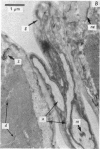
Abstract
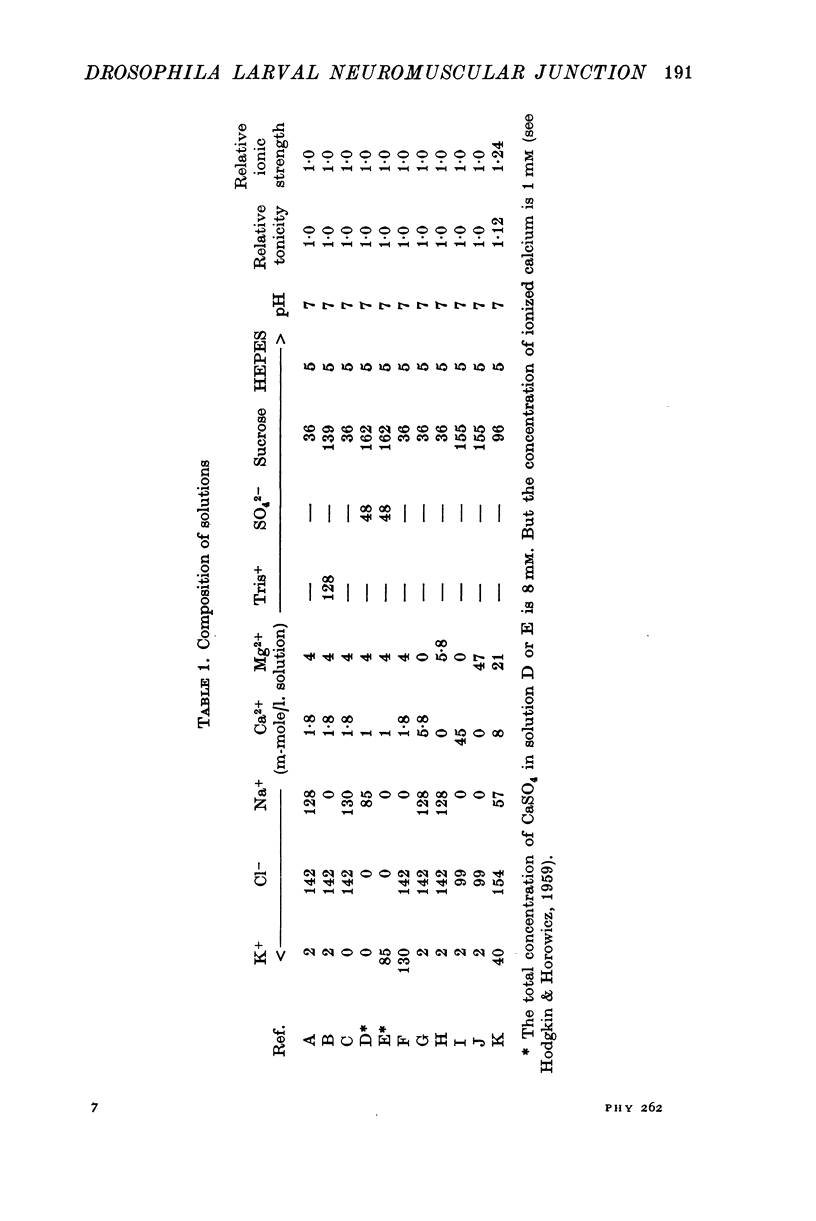
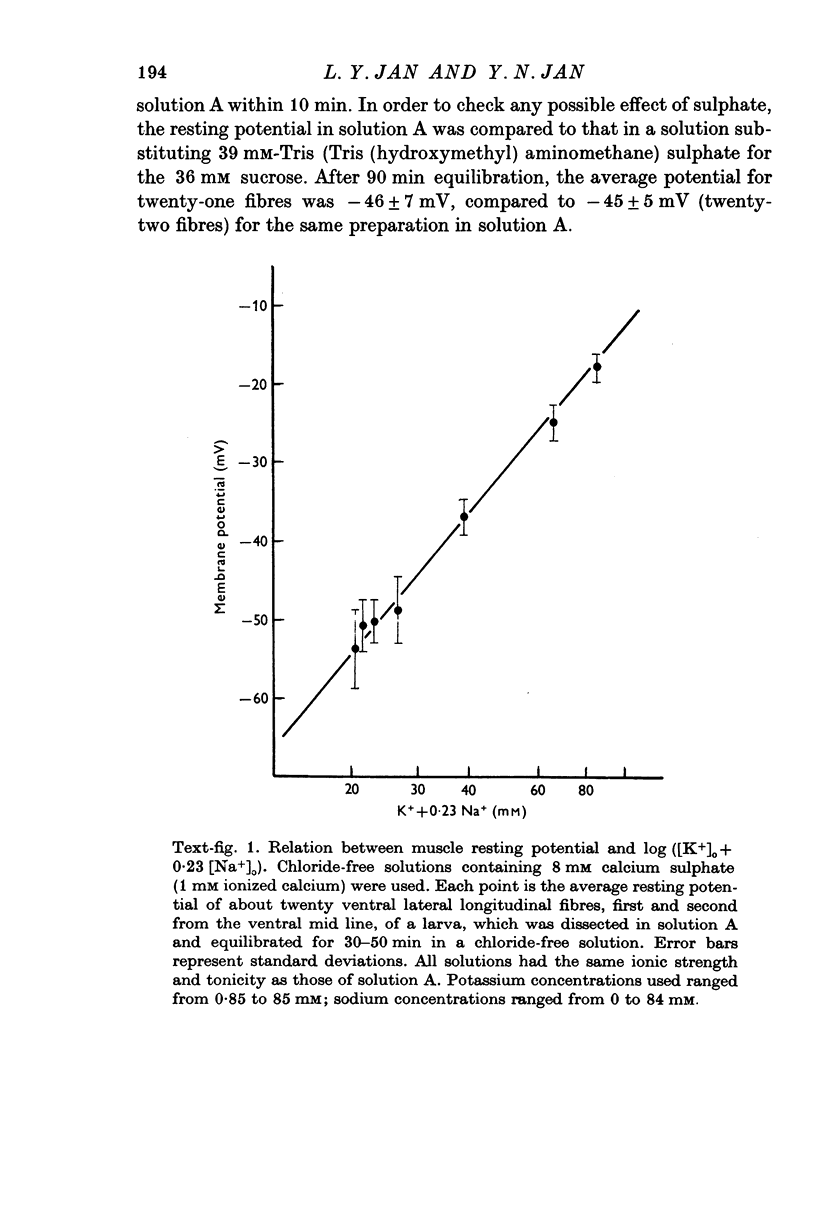
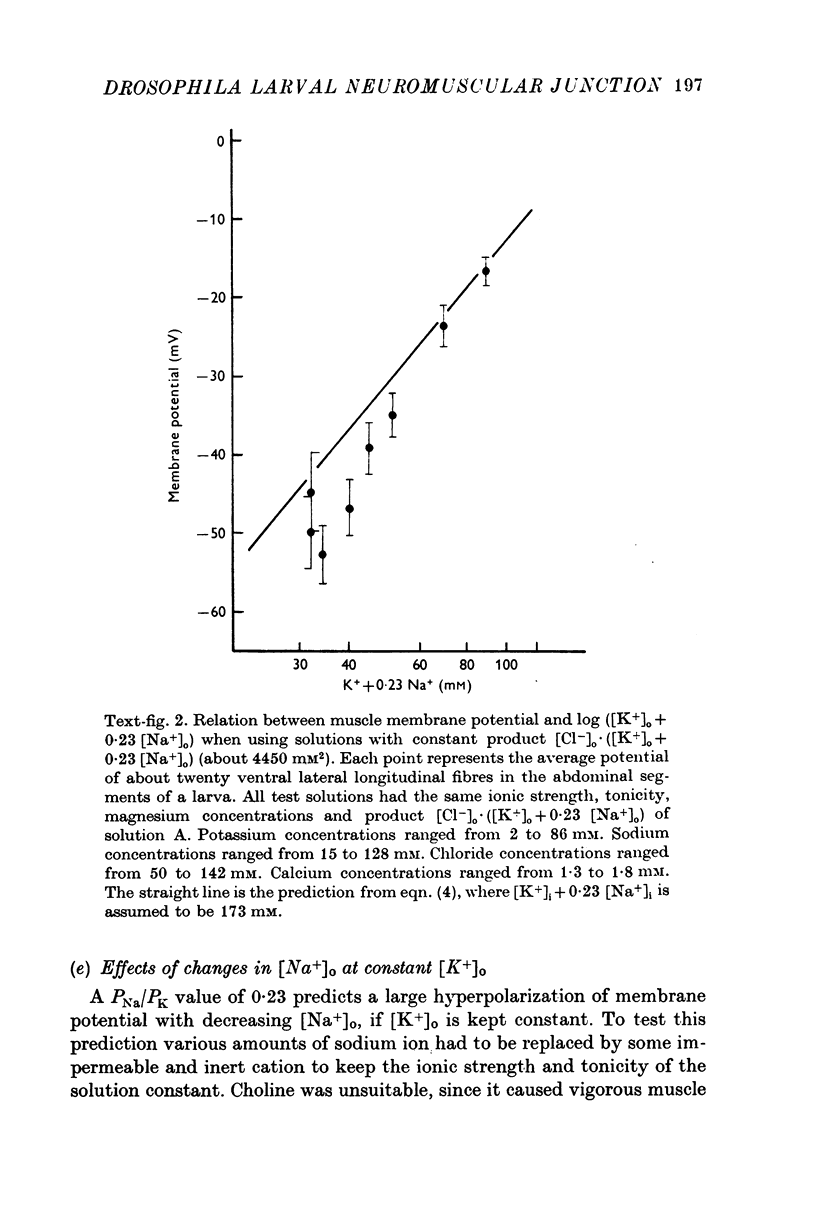
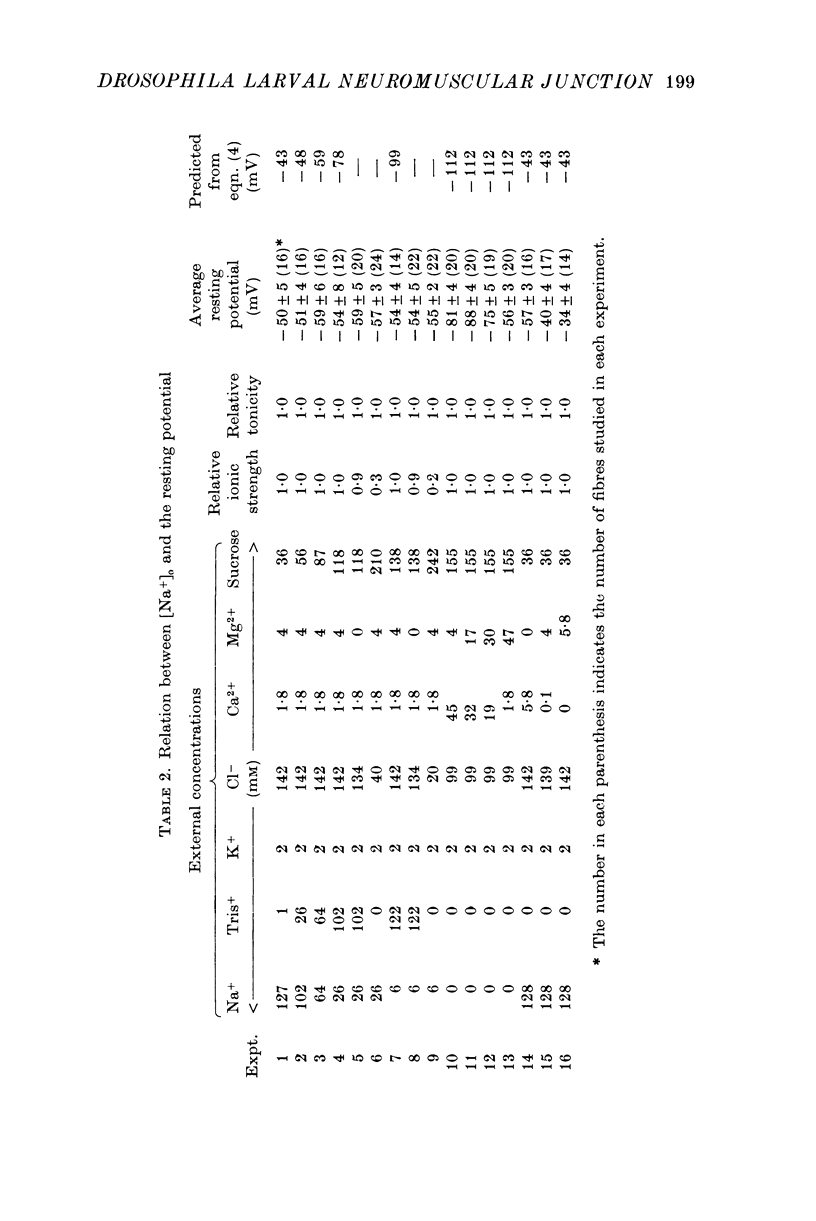
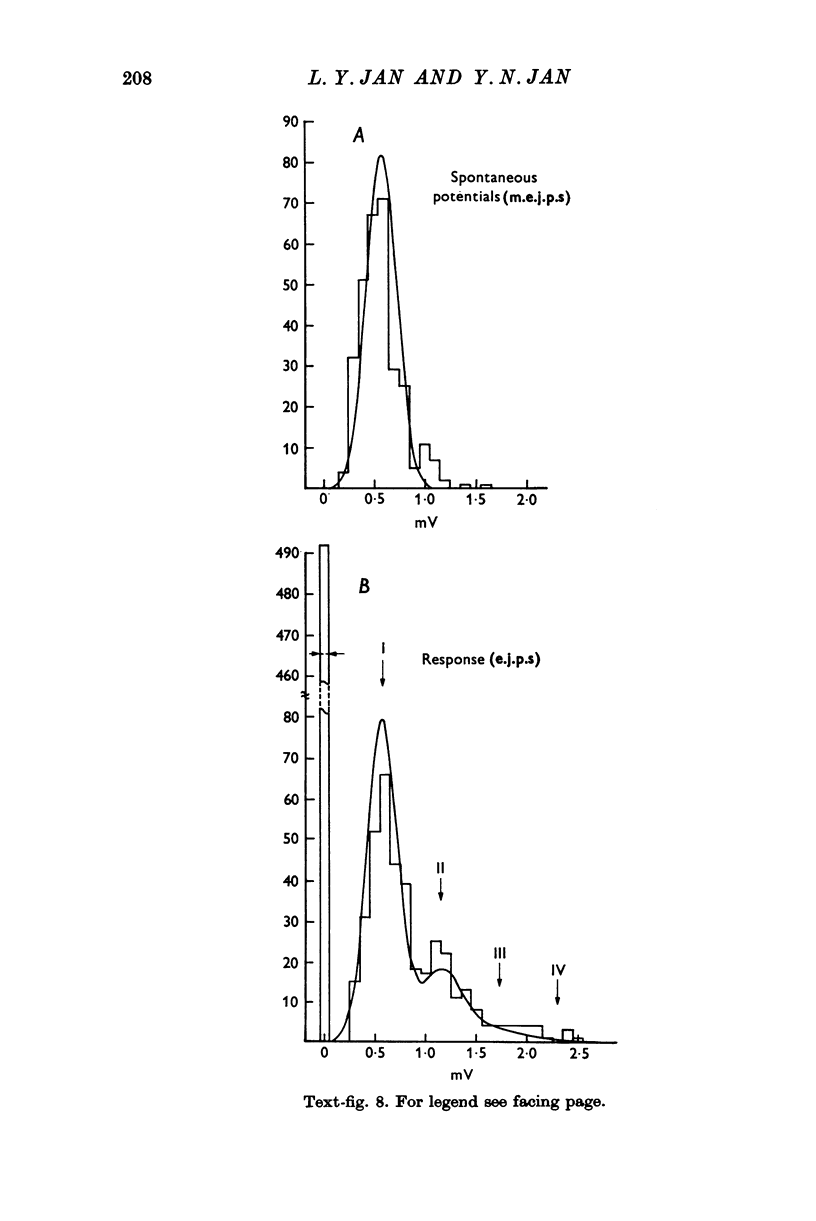
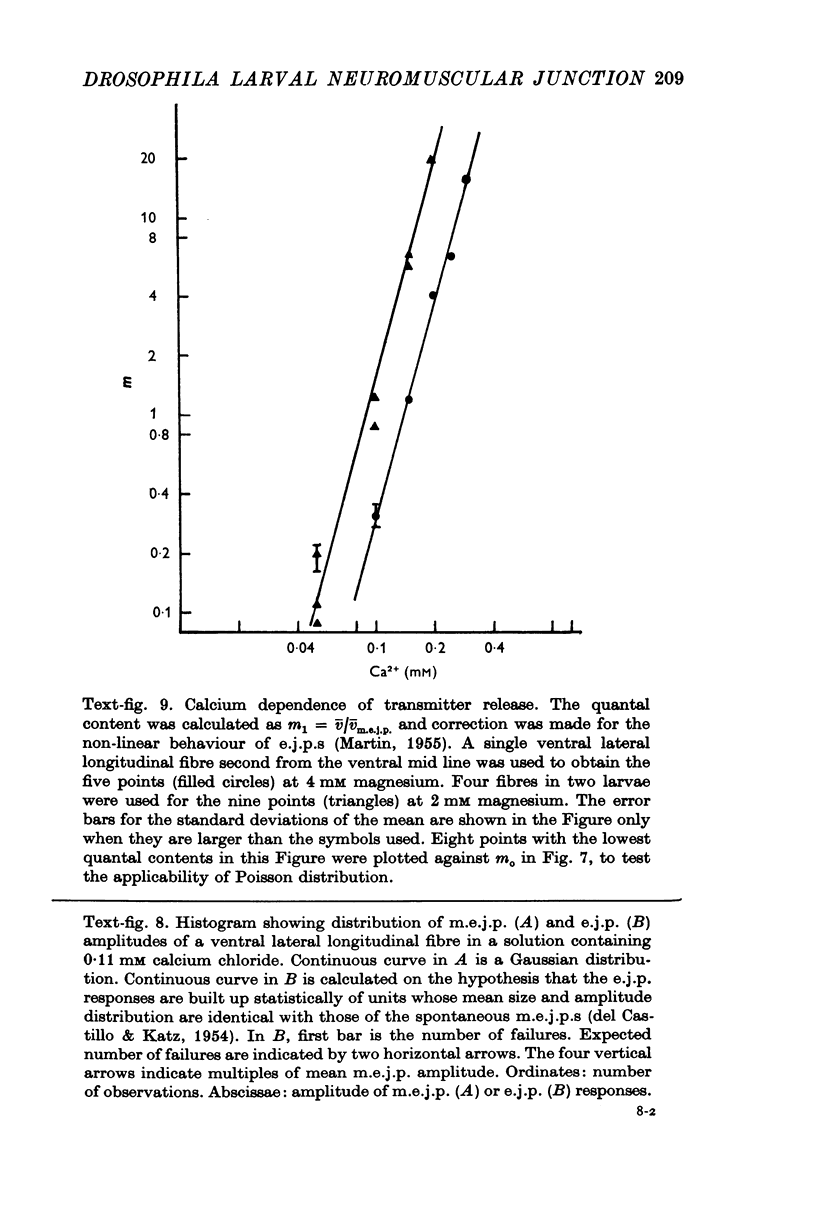
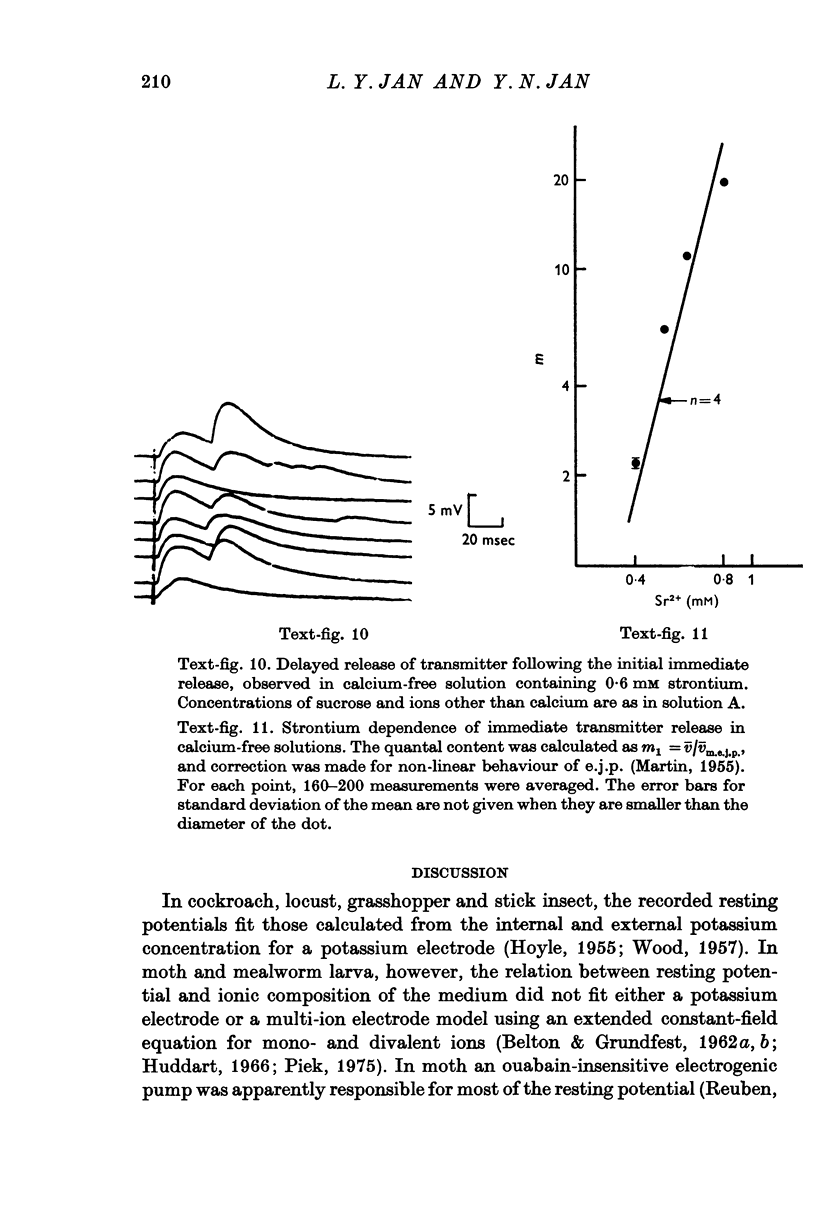
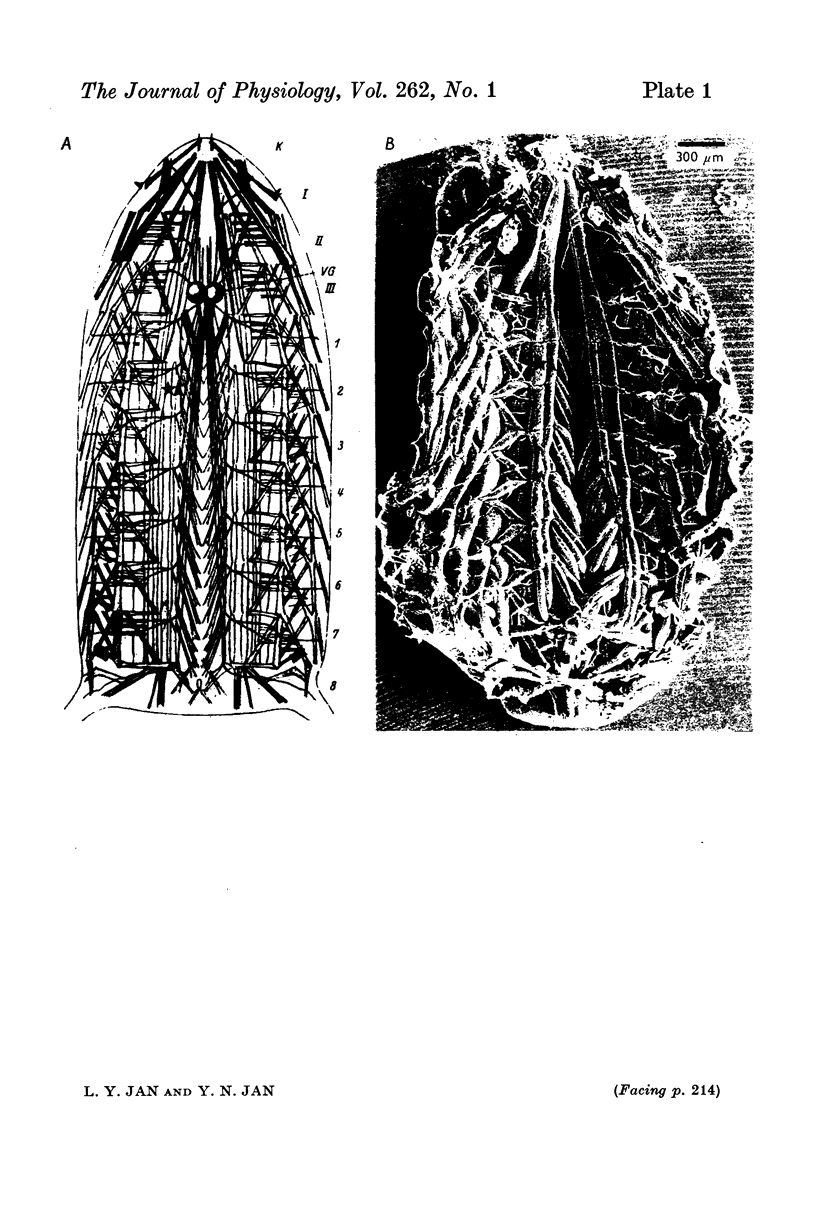
The anatomy and physiology of the Drosophila larval neuromuscular junction were studied. 2. The dependence of muscle resting potentials on [K+]o and [Na+]o follows the Goldman-Hodgkin-Katz equation (PNa/PK=0-23). Chloride ions distribute passively across the membrane. 3. The mean specific membrane resistance of muscle fibres is 4-3 X 10(3) omega cm2, and the mean specific membrane capacitance is 7-1 muF/cm2. The muscle fibre is virtually isopotential. 