Abstract
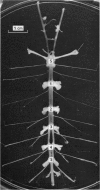
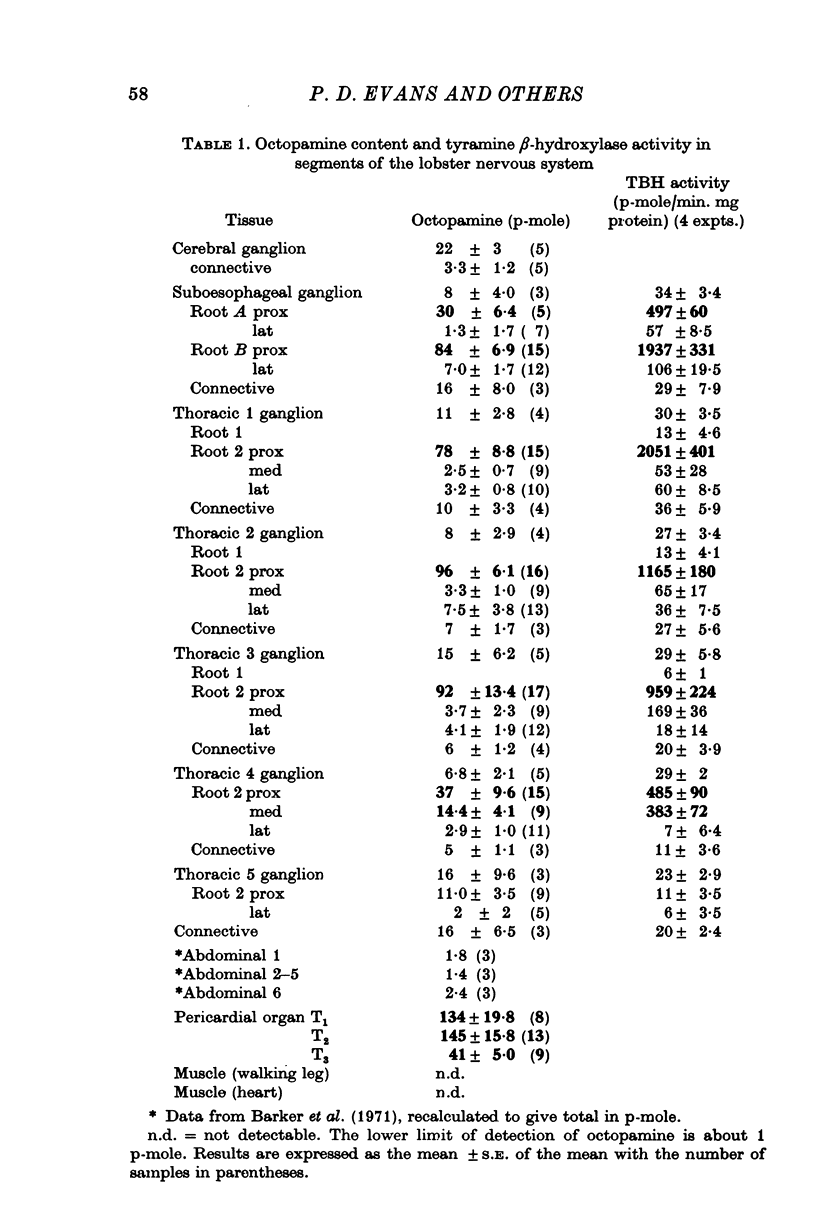
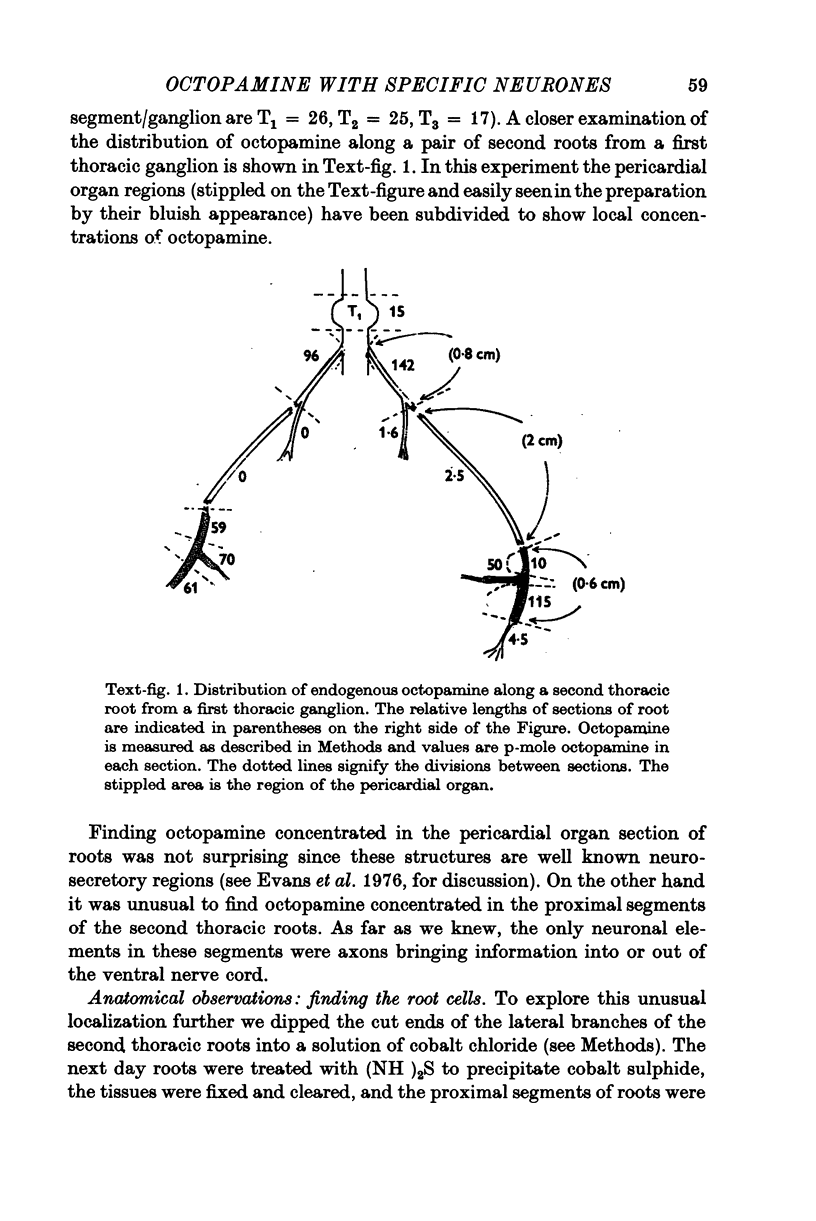
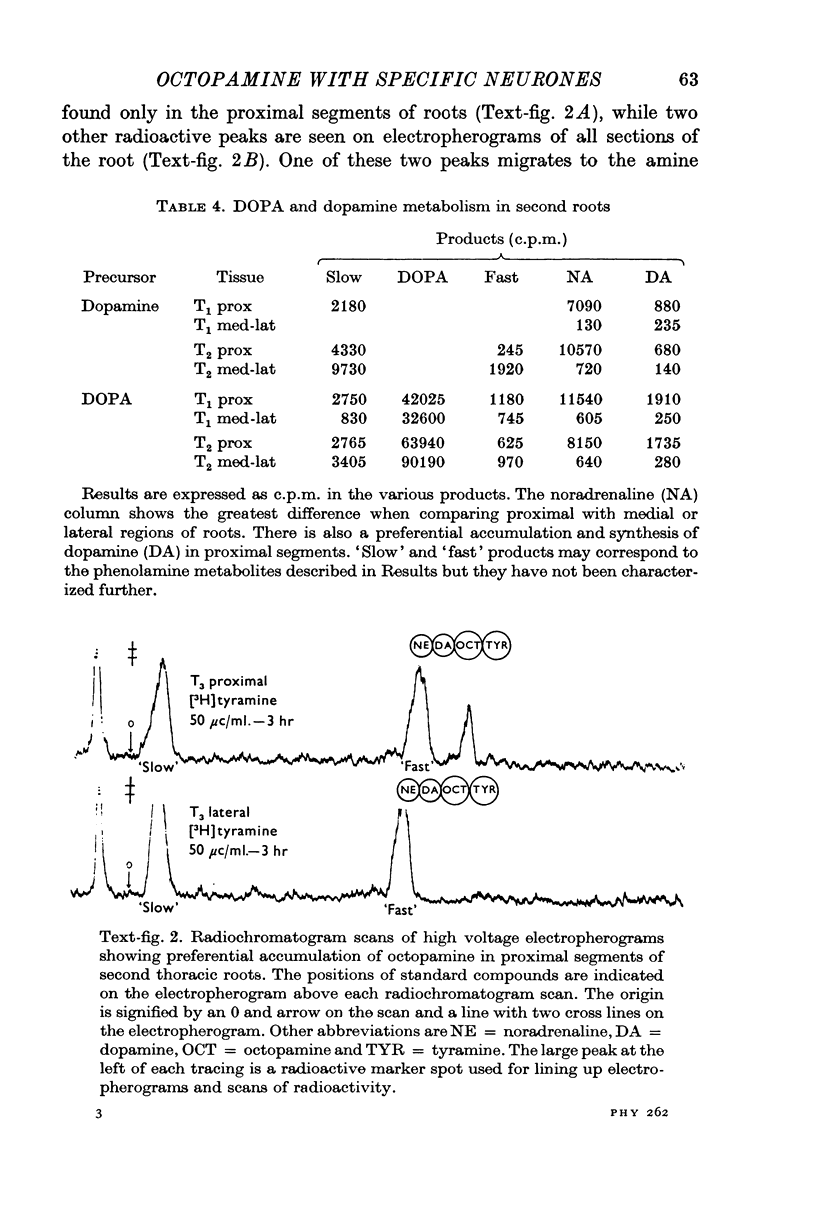
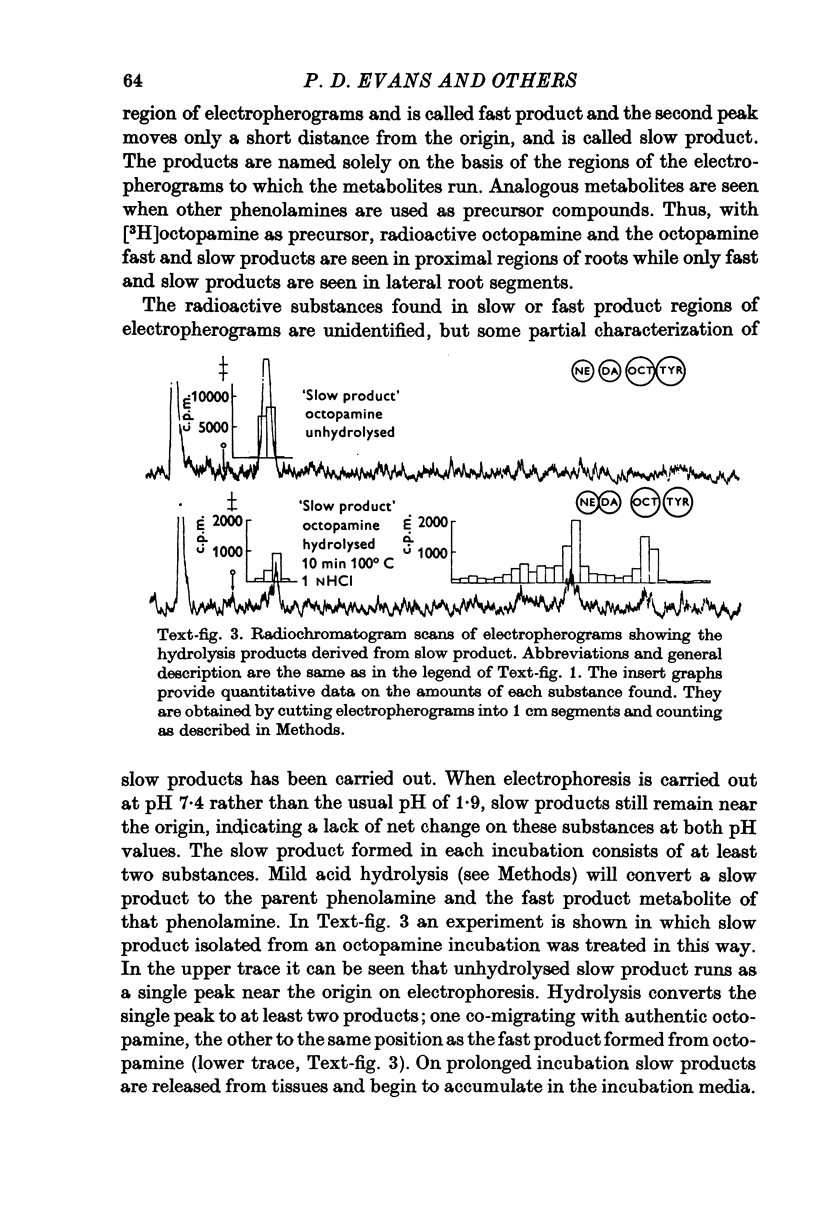
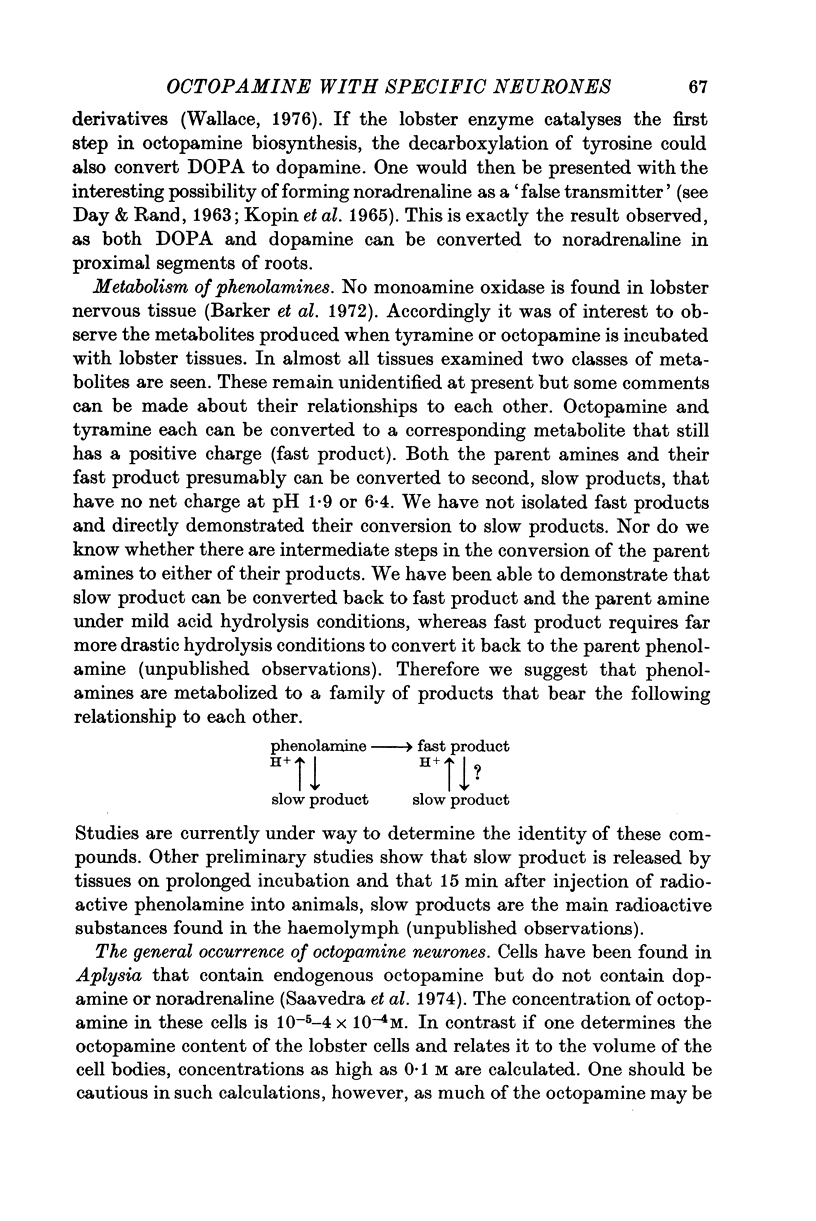
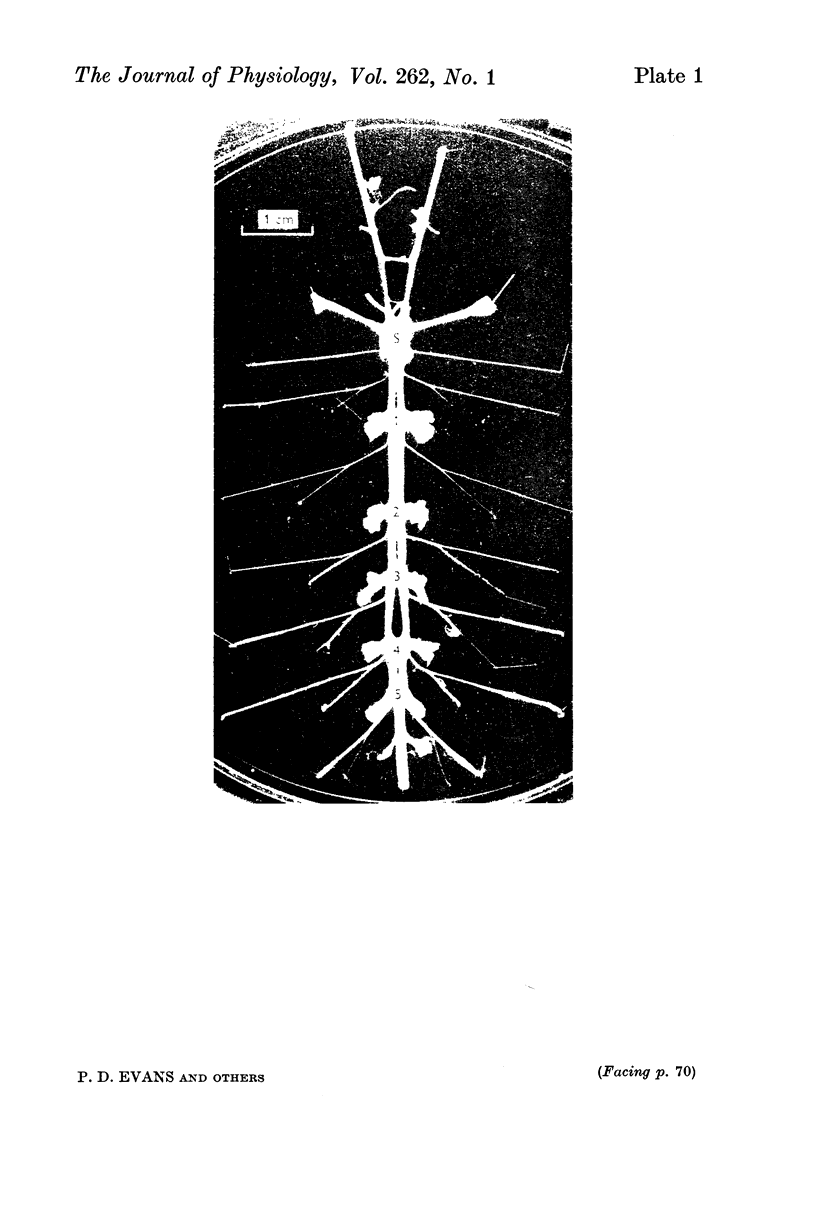
Octapamine and its synthetic enzyme, tyramine beta-hydroxylase (TBH), are found in high concentrations at two points along second thoracic nerve roots in lobsters. The first is in the proximal section of the second root between the ventral nerve cord and the bifurcation of the root into medial (to flexor muscles) and lateral (to extensors) branches. The second region of high concentration is within a well known crustacean neurosecretory system, the pericardial organ, located close to the ends of the lateral branches of the roots. 2. With several different staining procedures, small clusters of nerve cell bodies are found within the connective tissue sheath in the proximal regions of the second roots. No cell bodies are seen in the pericardial organ regions. Cell bodies are variable in number and position between corresponding roots in the same animal and homologous roots among different animals. The average numbers of cell bodies, however, correlate well with TBH and octopamine content, and with the synthesis of octopamine in these same regions of roots. 