Abstract
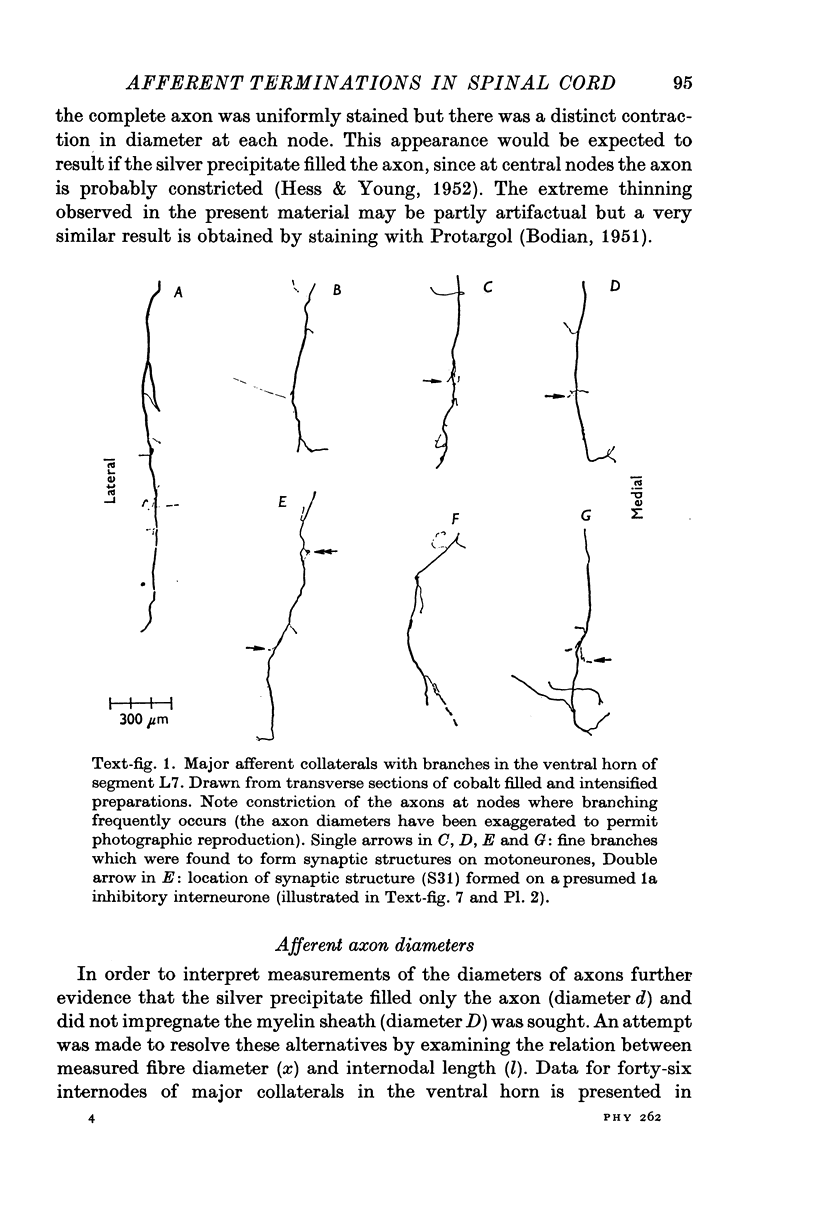
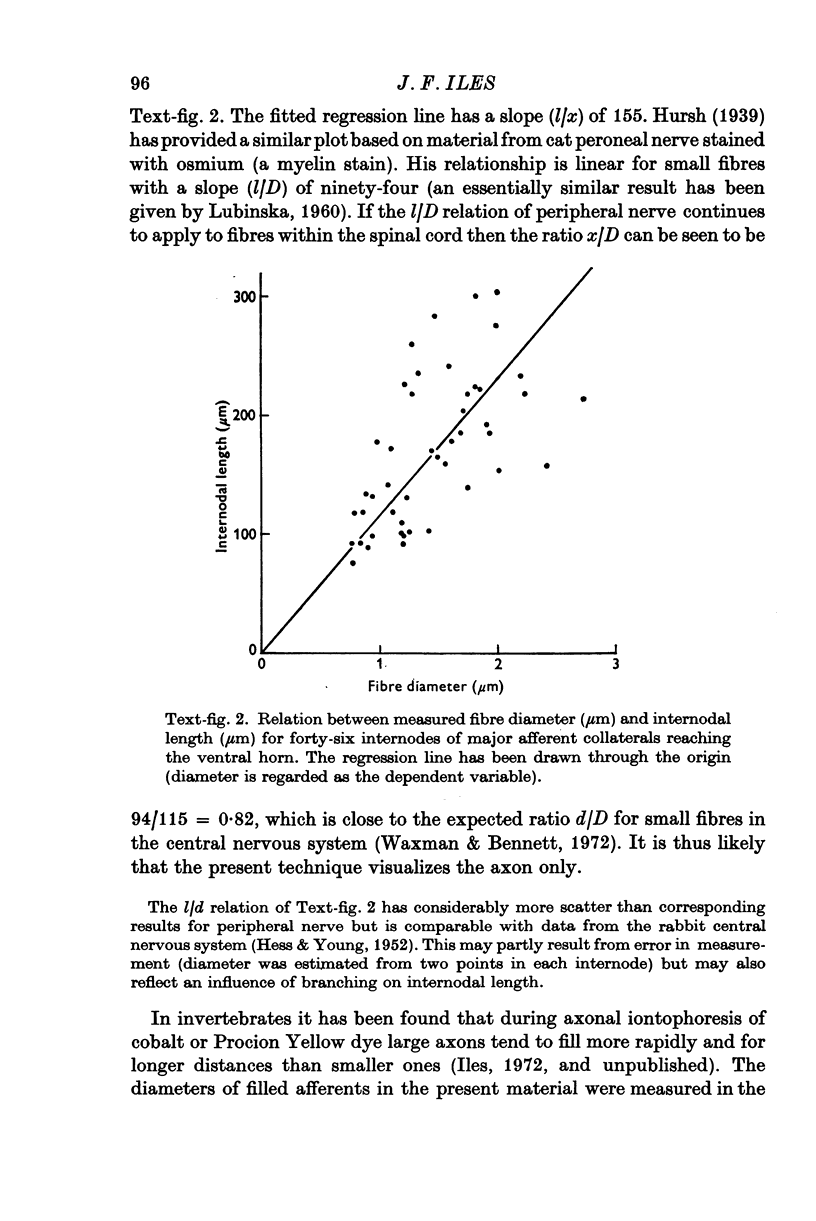
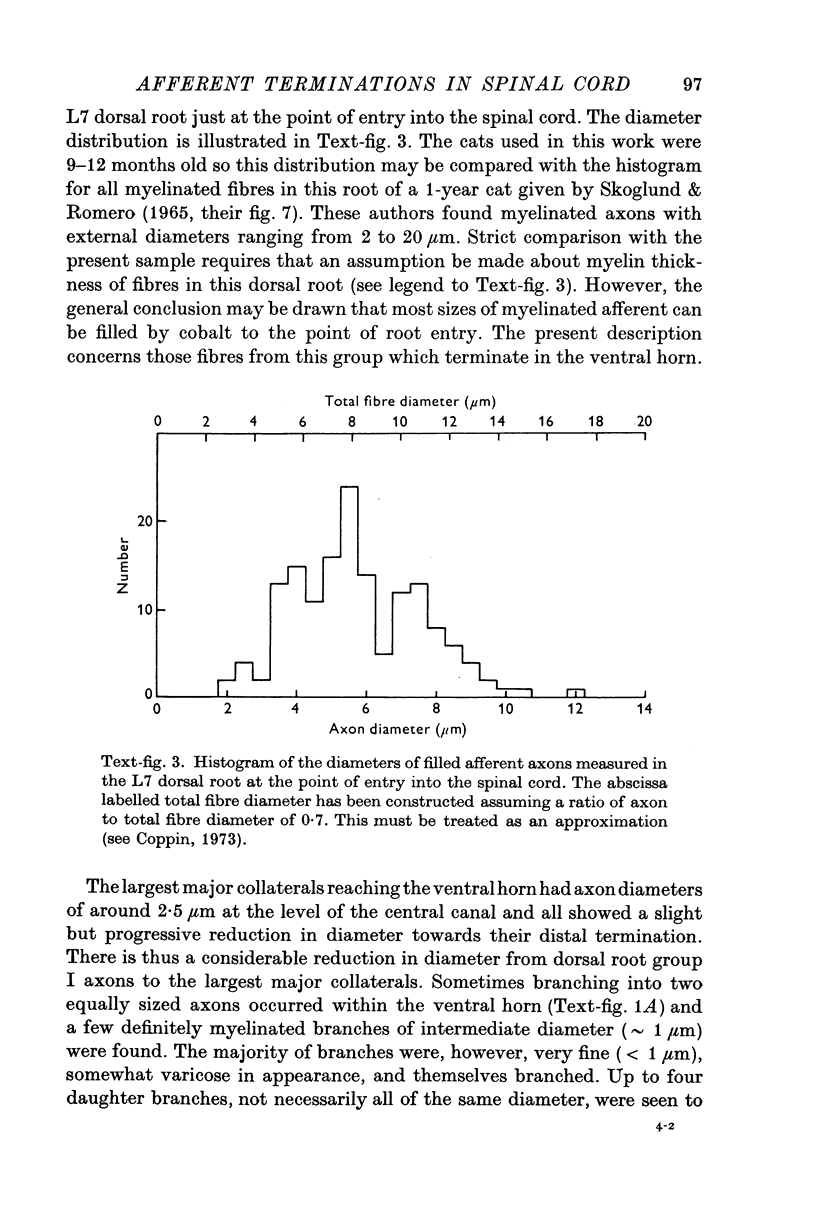
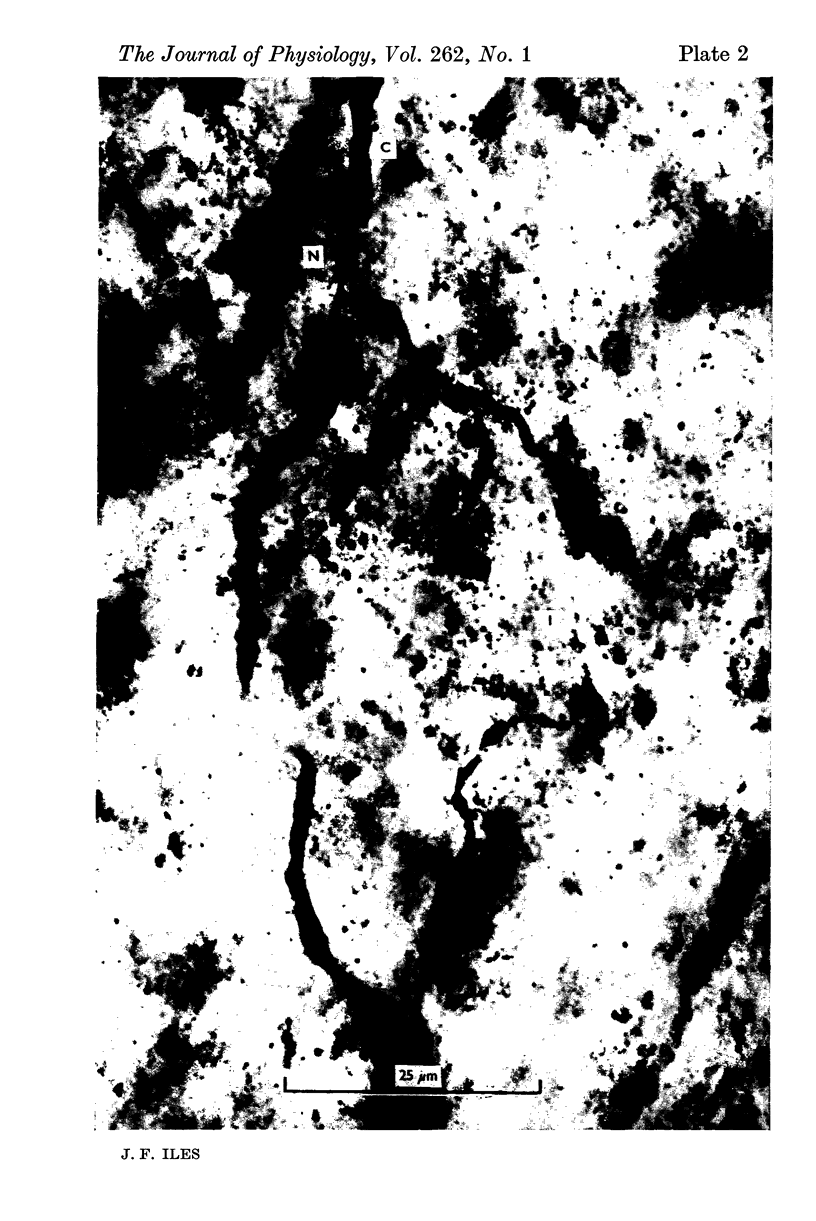
Intraspinal branches of primary afferent axons in the cat lumbar cord have been revealed by filling then with cobalt, followed by precipitation as cobalt sulphide and silver intensification. 2. Primary afferent collaterals which reached the ventral horn were myelinated and had axon diameters of around 2 mum. Up to four (mean 1) side branches occurred at nodes within the ventral horn. The finest branches were less than 1 mum in diameter. 3. The finest branches formed synaptic boutons on nerve cells. The total observed association between one afferent and a motoneurone (synaptic structure) consisted of up to six boutons (mean 1-85). The boutons constituting a synaptic structure were usually located close together on the soma or dendrites, but sometimes were spread along more than 60 mum of dendrite. It was assumed that these monosynaptic structures on moto-neurones were formed by muscle spindle afferents. 4. There was no correlation between the total contact area of a synaptic structure and its location on a motoneurone. 5. A computer model of the motoneurone was used to investigate the effect of multiple synaptic contact on electrophysiological estimates of synaptic location. It was concluded that the observed spread of boutons making up a synaptic structure would not significantly affect distance allocation of distal synapses but could lead to some proximal dendrite structures being incorrectly classified as somatic.
