Abstract
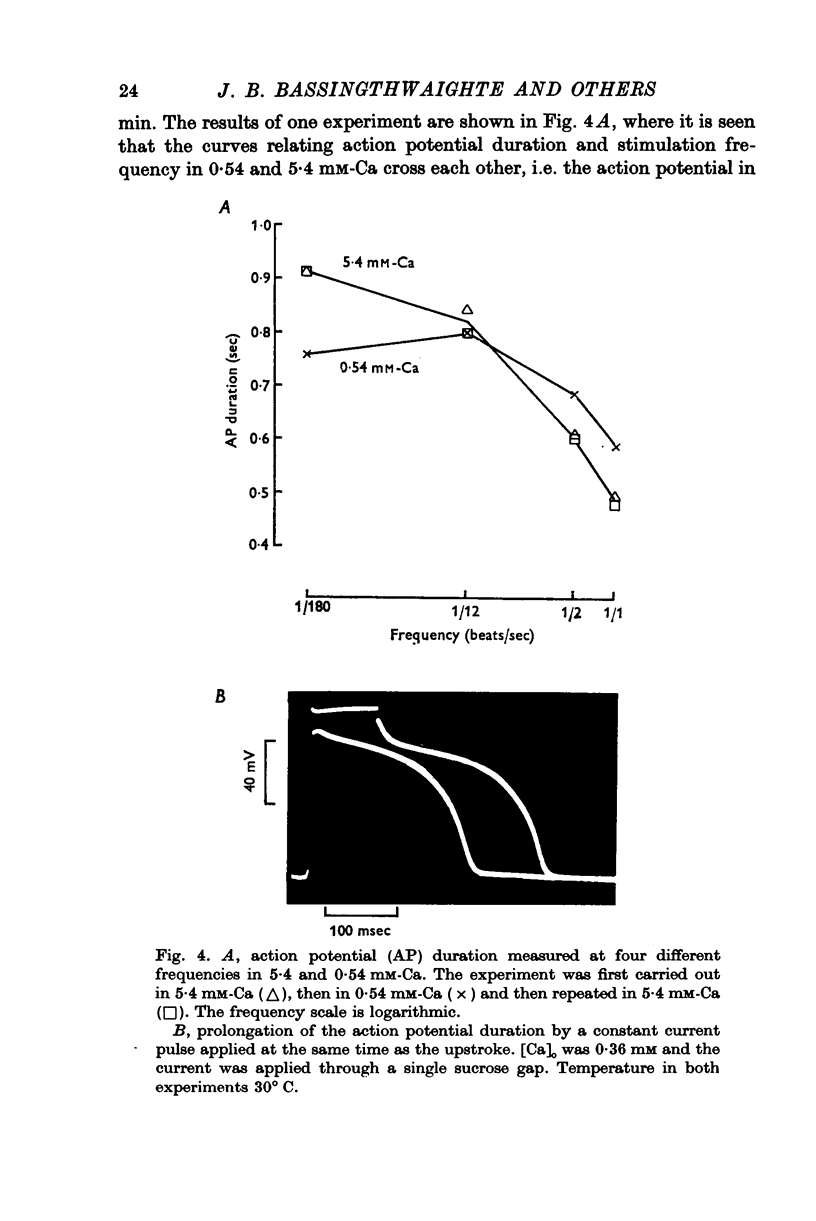
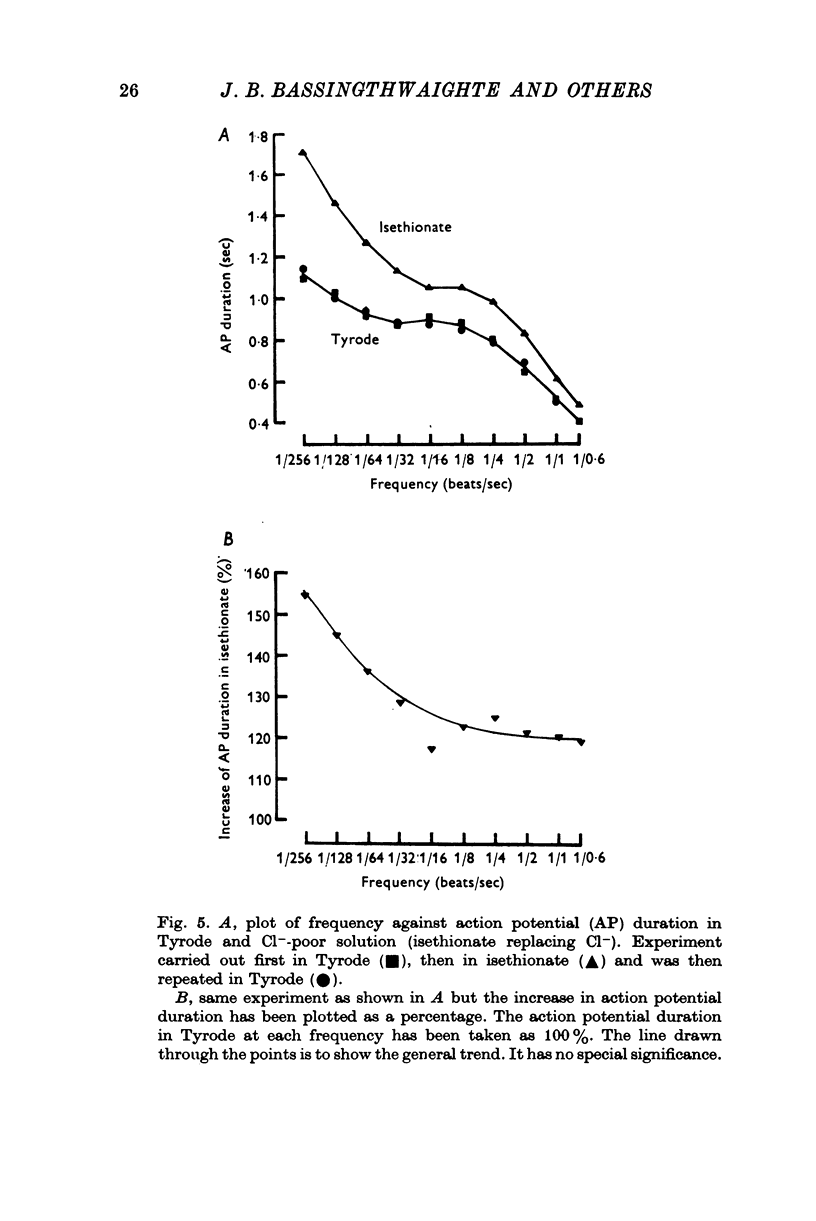
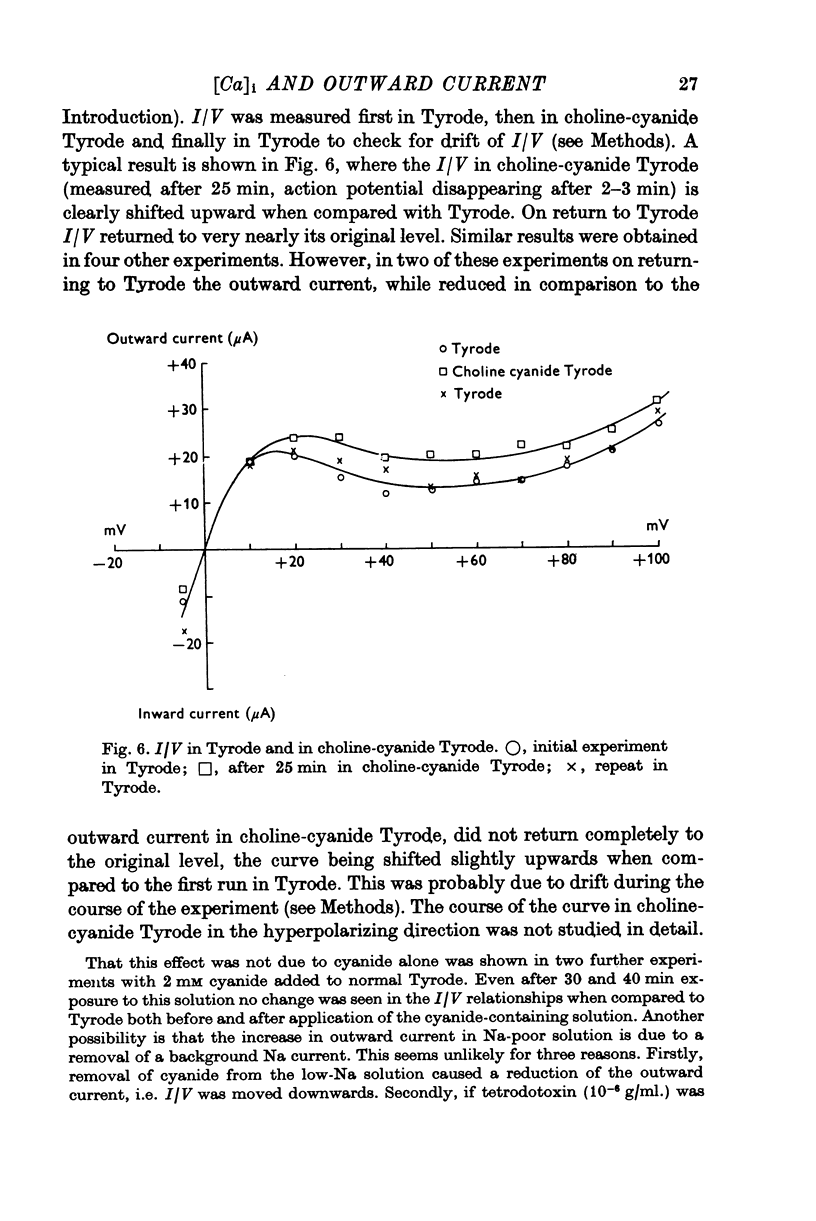
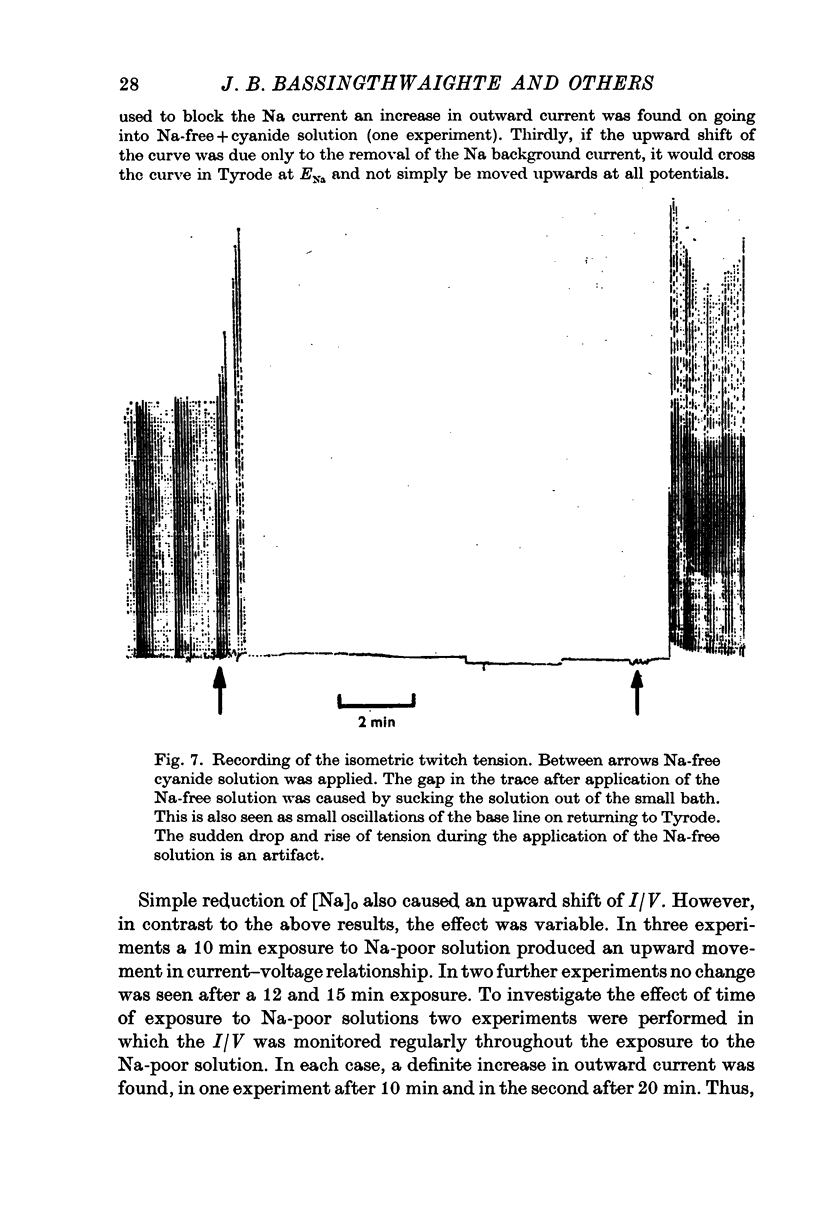
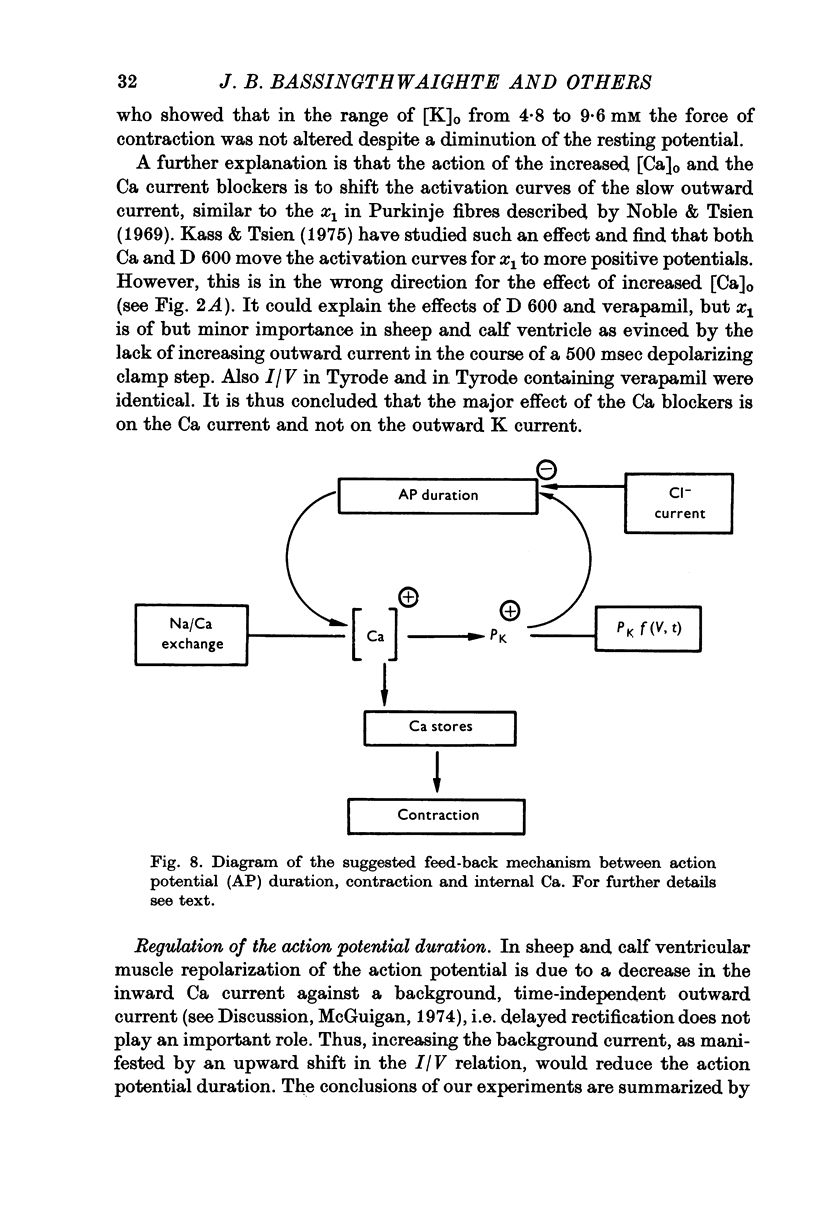
In sheep and calf ventricular bundles, increasing the internal calcium by increasing the frequency of voltage-clamping to plateau range potentials increased the time-independent outward current. This effect was more marked with higher [Ca]o, and was reduced if the Ca current blockers Verapamil or D 600 were used. 2. If the internal Ca was increased by the addition of cyanide and reduction of external sodium the outward current was also increased. The frequency-dependent increase in outward current also occurred in this Na-poor (12 mM) solution. 3. Tension measurement on the ventricular bundles showed that a Na-free solution with cyanide did not cause a contracture. On changing from Tyrode to a Na-free solution containing cyanide, and on changing back to Tyrode there was a potentiation of the twitch. 4. In Na-poor solution with cyanide, although no contracture was found, ECa was less positive, suggesting that under these circumstances Ca accumulates at the inner side of the membrane, but not around the myofibrils. 5. The prolongation of the action potential in Cl-free solution is frequency-dependent. A greater prolongation is seen at lower frequencies suggesting that Cl current is relatively more important for repolarization at lower frequencies of stimulation. 6. It is suggested that calcium at the inner side of the membrane sets the level of the background outward current. A feed-back mechanism on this basis is proposed for the control of the action potential duration. Various factors that could influence this basic mechanism are discussed.