4.Transmitter release is quantal. Both the miniature excitatory junctional potential and the evoked release follow the Poisson distribution. 5. Transmitter release depends on approximately the fourth power of [Ca2+]o. If Sr2+ replaces Ca2+, it depends on approximately the fourth power of [Sr2+]o. Mg2+ reduces transmitter release without altering the fourth power dependence on [Ca2+]o.
Full text
PDF















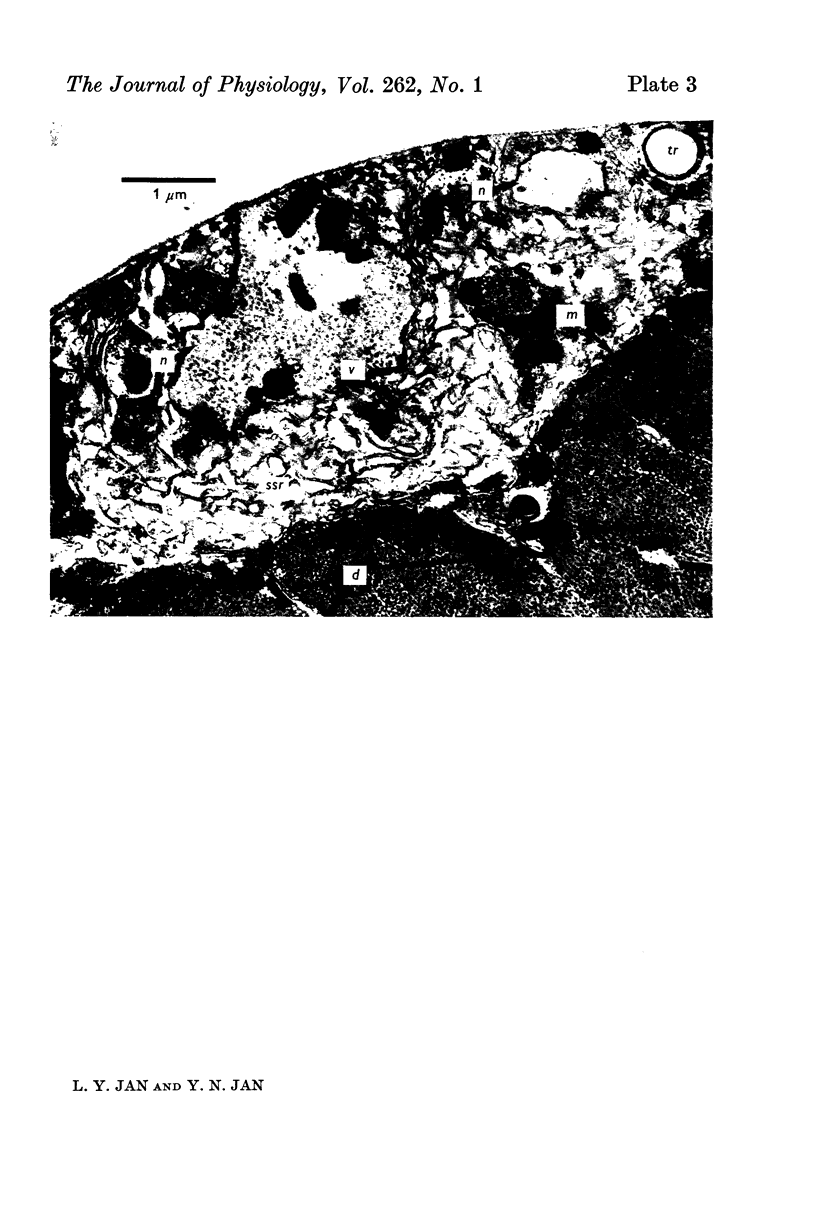
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELTON P., GRUNDFEST H. Potassium activation and K spikes in muscle fibers of the mealworm Iarva (Tenebrio molitor). Am J Physiol. 1962 Sep;203:588–594. doi: 10.1152/ajplegacy.1962.203.3.588. [DOI] [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. The end-plate potential in mammalian muscle. J Physiol. 