Full text
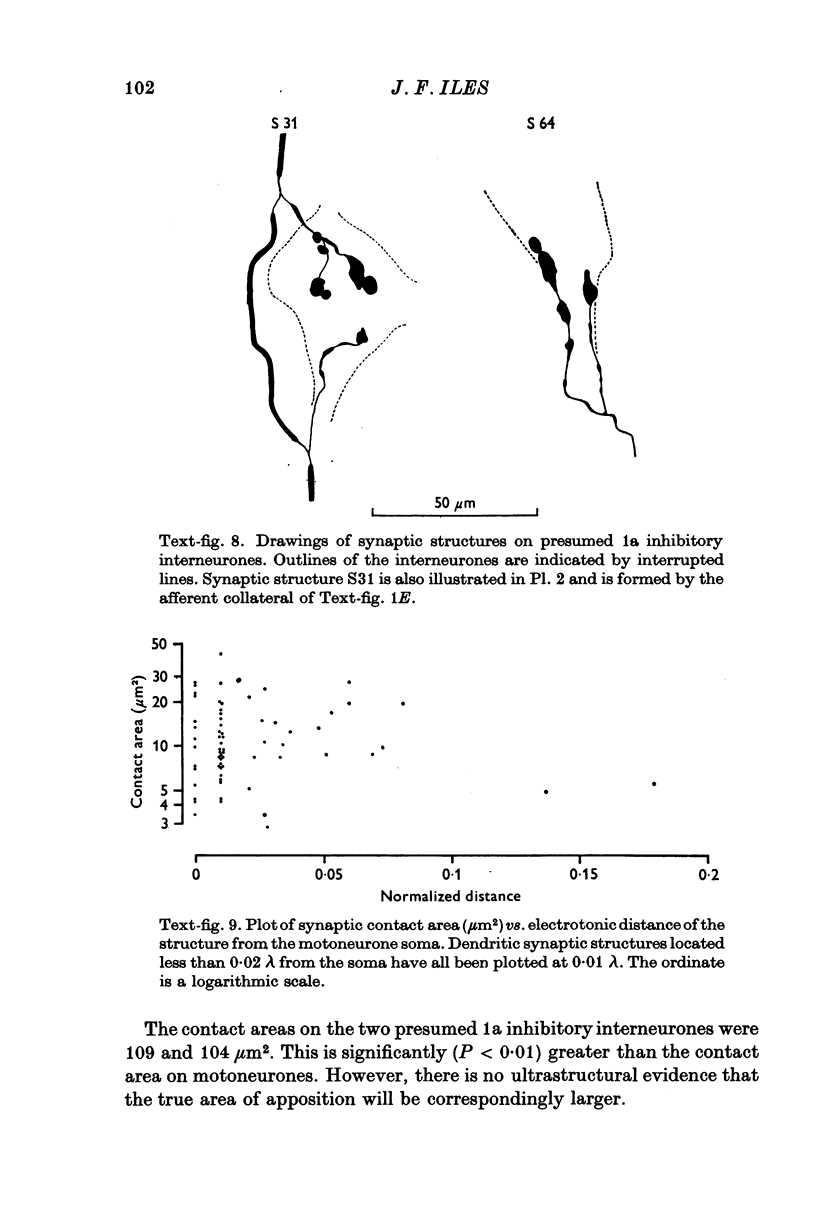
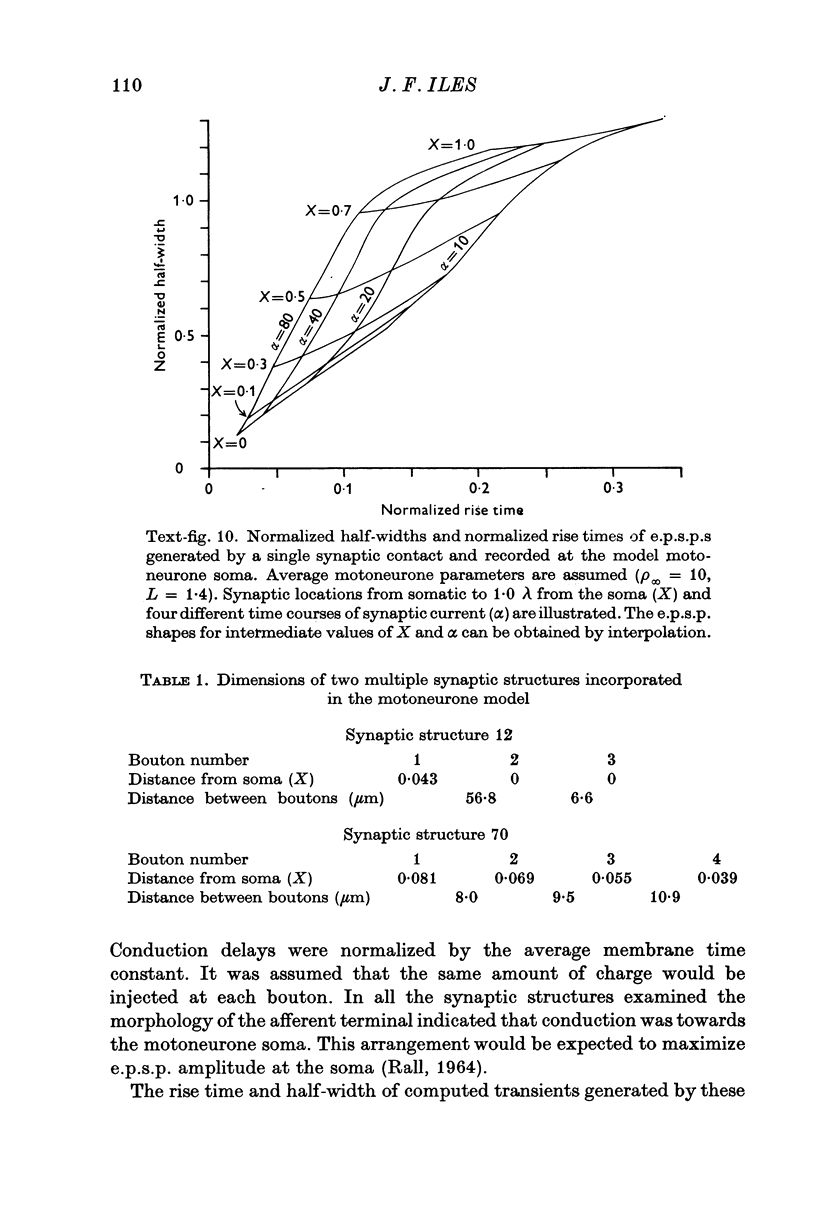
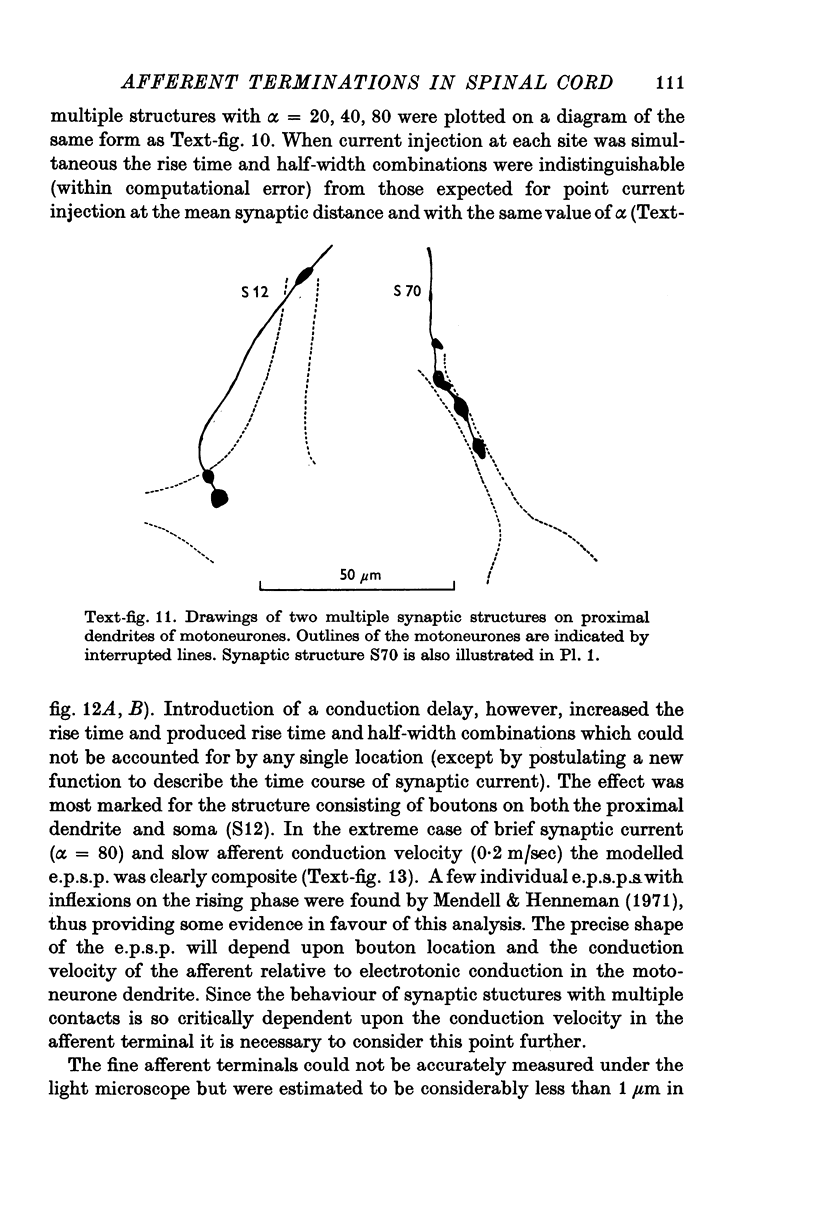
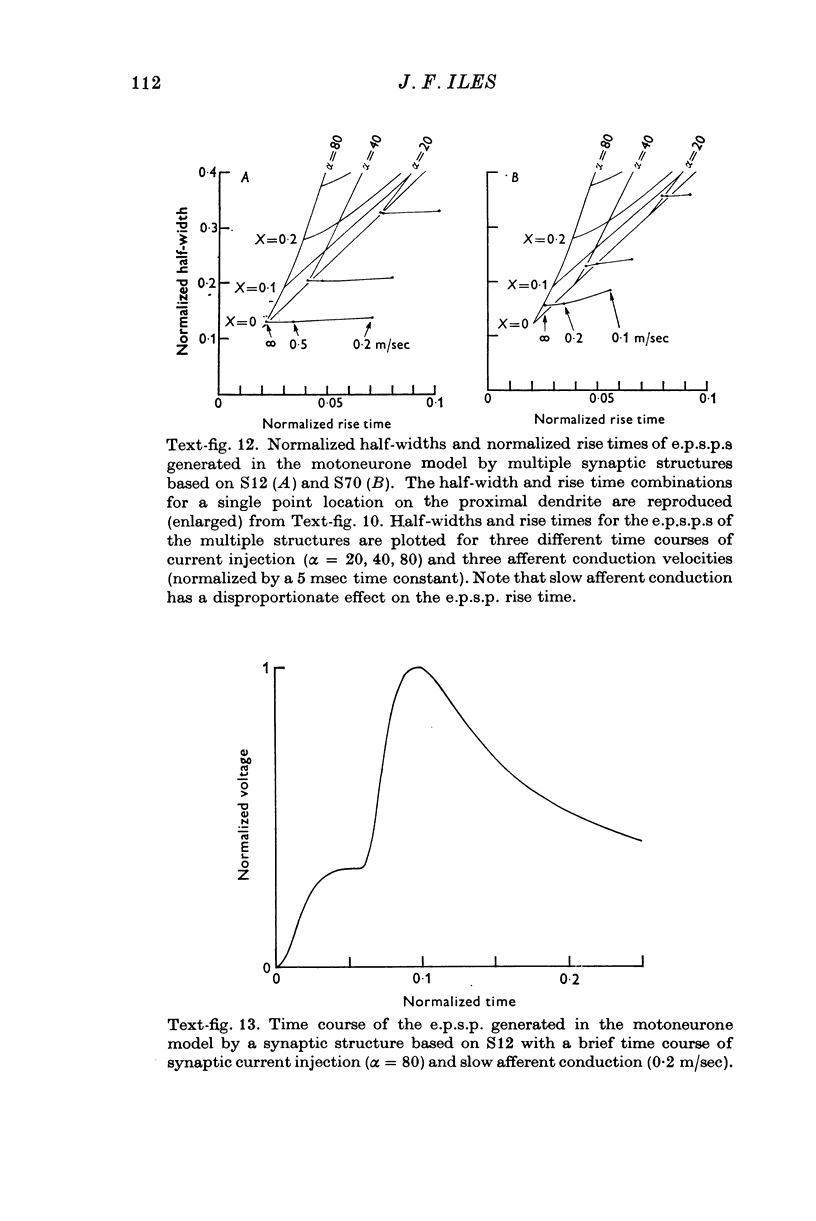
PDF




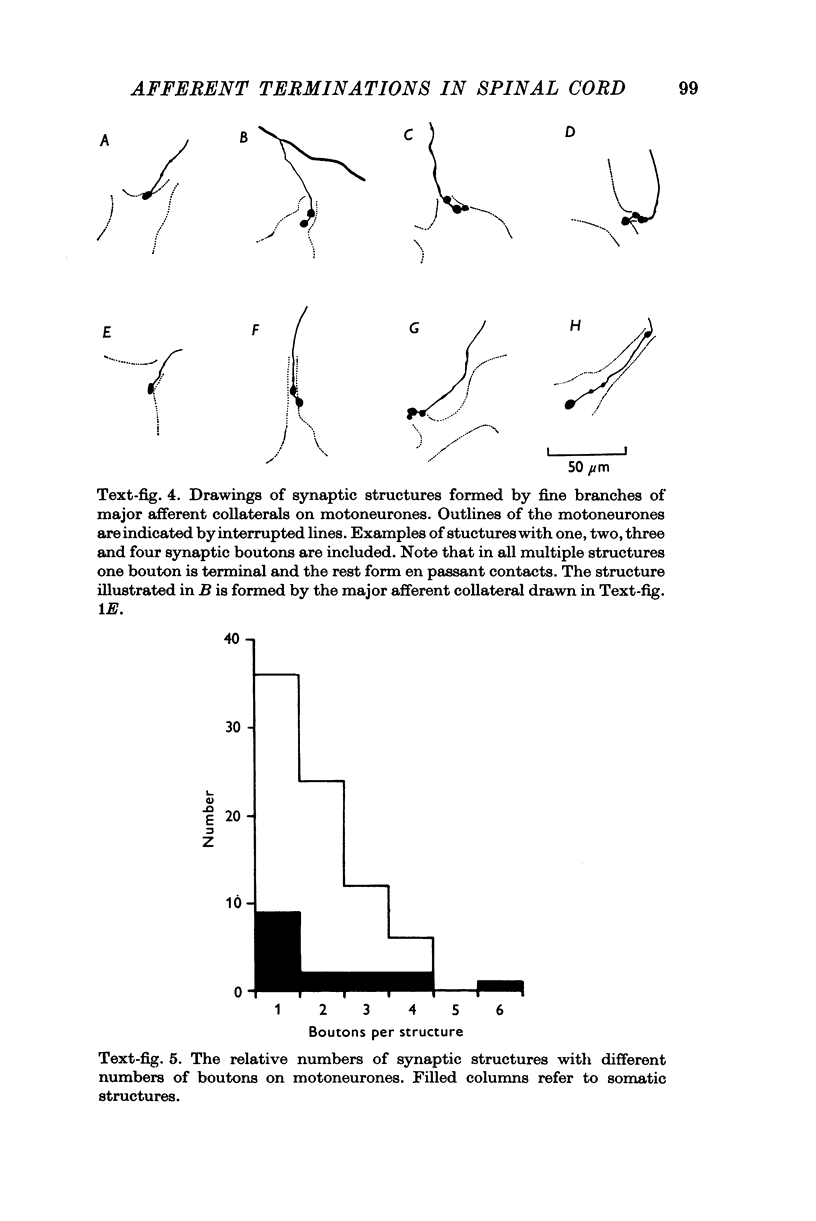













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
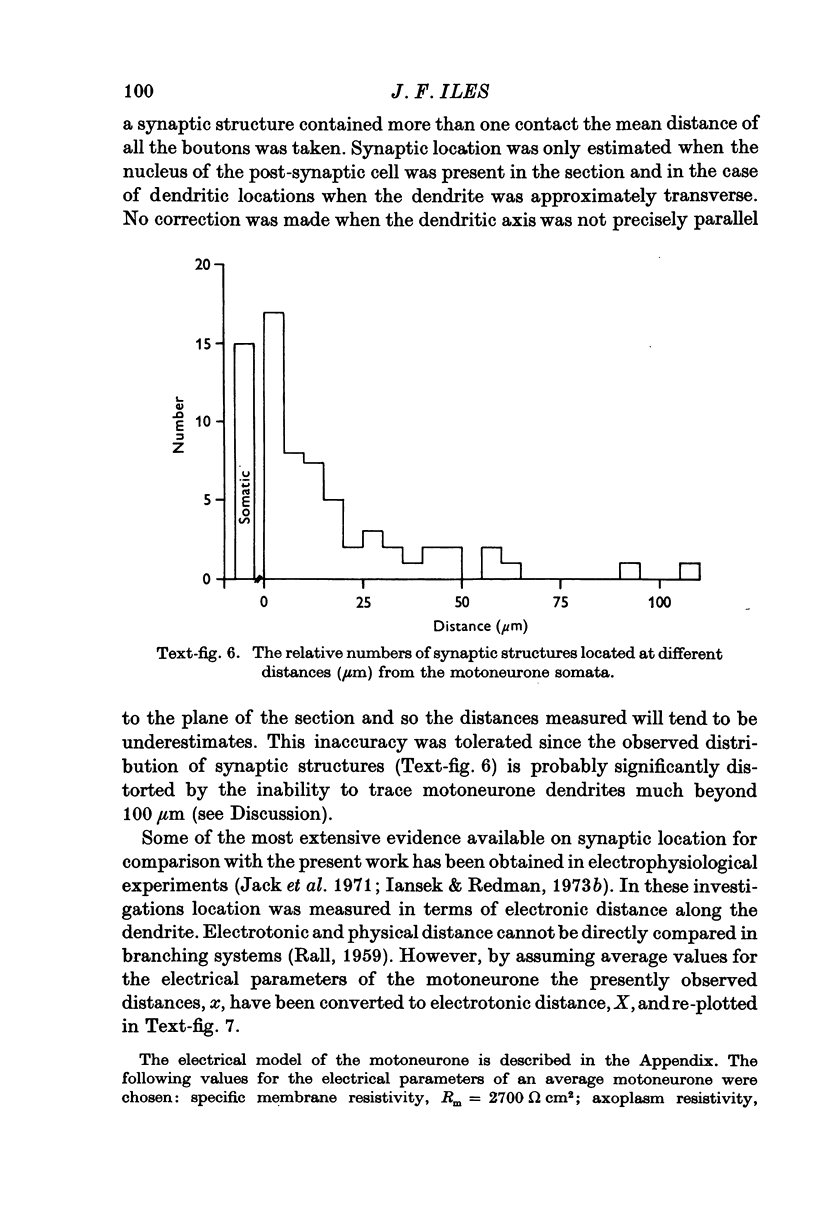
- BALTHASAR K. Morphologie der spinalen Tibialis- und Peronaeus-Kerne bei der Katze; Topographie, Architektonik, Axon- und Dendritenverlauf der Motoneurone und Zwischen-neurone in den Segmenten L6-S2. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1952;188(4):345–378. doi: 10.1007/BF00367982. [DOI] [PubMed] [Google Scholar]
- BODIAN D. A note on nodes of Ranvier in the central nervous system. J Comp Neurol. 1951 Jun;94(3):475–483. doi: 10.1002/cne.900940309. [DOI] [PubMed] [Google Scholar]
- Barrett J. N., Crill W. E. Specific membrane properties of cat motoneurones. J Physiol. 1974 Jun;239(2):301–324. doi: 10.1113/jphysiol.1974.sp010570. [DOI] [PMC free article] [PubMed] [Google Scholar]
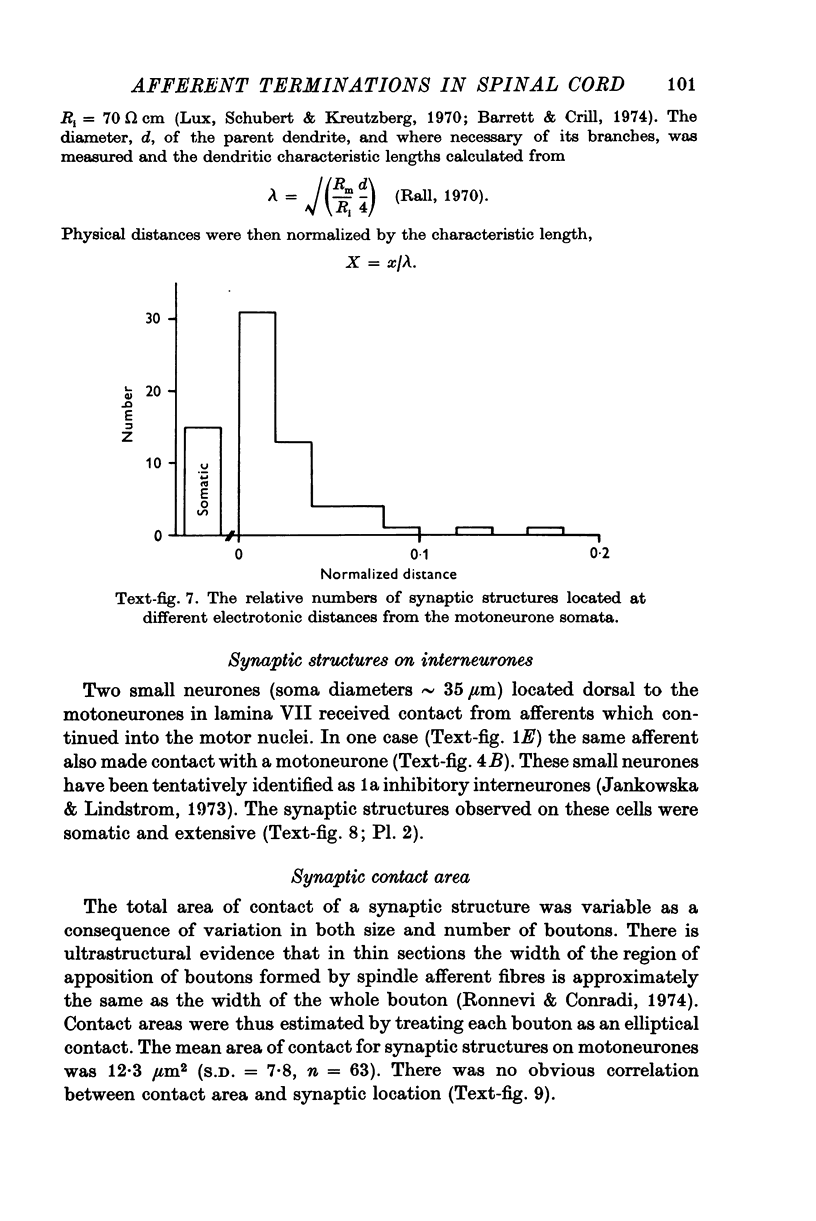
- Bodian D. Origin of specific synaptic types in the motoneuron neuropil of the monkey. J Comp Neurol. 1975 Jan 15;159(2):225–243. doi: 10.1002/cne.901590205. [DOI] [PubMed] [Google Scholar]
- Burke R., Lundberg A., Weight F. Spinal border cell origin of the ventral spinocerebellar tract. Exp Brain Res. 1971;12(3):283–294. doi: 10.1007/BF00237921. [DOI] [PubMed] [Google Scholar]
- Conradi S., Ronnevi L. O. Spontaneous elimination of synapses on cat spinal motoneurons after birth: do half of the synapses on the cell bodies disappear? Brain Res. 1975 Jul 18;92(3):505–510. doi: 10.1016/0006-8993(75)90338-8. [DOI] [PubMed] [Google Scholar]
- Conradi S. Ultrastructure of dorsal root boutons on lumbosacral motoneurons of the adult cat, as revealed by dorsal root section. Acta Physiol Scand Suppl. 1969;332:85–115. [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. Parallel fibre stimulation and the responses induced thereby in the Purkinje cells of the cerebellum. Exp Brain Res. 1966;1(1):17–39. doi: 10.1007/BF00235207. [DOI] [PubMed] [Google Scholar]