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. R., Foulks J. G. Effects of anions on frog ventricle. Can J Physiol Pharmacol. 1973 Oct;51(10):709–726. doi: 10.1139/y73-108. [DOI] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. Membrane calcium current in ventricular myocardial fibres. J Physiol. 1970 Mar;207(1):191–209. doi: 10.1113/jphysiol.1970.sp009056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begenisich T., Lynch C. Effects of internal divalent cations on voltage-clamped squid axons. J Gen Physiol. 1974 Jun;63(6):675–689. doi: 10.1085/jgp.63.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRANEFIELD P. F., HOFFMAN B. F. Propagated repolarization in heart muscle. J Gen Physiol. 1958 Mar 20;41(4):633–649. doi: 10.1085/jgp.41.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Daut J., Noble D. Proceedings: The influence of extracellular potassium ions on the action of ouabain on membrane currents in sheep Purkinje fibres. J Physiol. 1975 Jul;249(1):42P–43P. [PubMed] [Google Scholar]
- DUDEL J., TRAUTWEIN W. Elektrophysiologiche Messungen zur Strophanthinwirkung am Herzmuskel. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1958;232(2):393–407. [PubMed] [Google Scholar]
- De Mello W. C. Effect of intracellular injection of calcium and strontium on cell communication in heart. J Physiol. 1975 Sep;250(2):231–245. doi: 10.1113/jphysiol.1975.sp011051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Trautwein W. Temperature sensitivity of outward current in cardiac Purkinje fibers. Evidence of electrogenicity of active transport. Pflugers Arch. 1975 Jul 28;358(3):225–234. doi: 10.1007/BF00587219. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Trautwein W. The effect of dihydro-ouabain and lithium-ions on the outward current in cardiac Purkinje fibers. Evidence for electrogenicity of active transport. Pflugers Arch. 1974;350(1):41–54. doi: 10.1007/BF00586737. [DOI] [PubMed] [Google Scholar]
- Isnberg G. Is potassium conductance of cardiac Purkinje fibres controlled by (Ca2+)? Nature. 1975 Jan 24;253(5489):273–274. doi: 10.1038/253273a0. [DOI] [PubMed] [Google Scholar]
- Jundt H., Porzig H., Reuter H., Stucki J. W. The effect of substances releasing intracellular calcium ions on sodium-dependent calcium efflux from guinea-pig auricles. J Physiol. 1975 Mar;246(1):229–253. doi: 10.1113/jphysiol.1975.sp010888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASSEBAUM D. G. Electrophysiological effects of strophanthin in the heart. J Pharmacol Exp Ther. 1963 Jun;140:329–338. [PubMed] [Google Scholar]
- Kass R. S., Tsien R. W. Control of action potential duration by calcium ions in cardiac Purkinje fibers. J Gen Physiol. 1976 May;67(5):599–617. doi: 10.1085/jgp.67.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Tsien R. W. Multiple effects of calcium antagonists on plateau currents in cardiac Purkinje fibers. J Gen Physiol. 1975 Aug;66(2):169–192. doi: 10.1085/jgp.66.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhardt M., Bauer B., Krause H., Fleckenstein A. New selective inhibitors of the transmembrane Ca conductivity in mammalian myocardial fibres. Studies with the voltage clamp technique. Experientia. 1972 Mar 15;28(3):288–289. doi: 10.1007/BF01928693. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Krause H., Kübler M., Herdey A. Kinetics of inactivation and recovery of the slow inward current in the mammalian ventricular myocardium. Pflugers Arch. 1975 Mar 22;355(1):1–17. doi: 10.1007/BF00584795. [DOI] [PubMed] [Google Scholar]
