated by PCR with the 5′-Cy5-tagged forward (or reverse) primers using plasmids from the pD5-MF and pD5-MR sets as templates. Upon binding of pcPNAs, PleI restriction assays were performed as described above. The samples were resolved, along with the sequencing ladders, using Alf Express DNA Sequencer (Pharmacia Biotech).
AFM analysis.
An isolated 345-bp PvuII–PvuII fragment of pD5-10F or its complex with pcPNAs in the Mg++-containing buffer (Soldatenkov et al., 2002) were deposited on an anatomically flat mica surface, allowed to adsorb for 1 min, rinsed with deionized water and dried in a gentle nitrogen flow. The AFM images were obtained using a NanoScope IIIa device equipped with Escanner (Digital Instruments) operating in tapping mode in air. The tapping frequency of the 125 μm silicon cantilever was 300–400 kHz and the nominal scanning rate was set at 1–2 Hz.
The contour length of DNA molecules and bending angles were measured and quantitatively characterized using the NanoScope software. Two hundred and fifty-two PNA–DNA complexes and 100 'naked' DNA molecules were analyzed for DNA bending. In the case of PNA–DNA complexes, bending angles were determined by drawing straight lines through the axes of DNA molecules on both sides of the PNA intrusion (seen as a bump/protrusion during AFM scanning) and measuring the angle (Φ) at their intersection, as described by Rees et al. (1993). The DNA bending angle (θ) was defined as θ=180° – Φ.
In the control experiment with 'naked' DNA molecules when the PNA-binding site does not manifest itself in the AFM images, the bending angles were measured at ∼40 nm from both ends of DNA fragments. This position roughly corresponds to the PNA-binding site. A mask imitating the PNA–DNA invasion complex with the average apparent dimensions of the PNA intrusion was placed at these DNA locations and the bending angles were measured similar to the case with PNA–DNA complexes. The larger of two angular values thus determined for each DNA were taken for analysis.
Acknowledgments
We thank P.E. Nielsen for supplying pcPNAs, A. Dritschilo for providing the access to AFM facilities, and I.V. Smolina and C.J. Corcoran for technical assistance. This work was supported by the Human Frontier Science Program fellowship (E.P.), the Boston University Provost's Innovation Fund and SPRInG awards (V.V.D.), the US Army Medical Research and Development Command (V.S.), and the NIH General Medicine (M.D.F-.K.) and Cancer (V.S. and M.D.F-.K.) Divisions.
References
- Bath A.J., Milsom S.E., Gormley N.A. and Halford S.E. (2002) Many type IIs restriction endonucleases interact with two recognition sites before cleaving DNA. J. Biol. Chem., 277, 4024–4033. [DOI] [PubMed] [Google Scholar]
- Besnier C.E. and Kong H. (2001) Converting MlyI endonucleases into a nicking enzyme by changing its oligomerization state. EMBO rep., 2, 782–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitinaite J., Wah D.A., Aggarwal A.K. and Schildkraut I. (1998) FokI dimerization is required for DNA cleavage. Proc. Natl Acad. Sci. USA, 95, 10570–10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braasch D.A. and Corey D.R. (2002) Novel antisense and peptide nucleic acid strategies for controlling gene expression. Biochemistry, 41, 4504–4510. [DOI] [PubMed] [Google Scholar]
- Casey B.P. and Glazer P.M. (2001) Gene targeting via triple-helix formation. Prog. Nucleic Acid Res. Mol. Biol., 67, 163–192. [DOI] [PubMed] [Google Scholar]