- Edwards F. R., Redman S. J., Walmsley B. Non-quantal fluctuations and transmission failures in charge transfer at Ia synapses on spinal motoneurones. J Physiol. 1976 Aug;259(3):689–704. doi: 10.1113/jphysiol.1976.sp011489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T. C., Santini M., Schomburg E. D. Characteristics and distribution of spinal focal synaptic potentials generated by group II muscle afferents. Acta Physiol Scand. 1974 Jul;91(3):298–313. doi: 10.1111/j.1748-1716.1974.tb05686.x. [DOI] [PubMed] [Google Scholar]
- Fu T. C., Schomburg E. D. Electrophysiological investigation of the projection of secondary muscle spindle afferents in the cat spinal cord. Acta Physiol Scand. 1974 Jul;91(3):314–329. doi: 10.1111/j.1748-1716.1974.tb05687.x. [DOI] [PubMed] [Google Scholar]
- HESS A., YOUNG J. Z. The nodes of Ranvier. Proc R Soc Lond B Biol Sci. 1952 Nov 20;140(900):301–320. doi: 10.1098/rspb.1952.0063. [DOI] [PubMed] [Google Scholar]
- Ha H., Liu C. N. Cell origin of the ventral spinocerebellar tract. J Comp Neurol. 1968 Jun;133(2):185–206. doi: 10.1002/cne.901330204. [DOI] [PubMed] [Google Scholar]
- IGGO A. The electrophysiological identification of single nerve fibres, with particular reference to the slowest-conducting vagal afferent fibres in the cat. J Physiol. 1958 Jun 18;142(1):110–126. doi: 10.1113/jphysiol.1958.sp006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iansek R., Redman S. J. An analysis of the cable properties of spinal motoneurones using a brief intracellular current pulse. J Physiol. 1973 Nov;234(3):613–636. doi: 10.1113/jphysiol.1973.sp010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iansek R., Redman S. J. The amplitude, time course and charge of unitary excitatory post-synaptic potentials evoked in spinal motoneurone dendrites. J Physiol. 