- LANGER G. A., BRADY A. J. Calcium flux in the mammalian ventricular myocardium. J Gen Physiol. 1963 Mar;46:703–719. doi: 10.1085/jgp.46.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGER G. A. CALCIUM EXCHANGE IN DOG VENTRICULAR MUSCLE: RELATION TO FREQUENCY OF CONTRACTION AND MAINTENANCE OF CONTRACTILITY. Circ Res. 1965 Jul;17:78–89. doi: 10.1161/01.res.17.1.78. [DOI] [PubMed] [Google Scholar]
- Ladle R. O., Walker J. L. Intracellular chloride activity in frog heart. J Physiol. 1975 Oct;251(2):549–559. doi: 10.1113/jphysiol.1975.sp011107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer G. A. Heart: excitation-contraction coupling. Annu Rev Physiol. 1973;35:55–86. doi: 10.1146/annurev.ph.35.030173.000415. [DOI] [PubMed] [Google Scholar]
- Langer G. A. Ion fluxes in cardiac excitation and contraction and their relation to myocardial contractility. Physiol Rev. 1968 Oct;48(4):708–757. doi: 10.1152/physrev.1968.48.4.708. [DOI] [PubMed] [Google Scholar]
- Lederer W. J., Tsien R. W. Proceedings: Transient inward current underlying strophanthidin's enhancement of pace-maker activity in Purkinje fibres. J Physiol. 1975 Jul;249(1):40P–41P. [PubMed] [Google Scholar]
- Lew V. L. Effect of intracellular calcium on the potassium permeability of human red cells. J Physiol. 1970 Feb;206(2):35P–36P. [PubMed] [Google Scholar]