- Cherny D.I., Striker G., Subramaniam V., Jett S.D., Palecek E. and Jovin T.M. (1999) DNA bending due to specific p53 and p53 core domain–DNA interactions visualized by electron microscopy. J. Mol. Biol., 294, 1015–1026. [DOI] [PubMed] [Google Scholar]
- DeSanctis V., LaTerra S., Bianchi A., Shore D., Burderi L., DiMauro E. and Negri R. (2002) In vivo topography of Rap1p–DNA complex at Saccharomyces cerevisiae TEF2 UASRPG during transcriptional regulation. J. Mol. Biol., 318, 333–349. [DOI] [PubMed] [Google Scholar]
- Demidov V.V. (2002) PNA comes of age: from infancy to maturity. Drug Discov. Today, 7, 153–155. [DOI] [PubMed] [Google Scholar]
- Demidov V.V., Protozanova E., Izvolsky K.I., Price C., Nielsen P.E. and Frank-Kamenetskii M.D. (2002) Kinetics and mechanism of the DNA double helix invasion by pseudocomplementary PNAs. Proc. Natl Acad. Sci. USA, 99, 15953–15958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedhoff P., Lurz R., Lüder G. and Pingoud A. (2001) Sau3AI, a monomeric type II restriction endonuclease that dimerizes on the DNA and thereby induces DNA loops. J. Biol. Chem., 276, 23581–23588. [DOI] [PubMed] [Google Scholar]
- Gormley N.A., Hillberg A.L. and Halford S.E. (2002) The type IIs restriction endonuclease BspMI is a tetramer that acts concertedly at two copies of an asymmetric DNA sequence. J. Biol. Chem., 277, 4034–4041. [DOI] [PubMed] [Google Scholar]
- Hagerman P.J. (1988) Flexibility of DNA. Annu. Rev. Biophys. Biophys. Chem., 17, 265–286. [DOI] [PubMed] [Google Scholar]
- Halford S.E. (2001) Hopping, jumping and looping by restriction enzymes. Biochem. Soc. Trans., 29, 363–374. [DOI] [PubMed] [Google Scholar]
- Higgins L.S., Besnier C. and Kong H. (2001) The nicking endonuclease N.BstNBI is closely related to Type IIs restriction endonucleases MlyI and PleI. Nucleic Acids Res., 29, 2492–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izvolsky K.I., Demidov V.V., Nielsen P.E. and Frank-Kamenetskii M.D. (2000) Sequencespecific protection of duplex DNA against restriction and methylation enzymes by pseudocomplementary PNAs. Biochemistry, 39, 10908–10913. [DOI] [PubMed] [Google Scholar]
- Kahn J.D. and Crothers D.M. (1993) DNA bending in transcription initiation. Cold Spring Harbor Symp. Quant. Biol., 58, 115–122. [DOI] [PubMed] [Google Scholar]
- Kuhn H., Protozanova E. and Demidov V.V. (2002) Monitoring of single nicks in duplex DNA by gel electrophoretic mobilityshift assay. Electrophoresis, 23, 2384–2387. [DOI] [PubMed] [Google Scholar]
- Law S.M., Bellomy G.R., Schlax P.J. and Record M.T. Jr (1993) In vivo thermodynamic analysis of repression with and without looping in lac constructs. Estimates of free and local lac repressor concentrations and of physical properties of a region of supercoiled plasmid DNA in vivo. J. Mol. Biol., 230, 161–173. [DOI] [PubMed] [Google Scholar]
- Lee D.H. and Schleif R.F. (1989) In vivo DNA loops in araCBAD: size limits and helical repeat. Proc. Natl Acad. Sci. USA, 86, 476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Hatfield S., Li J., McMills M., Zhao Y. and Chen X. (2002) Seryl-histidine as an alternative DNA nicking agent in nick translation yields superior DNA probes and hybridizations. Bioorg. Med. Chem., 10, 667–673. [DOI] [PubMed] [Google Scholar]