1956 Apr 27;132(1):74–91. doi: 10.1113/jphysiol.1956.sp005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer S. From the gene to behavior. JAMA. 1971 Nov 15;218(7):1015–1022. [PubMed] [Google Scholar]
- Boyle P. J., Conway E. J. Potassium accumulation in muscle and associated changes. J Physiol. 1941 Aug 11;100(1):1–63. doi: 10.1113/jphysiol.1941.sp003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
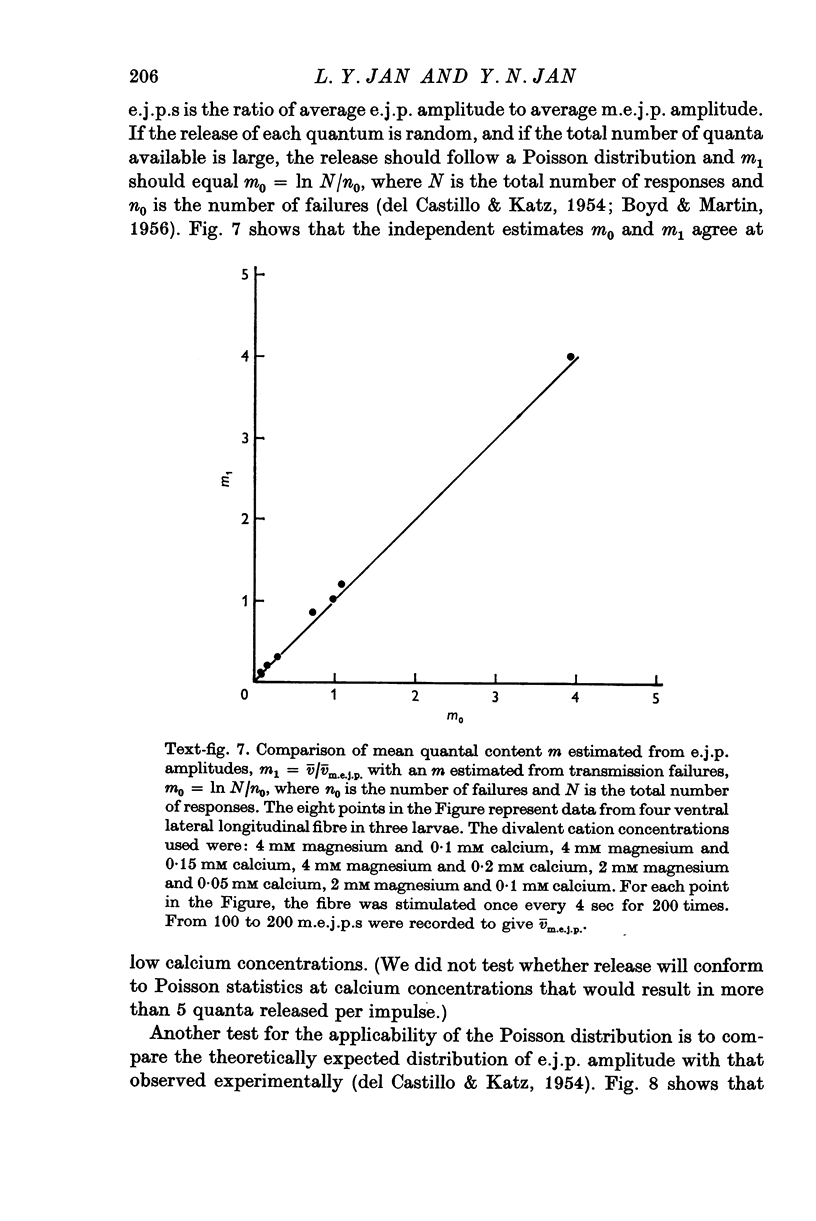
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. The quantal nature of transmission and spontaneous miniature potentials at the crayfish neuromuscular junction. J Physiol. 1961 Mar;155:514–529. doi: 10.1113/jphysiol.1961.sp006644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
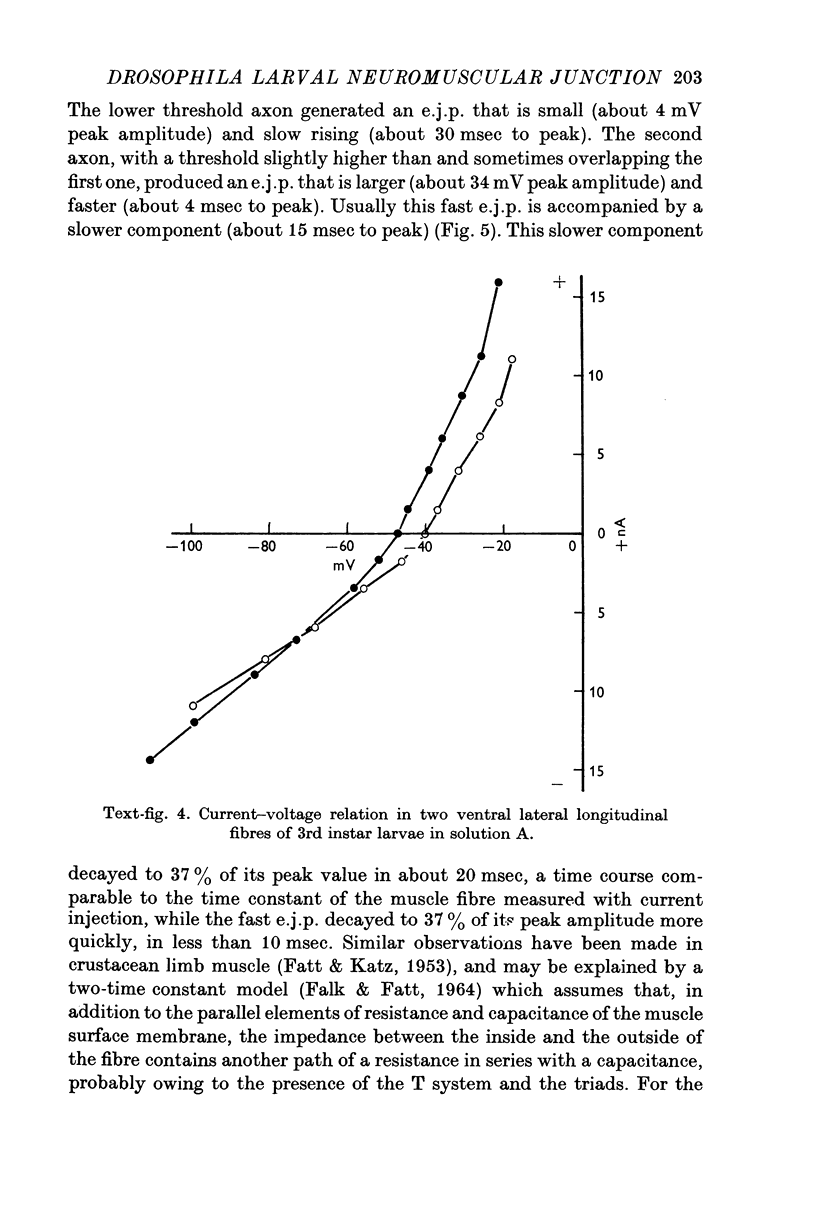