3. Small clusters of root cell bodies dissected from preparations have greater than 500-fold higher activities of TBH than isolated efferent excitatory and inhibitory or afferent sensory axons. 4. Along with octopamine, the preferential synthesis of acetylcholine and serotonin is also seen in proximal segments of roots. Acetylcholine synthesis in these regions may represent transmitter synthesized in the nerve terminals innervating the root cells. The role of serotonin in these regions is not understood at this time but the amounts of endogenous serotonin found are only a tenth of the amounts of octopamine present. 5. Dopamine is not synthesized from tyrosine in second thoracic roots. However, if DOPA or dopamine are used as precursor compounds, then noradrenaline, which is usually not found in lobsters, can be accumulated in proximal segments of roots. 6. Phenolamines are converted to two further metabolites by lobster tissues. The compounds are unidentified and are named fast and slow product on the basis of their migration on electrophoresis at acid pH. Some partial characterization of slow product reveals that it is a mixture of compounds that can be converted on mild acid hydrolysis to fast product and the parent phenolamine. 7. The several lines of evidence presented suggest that nerve cells found in the proximal segments of the second thoracic roots contain and can synthesize octopamine. Since not all the cells in any single root have been analysed for octopamine or TBH, however, the possibility that one or more of the cells contain physiologically interesting substances other than octopamine is not eliminated.
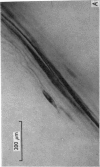
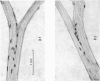
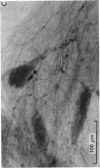
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akert K., Sandri C. An electron-microscopic study of zinc iodide-osmium impregnation of neurons. I. Staining of synaptic vesicles at cholinergic junctions. Brain Res. 1968 Feb;7(2):286–295. doi: 10.1016/0006-8993(68)90104-2. [DOI] [PubMed] [Google Scholar]
- Barker D. L., Herbert E., Hildebrand J. G., Kravitz E. A. Acetylcholine and lobster sensory neurones. J Physiol. 1972 Oct;226(1):205–229. doi: 10.1113/jphysiol.1972.sp009981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. L., Molinoff P. B., Kravitz E. A. Octopamine in the lobster nervous system. Nat New Biol. 1972 Mar 15;236(63):61–63. doi: 10.1038/newbio236061a0. [DOI] [PubMed] [Google Scholar]
