Abstract
Our aim was to evaluate the prevalence of bacterial vaginosis and decrease in lactobacillus colonization in women 40 years old or older in relation to menopausal status by evaluation of Gram-stained smears. A total of 1,486 smears from Italian Caucasian women aged 40 to 79 years were examined. Women were classified as follows: fertile (regular cycles) (n = 328), perimenopausal (irregular cycles) (n = 237), and postmenopausal (n = 921), including 331 women on estroprogestinic hormone replacement therapy (HRT). The prevalences of bacterial vaginosis (assessed as a Nugent score of ≥7) in fertile (9.8%) and perimenopausal (11.0%) women were not statistically different, whereas the prevalence was significantly lower overall in postmenopausal women (6.0%) (P = 0.02). Specifically, 6.3% of postmenopausal women without HRT and 5.4% of postmenopausal women with HRT were positive for bacterial vaginosis. The Nugent score system was not adequate for evaluating the normal and intermediate vaginal flora in women over the age of 40 years. High numbers of peri- and postmenopausal women had no lactobacilli and no bacterial-vaginosis-associated microorganisms. This nonpathological absence of lactobacilli in women with a Nugent score of 4 was scored as 4∗, and this group was considered separately from the intermediate flora group. A score of 4∗ was obtained for 2.1% of fertile women, 11.4% of perimenopausal women, 44.1% of postmenopausal women without HRT, and 6.9% of postmenopausal women with HRT. The physiological reduction in lactobacillus colonization of the vagina in postmenopausal women does not cause an increase in bacterial-vaginosis prevalence. Reversion of lactobacillus flora to premenopausal levels due to HRT does not increase the prevalence of bacterial vaginosis in postmenopausal women.
Bacterial vaginosis (BV) is a polymicrobial disorder characterized by an increase in the vaginal pH over 4.5, a reduction in or absence of lactobacillus colonization, and overgrowth of several facultatively and obligately anaerobic bacteria (10). BV is associated with adverse pregnancy outcomes, upper genital tract infections such as pelvic inflammatory disease, endometritis, post-gynecologic-surgery infections, cervicitis, urinary tract infections, cervical intraepithelial neoplasia, and increased risk of sexual acquisition of human immunodeficiency virus infection (7, 13, 16, 17, 19, 24, 25, 29-31). BV is usually diagnosed by the clinical Amsel's criteria (1) or by the Nugent method (26) of Gram stain interpretation. Both these methods, however, were developed to analyze the vaginal flora of pregnant women, and then their use was extended to evaluation of the vagina flora of fertile women. To our knowledge, no clear indications on how to diagnose vaginal flora alterations in peri- and postmenopausal women have been reported. In fact, in postmenopausal women BV cannot be clinically diagnosed by Amsel's criteria (1) because one of these criteria, the vaginal pH value, is constitutively elevated (3, 23); moreover, the scarcity of vaginal discharge makes it difficult to judge the kind of secretion. The Nugent method is based on the assumption that normal women have full vaginal colonization by lactobacilli (26); this is valid for pregnant and fertile women but not for postmenopausal women (3, 15, 23).
Very few studies have evaluated the prevalence of BV in postmenopausal women (3, 15), and no study has assessed the prevalence of BV in perimenopausal women. Moreover, no data are available on the effect of hormone replacement therapy (HRT) on BV prevalence.
The present study is a cohort study to determine the changes in vaginal flora and the prevalence of BV by standardized evaluation of Gram-stained smears for women 40 years old or older as a function of reproductive condition: fertile, perimenopausal, or postmenopausal with or without HRT.
MATERIALS AND METHODS
Study population.