- FALK G., FATT P. LINEAR ELECTRICAL PROPERTIES OF STRIATED MUSCLE FIBRES OBSERVED WITH INTRACELLULAR ELECTRODES. Proc R Soc Lond B Biol Sci. 1964 Apr 14;160:69–123. doi: 10.1098/rspb.1964.0030. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
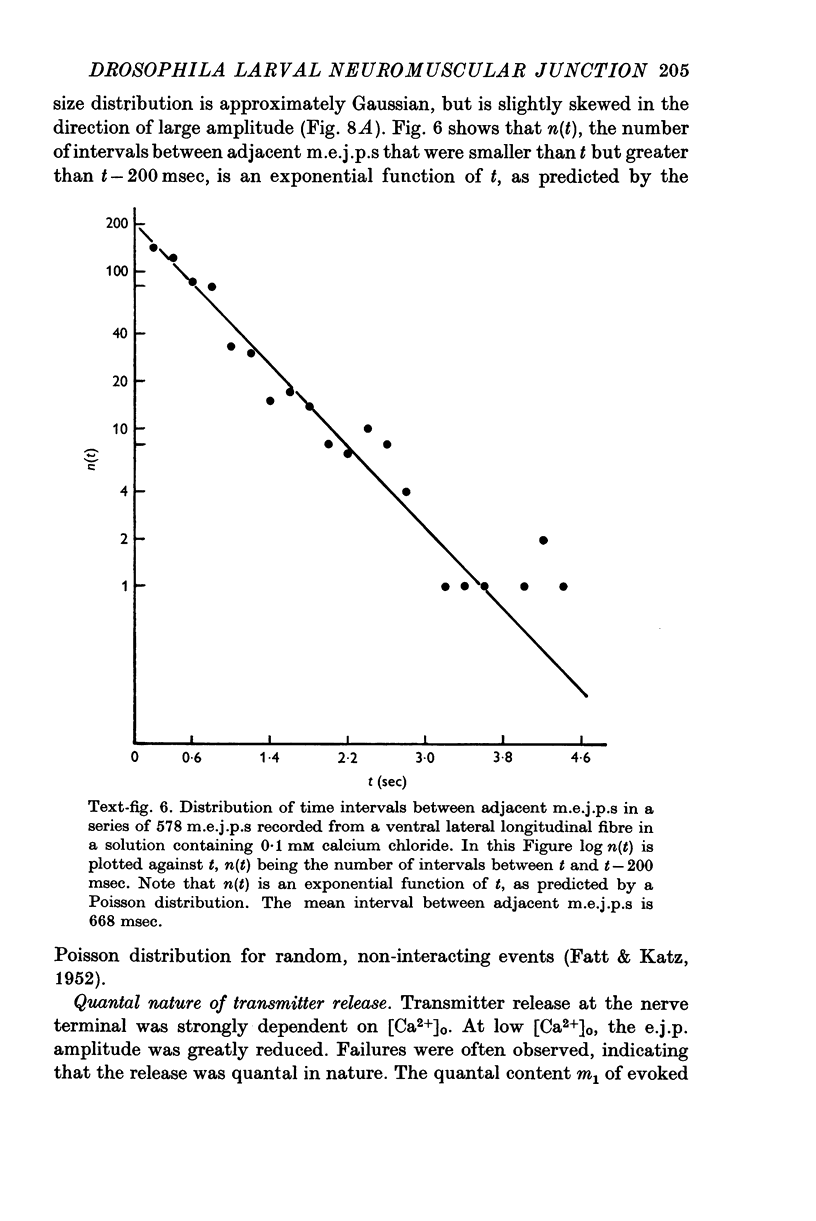
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Grigliatti T. A., Hall L., Rosenbluth R., Suzuki D. T. Temperature-sensitive mutations in Drosophila melanogaster. XIV. A selection of immobile adults. Mol Gen Genet. 1973 Jan 24;120(2):107–114. doi: 10.1007/BF00267238. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYLE G. The effects of some common cations on neuromuscular transmission in insects. J Physiol. 