1973 Nov;234(3):665–688. doi: 10.1113/jphysiol.1973.sp010366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles J. F., Mulloney B. Procion yellow staining of cockroach motor neurones without the use of microelectrodes. Brain Res. 1971 Jul 23;30(2):397–400. doi: 10.1016/0006-8993(71)90089-8. [DOI] [PubMed] [Google Scholar]
- Iles J. F. Proceedings: Demonstration of afferent terminations in the cat spinal cord. J Physiol. 1973 Oct;234(2):22P–24P. [PubMed] [Google Scholar]
- Iles J. F. Structure and synaptic activation of the fast coxal depressor motoneurone of the cockroach, Periplaneta americana. J Exp Biol. 1972 Jun;56(3):647–656. doi: 10.1242/jeb.56.3.647. [DOI] [PubMed] [Google Scholar]
- Illis L. The relative densities of monosynaptic pathways to the cell bodies and dendrites of the cat ventral horn. J Neurol Sci. 1967 Mar-Apr;4(2):259–270. doi: 10.1016/0022-510x(67)90104-9. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Miller S., Porter R., Redman S. J. The time course of minimal excitory post-synaptic potentials evoked in spinal motoneurones by group Ia afferent fibres. J Physiol. 1971 Jun;215(2):353–380. doi: 10.1113/jphysiol.1971.sp009474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J. Physiology of peripheral nerve fibres in relation to their size. Br J Anaesth. 1975 Feb;47 Suppl:173–182. [PubMed] [Google Scholar]
- Jack J. J., Redman S. J. An electrical description of the motoneurone, and its application to the analysis of synaptic potentials. J Physiol. 1971 Jun;215(2):321–352. doi: 10.1113/jphysiol.1971.sp009473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Lindström S. Morphology of interneurones mediating Ia reciprocal inhibition of motoneurones in the spinal cord of the cat. J Physiol. 1972 Nov;226(3):805–823. doi: 10.1113/jphysiol.1972.sp010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Ritchie J. M. The movements of labelled ions in mammalian non-myelinated nerve fibres. J Physiol. 1965 Jul;179(2):333–367. doi: 10.1113/jphysiol.1965.sp007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Monosynaptic excitation of motoneurones from secondary endings of muscle spindles. Nature. 1974 Nov 15;252(5480):243–244. doi: 10.1038/252243a0. [DOI] [PubMed] [Google Scholar]