Nonpregnant women aged 40 to 79 years were consecutively recruited during routine gynecologic examinations (Papanicolaou [Pap] smear tests) in three clinics (located in Udine, Bologna, and Trieste) in northern Italy from April 1998 to April 2001. Women were enrolled after giving informed consent according to local Ethics Committee guidelines, and clinical research was conducted in accordance with guidelines for human experimentation issued by the authors' institutions. As a routine practice, at the time of scheduling of the Pap test, patients were requested to refrain from sexual intercourse and from any vaginal treatment for 3 days prior to their checkups. All women were Caucasian; 92% had ≥8 years of education, and 67% had ≥13 years of education. All women included were eligible for the Pap test (no bleeding or major vaginal inflammatory signs) and had no malignancies or severe medical illnesses. None had overt yeast vaginitis, and none was positive for Neisseria gonorrhoeae infection. Women with positive Trichomonas vaginalis results (on a wet smear and/or Pap smear exam) were excluded. A Chlamydia trachomatis test by ligase chain reaction (LCx; Abbott Diagnostics, Rome, Italy) was performed on the first 500 women enrolled. Because only one woman was positive (0.2% C. trachomatis prevalence), further tests were not carried out. Further exclusion criteria were partial or total hysterectomy, menopause induced by drug treatments, HRT terminated less than 6 months earlier, HRT taken for less than 3 months, vaginal estrogenic treatment, progestinic treatment in perimenopause, and tamoxifen or analogous antiestrogen drug therapy. When interviewed at the times of their visits, women included in the study said that they had not had sexual intercourse or engaged in any vaginal practice (such as douching or using vaginal suppositories) in the past 3 days and had not used antibiotics in the past 2 weeks. Among 1,557 eligible women over the age of 40, 71 were excluded for reasons such as an incomplete questionnaire, positive cervical intraepithelial neoplasia discovered as a result of the Pap test performed at the time of the visit, or an inadequate vaginal secretion smear, leaving a study base of 1,486 participants for analysis.
The mean age ± standard deviation of the 1,486 women enrolled was 53.4 ± 7.0 years.
Groups of women.
Postmenopausal condition was defined as the absence of natural menses for at least 12 months.
From the study base of 1,486 women, four different groups of participants were identified. The first group consisted of 328 fertile women who had regular menses. The mean age of this group was 45.3 ± 3.7 years (range, 40 to 55 years);
The second group consisted of 237 perimenopausal women who had irregular menses, or absence of menstruation for less than 12 months (8). The mean age was 50.1 ± 3.0 years (range, 40 to 58 years);
The third group consisted of 590 postmenopausal women who had not received HRT in the past 6 months (95% had never received HRT). The mean age was 58.1 ± 6.0 years (range, 42 to 79 years); the mean age of onset of menopausal status was 50.2 ± 3.6 years (range, 33 to 59 years); the average number of postmenopausal years was 7.9 ± 6.1 (range, 1 to 34 years);
The fourth group consisted of 331 postmenopausal women currently receiving oral or transdermal estroprogestinic replacement therapy. The mean age was 55.4 ± 4.4 years (range, 42 to 69 years); the mean onset of menopausal status was 49.3 ± 4.1 years (range, 32 to 58 years); the average number of postmenopausal years was 6.1 ± 3.5 (range, 1 to 26 years). The average time of HRT was 3.9 ± 2.6 years. One hundred ninety-eight of these women were receiving transdermal estrogenic treatment, and 133 were on oral treatment; 9 of those on oral treatment were receiving tibolone. Progestinic treatment was transdermal in 25 cases, vaginal in 2 cases, and oral in the remaining cases. Two hundred sixty-eight protocols were sequential; the rest were combined estroprogestinic protocols.
Overall, 921 women were postmenopausal, with a physiological termination of menstruation. Their mean age was 57.1 ± 5.6 years (range, 42 to 79 years); the mean age of onset of menopausal status was 49.9 ± 3.8 years (range, 32 to 59 years); the average number of postmenopausal years was 7.3 ± 5.4 (range, 1 to 34 years).
Vaginal sample collection.
Samples were collected from the posterior fornix or lateral vaginal wall of nonbleeding women with an Ayre's spatula using a nonlubricated speculum to perform a smear on a glass slide. The smear was then stained according to the Gram procedure. Clue cells and vaginal flora were evaluated on the Gram-stained smear.
Gram-stained-smear evaluation.
The vaginal flora was determined by evaluation of five different fields under oil immersion (magnification, ×1,000). For the first analysis we adopted the Nugent score method (26). Three different morphotypes are quantitatively evaluated: lactobacilli, Gardnerella-like species (including Gardnerella vaginalis, Bacteroides spp., Prevotella spp., and Porphyromonas spp.), and Mobiluncus spp. Lactobacilli and Gardnerella-like morphotypes are ranked by the average number in five fields as follows: 0, <1, 1 to 4, 5 to 30, and >30. These correspond to 4 to 0 points, respectively, for the lactobacillus morphotype and to 0 to 4 points, respectively, for the Gardnerella-like morphotype. The Mobiluncus morphotype (curved rods) is ranked as 0, <1 to 4, and ≥5, corresponding to 0, 1, and 2 points, respectively. The sum of the points assigned to each of the three morphotypes is the final Nugent score of the patient, which is classified as normal (a Nugent score from 0 to 3), intermediate altered flora (a Nugent score from 4 to 6), or full BV (a Nugent score from 7 to 10).