- McGuigan J. A. Some limitations of the double sucrose gap, and its use in a study of the slow outward current in mammalian ventricular muscle. J Physiol. 1974 Aug;240(3):775–806. doi: 10.1113/jphysiol.1974.sp010634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. The sensitivity of Helix aspersa neurones to injected calcium ions. J Physiol. 1974 Mar;237(2):259–277. doi: 10.1113/jphysiol.1974.sp010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERGERKE R. The staircase phenomenon and the action of calcium on the heart. J Physiol. 1956 Dec 28;134(3):569–583. doi: 10.1113/jphysiol.1956.sp005666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New W., Trautwein W. The ionic nature of slow inward current and its relation to contraction. Pflugers Arch. 1972;334(1):24–38. doi: 10.1007/BF00585998. [DOI] [PubMed] [Google Scholar]
- Niedergerke R., Orkand R. K. The dependence of the action potential of the frog's heart on the external and intracellular sodium concentration. J Physiol. 1966 May;184(2):312–334. doi: 10.1113/jphysiol.1966.sp007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. Outward membrane currents activated in the plateau range of potentials in cardiac Purkinje fibres. J Physiol. 1969 Jan;200(1):205–231. doi: 10.1113/jphysiol.1969.sp008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porzig H. Comparative study of the effects of propranolol and tetracaine on cation movements in resealed human red cell ghosts. J Physiol. 1975 Jul;249(1):27–49. doi: 10.1113/jphysiol.1975.sp011001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K. Influence of energy supply and calcium on the low sodium induced changes in the transmembrane potential and contraction of guinea pig papillary muscle. Can J Physiol Pharmacol. 1970 Apr;48(4):241–253. doi: 10.1139/y70-042. [DOI] [PubMed] [Google Scholar]