- Kuno M., Muñoz-Martinez E. J., Randić M. Synaptic action on Clarke's column neurones in relation to afferent terminal size. J Physiol. 1973 Jan;228(2):343–360. doi: 10.1113/jphysiol.1973.sp010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Turkanis S. A., Weakly J. N. Correlation between nerve terminal size and transmitter release at the neuromuscular junction of the frog. J Physiol. 1971 Mar;213(3):545–556. doi: 10.1113/jphysiol.1971.sp009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason C. A. Delineation of the rat visual system by the axonal iontophoresis-cobalt sulfide precipitation technique. Brain Res. 1975 Feb 28;85(2):287–293. doi: 10.1016/0006-8993(75)90083-9. [DOI] [PubMed] [Google Scholar]
- McLaughlin B. J. Dorsal root projections to the motor nuclei in the cat spinal cord. J Comp Neurol. 1972 Apr;144(4):461–474. doi: 10.1002/cne.901440405. [DOI] [PubMed] [Google Scholar]
- Mendell L. M., Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J Neurophysiol. 1971 Jan;34(1):171–187. doi: 10.1152/jn.1971.34.1.171. [DOI] [PubMed] [Google Scholar]
- Nelson P. G., Lux H. D. Some electrical measurements of motoneuron parameters. Biophys J. 1970 Jan;10(1):55–73. doi: 10.1016/S0006-3495(70)86285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkovits M., Magyar P., Szentágothai J. Quantitative histological analysis of the cerebellar cortex in the cat. 3. Structural organization of the molecular layer. Brain Res. 1971 Nov;34(1):1–18. doi: 10.1016/0006-8993(71)90347-7. [DOI] [PubMed] [Google Scholar]
- Pitman R. M., Tweedle C. D., Cohen M. J. Branching of central neurons: intracellular cobalt injection for light and electron microscopy. Science. 1972 Apr 28;176(4033):412–414. doi: 10.1126/science.176.4033.412. [DOI] [PubMed] [Google Scholar]