- Carpenter D. O., Gaubatz G. L. Octopamine receptors on Aplysia neurones mediate hyperpolarisation by increasing membrane conductance. Nature. 1974 Dec 6;252(5483):483–485. doi: 10.1038/252483a0. [DOI] [PubMed] [Google Scholar]
- DAY M. D., RAND M. J. A hypothesis for the mode of action of alpha-methyldopa in relieving hypertension. J Pharm Pharmacol. 1963 Apr;15:221–224. doi: 10.1111/j.2042-7158.1963.tb12778.x. [DOI] [PubMed] [Google Scholar]
- ERSPAMER V., BORETTI G. Identification and characterization, by paper chromatography, of enteramine, octopamine, tyramine, histamine and allied substances in extracts of posterior salivary glands of octopoda and in other tissue extracts of vertebrates and invertebrates. Arch Int Pharmacodyn Ther. 1951 Dec;88(3):296–332. [PubMed] [Google Scholar]
- Evans P. D., Kravitz E. A., Talamo B. R. Octopamine release at two points along lobster nerve trunks. J Physiol. 1976 Oct;262(1):71–89. doi: 10.1113/jphysiol.1976.sp011586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. D., Talamo B. R., Kravitz E. A. Octopamine neurons: morphology, release of octopamine and possible physiological role. Brain Res. 1975 Jun 13;90(2):340–347. doi: 10.1016/0006-8993(75)90317-0. [DOI] [PubMed] [Google Scholar]