- Reiter M., Seibel K., Stickel F. J. Sodium dependence of the inotropic effect of a reduction in extracellular potassium concentration. Naunyn Schmiedebergs Arch Pharmakol. 1971;268(4):361–378. doi: 10.1007/BF00997062. [DOI] [PubMed] [Google Scholar]
- Reiter M., Stickel F. J. Der Einfluss der Kontraktionsfrequenz auf das Aktionspotential des Meerschweinchem-Papillarmuskels. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968;260(4):342–365. [PubMed] [Google Scholar]
- Reuter H. Divalent cations as charge carriers in excitable membranes. Prog Biophys Mol Biol. 1973;26:1–43. doi: 10.1016/0079-6107(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. Time- and voltage-dependent contractile responses in mammalian cardiac muscle. Eur J Cardiol. 1973 Dec;1(2):177–181. [PubMed] [Google Scholar]
- Romero P. J., Whittam R. The control by internal calcium of membrane permeability to sodium and potassium. J Physiol. 1971 May;214(3):481–507. doi: 10.1113/jphysiol.1971.sp009445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. Permeability of cell junction depends on local cytoplasmic calcium activity. Nature. 1975 Mar 20;254(5497):250–252. doi: 10.1038/254250a0. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y. Membrane characteristics of the canine papillary muscle fiber. J Gen Physiol. 1969 Dec;54(6):765–781. doi: 10.1085/jgp.54.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz H. Uber Unterschiede im Kontrakturverhalten bei Ventrikel- und Vorhofspräparaten aus Warmblüterherzen. Pflugers Arch. 1969;312(3):63–81. doi: 10.1007/BF00588532. [DOI] [PubMed] [Google Scholar]