1955 Jan 28;127(1):90–103. doi: 10.1113/jphysiol.1955.sp005240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huddart H. The effect of potassium ions on resting and action potentials in lepidopteran muscle. Comp Biochem Physiol. 1966 May;18(1):131–140. doi: 10.1016/0010-406x(66)90337-9. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Kaplan W. D. Patterned neural activity of a mutant Drosophila melanogaster. Proc Natl Acad Sci U S A. 1970 Jul;66(3):765–772. doi: 10.1073/pnas.66.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K., Ozawa S., Hagiwara S. Synaptic transmission reversibly conditioned by single-gene mutation in Drosophila melanogaster. Nature. 1976 Feb 12;259(5543):489–491. doi: 10.1038/259489a0. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. L-glutamate as an excitatory transmitter at the Drosophila larval neuromuscular junction. J Physiol. 1976 Oct;262(1):215–236. doi: 10.1113/jphysiol.1976.sp011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan W. D., Trout W. E., 3rd The behavior of four neurological mutants of Drosophila. Genetics. 1969 Feb;61(2):399–409. doi: 10.1093/genetics/61.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiri U., Rahamimoff R. Activation of transmitter release by strontium and calcium ions at the neuromuscular junction. J Physiol. 1971 Jul;215(3):709–726. doi: 10.1113/jphysiol.1971.sp009493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas I., Rahamimoff R., Sarney Tonic release of transmitter at the neuromuscular junction of the crab. J Physiol. 1975 Sep;250(2):275–286. doi: 10.1113/jphysiol.1975.sp011054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamimoff R., Yaari Y. Delayed release of transmitter at the frog neuromuscular junction. J Physiol. 