- Hildebrand J. G., Barker D. L., Herbert E., Kravitz E. A. Screening for neurotransmitters: a rapid radiochemical procedure. J Neurobiol. 1971;2(3):231–246. doi: 10.1002/neu.480020305. [DOI] [PubMed] [Google Scholar]
- Hoyle G., Barker D. L. Synthesis of octopamine by insect dorsal median unpaired neurons. J Exp Zool. 1975 Sep;193(3):433–439. doi: 10.1002/jez.1401930322. [DOI] [PubMed] [Google Scholar]
- Juorio A. V., Molinoff P. B. The normal occurrence of octopamine in neural tissue of the Octopus and other cephalopods. J Neurochem. 1974 Feb;22(2):271–280. doi: 10.1111/j.1471-4159.1974.tb11590.x. [DOI] [PubMed] [Google Scholar]
- KAKIMOTO Y., ARMSTRONG M. D. On the identification of octopamine in mammals. J Biol Chem. 1962 Feb;237:422–427. [PubMed] [Google Scholar]
- KOPIN I. J., FISCHER J. E., MUSACCHIO J. M., HORST W. D., WEISE V. K. "FALSE NEUROCHEMICAL TRANSMITTERS" AND THE MECHANISM OF SYMPATHETIC BLOCKADE BY MONOAMINE OXIDASE INHIBITORS. J Pharmacol Exp Ther. 1965 Feb;147:186–193. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levitan I. B., Barondes S. H. Octopamine- and serotonin-stimulated phosphorylation of specific protein in the abdominal ganglion of Aplysia californica. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1145–1148. doi: 10.1073/pnas.71.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinoff P. B., Axelrod J. Distribution and turnover of octopamine in tissues. J Neurochem. 1972 Jan;19(1):157–163. doi: 10.1111/j.1471-4159.1972.tb01265.x. [DOI] [PubMed] [Google Scholar]
- Molinoff P. B., Landsberg L., Axelrod J. An enzymatic assay for octopamine and other beta-hydroxylated phenylethylamines. J Pharmacol Exp Ther. 1969 Dec;170(2):253–261. [PubMed] [Google Scholar]
- Molinoff P., Axelrod J. Octopamine: normal occurrence in sympathetic nerves of rats. Science. 1969 Apr 25;164(3878):428–429. doi: 10.1126/science.164.3878.428. [DOI] [PubMed] [Google Scholar]
- Nathanson J. A., Greengard P. Octopamine-sensitive adenylate cyclse: evidence for a biological role of octopamine in nervous tissue. Science. 1973 Apr 20;180(4083):308–310. doi: 10.1126/science.180.4083.308. [DOI] [PubMed] [Google Scholar]
- Orkand P. M., Kravitz E. A. Localization of the sites of gamma-aminobutyric acid (GABA) uptake in lobster nerve-muscle preparations. J Cell Biol. 1971 Apr;49(1):75–89. doi: 10.1083/jcb.49.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. A., Steele J. E. Octopamine in the insect central nervous system: distribution, biosynthesis and possible physiological role. J Physiol. 1974 Mar;237(2):34P–35P. [PubMed] [Google Scholar]
- Saavedra J. M., Brownstein M. J., Carpenter D. O., Axelrod J. Octopamine: presence in single neurons of Aplysia suggests neurotransmitter function. Science. 1974 Jul 26;185(4148):364–365. doi: 10.1126/science.185.4148.364. [DOI] [PubMed] [Google Scholar]
- Walker R. J., Ramage A. G., Woodruff G. N. The presence of octopamine in the brain of Helix aspersa and its action on specific snail neurones. Experientia. 1972 Oct 15;28(10):1173–1174. doi: 10.1007/BF01946151. [DOI] [PubMed] [Google Scholar]
- Wallace B. G., Talamo B. R., Evans P. D., Kravitz E. A. Octopamine: selective association with specific neurons in the lobster nervous system. Brain Res. 1974 Jul 12;74(2):349–355. doi: 10.1016/0006-8993(74)90590-3. [DOI] [PubMed] [Google Scholar]
- Wallace B. G. The biosynthesis of octopamine--characterization of lobster tyramine beta-hydroxylase. J Neurochem. 1976 Apr;26(4):761–770. doi: 10.1111/j.1471-4159.1976.tb04449.x. [DOI] [PubMed] [Google Scholar]