- Prior D. J., Fuller P. M. The use of a cobalt iontophoresis technique for identification of the mesencephalic trigeminal nucleus. Brain Res. 1973 Dec 21;64:472–475. doi: 10.1016/0006-8993(73)90208-4. [DOI] [PubMed] [Google Scholar]
- ROMANES G. J. The motor cell columns of the lumbo-sacral spinal cord of the cat. J Comp Neurol. 1951 Apr;94(2):313–363. doi: 10.1002/cne.900940209. [DOI] [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol. 1967 Sep;30(5):1138–1168. doi: 10.1152/jn.1967.30.5.1138. [DOI] [PubMed] [Google Scholar]
- Rall W. Time constants and electrotonic length of membrane cylinders and neurons. Biophys J. 1969 Dec;9(12):1483–1508. doi: 10.1016/S0006-3495(69)86467-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnevi L. O., Conradi S. Ultrastructural evidence for spontaneous elimination of synaptic terminals on spinal motoneurons in the kitten. Brain Res. 1974 Nov 15;80(2):335–339. doi: 10.1016/0006-8993(74)90696-9. [DOI] [PubMed] [Google Scholar]
- Réthelyi M. The Golgi architecture of Clarke's column. Acta Morphol Acad Sci Hung. 1968;16(3):311–330. [PubMed] [Google Scholar]
- SPRAGUE J. M., HONGCHIEN H. A. THE TERMINAL FIELDS OF DORSAL ROOT FIBERS IN THE LUMBOSACRAL SPINAL CORD OF THE CAT, AND THE DENDRITIC ORGANIZATION OF THE MOTOR NUCLEI. Prog Brain Res. 1964;11:120–154. doi: 10.1016/s0079-6123(08)64046-7. [DOI] [PubMed] [Google Scholar]
- SPRAGUE J. M. The distribution of dorsal root fibres on motor cells in the lumbosacral spinal cord of the cat, and the site of excitatory and inhibitory terminals in monosynaptic pathways. Proc R Soc Lond B Biol Sci. 1958 Dec 24;149(937):534–556. doi: 10.1098/rspb.1958.0091. [DOI] [PubMed] [Google Scholar]
- Saito K. Electron microscopic observations on terminal boutons and synaptic structures in the anterior horn of the spinal cord in the adult cat. Okajimas Folia Anat Jpn. 1972 Mar;48(6):361–411. doi: 10.2535/ofaj1936.48.6_361. [DOI] [PubMed] [Google Scholar]