- Simons T. J. Resealed ghosts used to study the effect of intracellular calcium ions on the potassium permeability of human red cell membranes. J Physiol. 1975 Mar;246(2):52P–54P. [PubMed] [Google Scholar]
- Sumbera J. Induced changes of action potential on cardiac contraction. Experientia. 1970;26(7):738–739. doi: 10.1007/BF02232516. [DOI] [PubMed] [Google Scholar]
- Tasaki I., Watanabe A., Lerman L. Role of divalent cations in excitation of squid giant axons. Am J Physiol. 1967 Dec;213(6):1465–1474. doi: 10.1152/ajplegacy.1967.213.6.1465. [DOI] [PubMed] [Google Scholar]
- Tomita T., Watanabe H. Factors controlling myogenic activity in smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):73–85. doi: 10.1098/rstb.1973.0010. [DOI] [PubMed] [Google Scholar]
- Trautwein W., McDonald T. F., Tripathi O. Calcium conductance and tension in mammalian ventricular muscle. Pflugers Arch. 1975;354(1):55–74. doi: 10.1007/BF00584503. [DOI] [PubMed] [Google Scholar]
- Weidmann S. Electrical constants of trabecular muscle from mammalian heart. J Physiol. 1970 Nov;210(4):1041–1054. doi: 10.1113/jphysiol.1970.sp009256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam R. Control of membrane permeability to potassium in red blood cells. Nature. 1968 Aug 10;219(5154):610–610. doi: 10.1038/219610a0. [DOI] [PubMed] [Google Scholar]
- Wood E. H., Heppner R. L., Weidmann S. Inotropic effects of electric currents. I. Positive and negative effects of constant electric currents or current pulses applied during cardiac action potentials. II. Hypotheses: calcium movements, excitation-contraction coupling and inotropic effects. Circ Res. 1969 Mar;24(3):409–445. doi: 10.1161/01.res.24.3.409. [DOI] [PubMed] [Google Scholar]