- Liberles D.A. and Dervan P.B. (1996) Design of artificial sequencespecific DNA bending ligands. Proc. Natl Acad. Sci. USA, 93, 9510–9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse J., Dahl O. and Nielsen P.E. (1999) Double duplex invasion by peptide nucleic acid: a general principle for sequencespecific targeting of double-stranded DNA. Proc. Natl Acad. Sci. USA, 96, 11804–11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J., Oehler S. and Müller-Hill B. (1996) Repression of lac promoter as a function of distance, phase and quality of an auxiliary lac operator. J. Mol. Biol., 257, 21–29. [DOI] [PubMed] [Google Scholar]
- Nielsen P.E. (2001) Peptide nucleic acid targeting of doublestranded DNA. Methods Enzymol., 340, 329–340. [DOI] [PubMed] [Google Scholar]
- Pooga M., Land T., Bartfai T. and Langel Ü. (2001) PNA oligomers as tools for specific modulation of gene expression. Biomol. Eng., 17, 183–192. [DOI] [PubMed] [Google Scholar]
- Ptashne M. and Gann A. (2002) Genes and Signals. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Rees W.A., Keller R.W., Vesenka J.P., Yang G. and Bustamante C. (1993) Evidence of DNA bending in transcription complexes imaged by scanning force microscopy. Science, 260, 1646–1649. [DOI] [PubMed] [Google Scholar]
- Rippe K., Guthold M., vonHippel P.H. and Bustamante C. (1997) Transcriptional activation via DNA-looping: visualization of intermediates in the activation pathway of E. coli RNA polymerase.σ54 holoenzyme by scanning force microscopy. J. Mol. Biol., 270, 125–138. [DOI] [PubMed] [Google Scholar]
- Roberts R.J. and Macelis D. (2001) REBASE—restriction enzymes and methylases. Nucleic Acids Res., 29, 268–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleif R. (2000) Regulation of the L-arabinose operon of Escherichia coli. Trends Genet., 16, 559–565. [DOI] [PubMed] [Google Scholar]
- Shortle D., Grisafi P., Benkovic S.J. and Botstein D. (1982) Gap misrepair mutagenesis: efficient site-directed induction of transition, transversion, and frameshift mutations in vitro. Proc. Natl Acad. Sci. USA, 79, 1588–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden R.R. (1994) DNA Structure and Function. Academic Press, San Diego, CA. [Google Scholar]
- Soldatenkov V.A., Chasovskikh S., Potaman V.N., Trofimova I., Smulson M.E. and Dritschilo A. (2002) Transcriptional repression by binding of poly(ADP-ribose) polymerase to promoter sequences. J. Biol. Chem., 277, 665–670. [DOI] [PubMed] [Google Scholar]
- Stahl F., Wende W., Jeltsch A. and Pingoud A. (1996) Introduction of asymmetry in the naturally symmetric restriction endonuclease EcoRV to investigate intersubunit communication in the homodimeric protein. Proc. Natl Acad. Sci. USA, 93, 6175–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanamee É.S., Santagata S. and Aggarwal A.K. (2001) FokI requires two specific DNA sites for cleavage. J. Mol. Biol., 309, 69–78. [DOI] [PubMed] [Google Scholar]
- Walker G.T., Little M.C., Nadeau J.G. and Shank D.D. (1992) Isothermal in vitro amplification of DNA by a restriction enzyme/DNA polymerase system. Proc. Natl Acad. Sci. USA, 89, 392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. and Hays J.B. (2001) Simple and rapid preparation of gapped plasmid DNA for incorporation of oligomers containing specific DNA lesions. Mol. Biotechnol., 19, 133–140. [DOI] [PubMed] [Google Scholar]
- Winters T.A. (2000) Gene targeted agents: new opportunities for rational drug development. Curr. Opin. Mol. Ther., 2, 670–681. [PubMed] [Google Scholar]
- Xu Y., Lunnen K.D. and Kong H. (2001) Engineering a nicking endonuclease N.AlwI by domain swapping. Proc. Natl Acad. Sci. USA, 98, 12990–12995. [DOI] [PMC free article] [PubMed] [Google Scholar]