- Scheibel M. E., Scheibel A. B. Organization of spinal motoneuron dendrites in bundles. Exp Neurol. 1970 Jul;28(1):106–112. doi: 10.1016/0014-4886(70)90165-2. [DOI] [PubMed] [Google Scholar]
- Scheibel M. E., Scheibel A. B. Terminal patterns in cat spinal cord. 3. Primary afferent collaterals. Brain Res. 1969 May;13(3):417–443. doi: 10.1016/0006-8993(69)90258-3. [DOI] [PubMed] [Google Scholar]
- Silakov V. L., Zarkeshev E. G. Vliianie vnekletochnoi poliarizatsii na aktivnost' neironov naruzhnykh kolenhatykh tel. Neirofiziologiia. 1972 Mar-Apr;4(2):130–140. [PubMed] [Google Scholar]
- Skoglund S., Romero C. Postnatal growth of spinal nerves and roots. A morphological study in the cat with physiological correlations. Acta Physiol Scand Suppl. 1965;260:1–50. [PubMed] [Google Scholar]
- Sterling P., Kuypers H. G. Anatomical organization of the brachial spinal cord of the cat. I. The distribution of dorsal root fibers. Brain Res. 1967 Feb;4(1):1–15. doi: 10.1016/0006-8993(67)90144-8. [DOI] [PubMed] [Google Scholar]
- Székely G. The morphology of motoneurons and dorsal root fibers in the frog's spinal cord. Brain Res. 1976 Feb 20;103(2):275–290. doi: 10.1016/0006-8993(76)90799-x. [DOI] [PubMed] [Google Scholar]
- Tyrer N. M., Bell E. M. The intensification of cobalt-filled neurone profiles using a modification of Timm's sulphide-silver method. Brain Res. 1974 Jun 14;73(1):151–155. doi: 10.1016/0006-8993(74)91014-2. [DOI] [PubMed] [Google Scholar]
- Van Buren J. M., Frank K. Correlation between the morphology and potential field of a spinal motor nucleus in the cat. Electroencephalogr Clin Neurophysiol. 1965 Aug;19(2):112–126. doi: 10.1016/0013-4694(65)90222-1. [DOI] [PubMed] [Google Scholar]
- Waxman S. G., Bennett M. V. Relative conduction velocities of small myelinated and non-myelinated fibres in the central nervous system. Nat New Biol. 1972 Aug 16;238(85):217–219. doi: 10.1038/newbio238217a0. [DOI] [PubMed] [Google Scholar]