1973 Jan;228(1):241–257. doi: 10.1113/jphysiol.1973.sp010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheuben M. B. The resting potential of moth muscle fibre. J Physiol. 1972 Sep;225(3):529–554. doi: 10.1113/jphysiol.1972.sp009954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani E., Steinbach A. B. Resting potential and electrical properties of frog slow muscle fibres. Effect of different external solutions. J Physiol. 1969 Aug;203(2):383–401. doi: 10.1113/jphysiol.1969.sp008869. [DOI] [PMC free article] [PubMed] [Google Scholar]
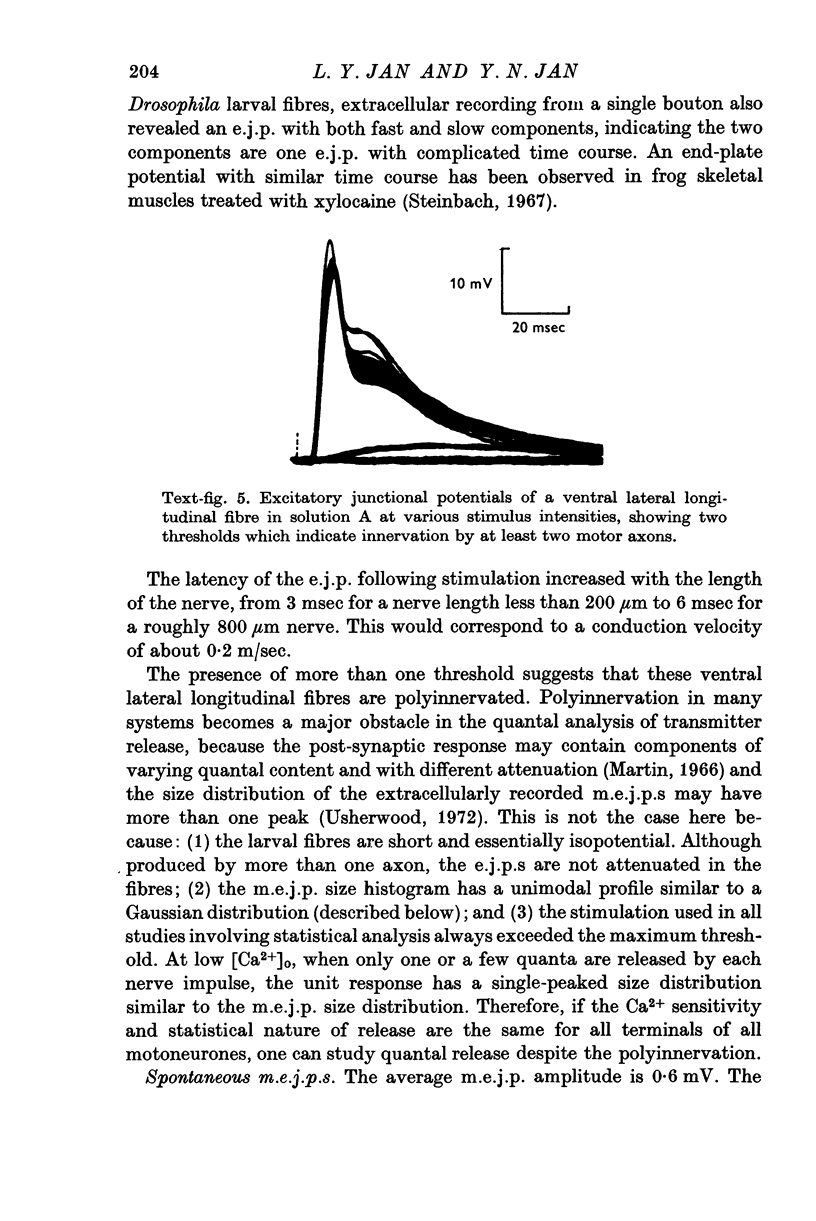
- Steinbach A. B. Unusual endplate potentials which reflect the complexity of muscle structure. Nature. 1967 Dec 30;216(5122):1331–1333. doi: 10.1038/2161331a0. [DOI] [PubMed] [Google Scholar]
- Usherwood P. N. Transmitter release from insect excitatory motor nerve terminals. J Physiol. 1972 Dec;227(2):527–551. doi: 10.1113/jphysiol.1972.sp010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDMANN S. The electrical constants of Purkinje fibres. J Physiol. 1952 Nov;118(3):348–360. doi: 10.1113/jphysiol.1952.sp004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD D. W. The effect of ions upon neuromuscular transmission in a herbivorous insect. J Physiol. 1957 Aug 29;138(1):119–139. doi: 10.1113/jphysiol.1957.sp005841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S. Characteristics of crayfish neuromuscular facilitation and their calcium dependence. J Physiol. 1974 Aug;241(1):91–110. doi: 10.1113/jphysiol.1974.sp010642. [DOI] [PMC free article] [PubMed] [Google Scholar]