In a more-refined analysis, the absence of lactobacilli, of Gardnerella-like bacteria, and of Mobiluncus spp., which is very frequent in peri- or postmenopausal women, was considered apart from the group with intermediate flora (which denotes a partially pathological change in the vaginal flora). For most of these women only epithelial host cells are visible in the smear; occasionally gram-positive cocci and/or enterobacteriaceae are detectable. In fact, the decrease in lactobacilli is a commonly observed phenomenon in healthy postmenopausal women. Since such a physiological condition characterizes a subgroup of women with a Nugent score of 4, we refer to it below as score 4∗. None of the women with score 4∗ had clue cells.
Women with Nugent scores from 4 to 10 who were positive for clue cells were considered to have abnormal anaerobic vaginal flora (6, 32).
Full lactobacillus colonization was defined as more than 30 lactobacilli observed in all the five (magnification, ×1,000) fields examined.
All the Gram smear evaluations were performed by two independent investigators, with more than 90% agreement; discrepant readings were reexamined, and a third investigator was consulted in case of persistent disagreement.
Statistical analysis.
Univariate comparisons of proportions were carried out by using the Pearson χ2 test. Any P value of <0.05 was considered statistically significant. The SPSS (Statistical Package for Social Sciences) software package was used for data analysis.
RESULTS
Gram-stained smear scores are shown in Table 1, subdivided as Nugent scores 0 to 3, 4 to 6, and 7 to 10 (normal, intermediate, and full BV flora, according to Nugent et al. [26], respectively) and abnormal anaerobic flora (a Nugent score of ≥4 and the presence of clue cells); values are reported for all women as well as for specific subgroups of women defined as fertile, perimenopausal, postmenopausal, and postmenopausal with or without HRT (see Materials and Methods). The overall prevalence of full BV was 7.6%, and that of abnormal anaerobic vaginal flora was 9.9%.
TABLE 1.
Prevalence of normal, intermediate, and full BV vaginal floraa and of abnormal anaerobic florab in specific subgroups women over the age of 40 years
| Group | Total no. of patients | % (no.) of women with a Nugent score of:
|
|||
|---|---|---|---|---|---|
| 0-3 (normal flora) | 4-6 (intermediate flora) | 7-10 (full BV flora) | ≥4 plus clue cells (abnormal anaerobic flora) | ||
| All aged ≥40 yr | 1,486 | 67.8 (1,008) | 24.6 (365) | 7.6 (113) | 9.9 (147) |
| Fertile | 328 | 84.1 (276) | 6.1 (20) | 9.8 (32) | 12.5 (41) |
| Perimenopausal | 237 | 75.1 (178)c | 13.9 (33)c | 11.0 (26) | 13.1 (31) |
| All postmenopausal | 921 | 60.2 (554)c | 33.9 (312)c | 6.0 (55)d | 8.1 (75)d |
| Postmenopausal without HRT | 590 | 46.3 (273)c | 47.5 (280)c | 6.3 (37)d | 8.5 (50)d |
| Postmenopausal with HRT | 331 | 84.9 (281) | 9.7 (32) | 5.4 (18)d | 7.6 (25)d |
According to Nugent (26).
See Materials and Methods.
Significantly different from values obtained for the subgroup of fertile women (P ≤ 0.01).
Significantly different from values obtained for the subgroup of fertile women (P ≤ 0.05).
Fertile and perimenopausal women showed no statistically significant differences in the prevalences of BV and abnormal anaerobic flora (9.8 versus 11.0% [P = 0.639] for BV; 12.5 versus 13.1% [P = 0.838] for abnormal anaerobic flora). For all postmenopausal women together, the prevalences of BV (6.0%) and abnormal vaginal flora (8.1%) were lower than those in fertile women (P = 0.021 and P = 0.020, respectively) and perimenopausal women (P = 0.007 and P = 0.019, respectively). The subgroup of postmenopausal women with HRT had slightly lower prevalences of BV (5.4%) and abnormal anaerobic flora (7.6%) than postmenopausal women without HRT (6.3 and 8.5%, respectively), but the differences were not statistically significant (P = 0.609 for full BV; P = 0.624 for abnormal anaerobic flora).
Postmenopausal women's smears analyzed according to the method of Nugent showed clearly disproportionate figures for the normal and intermediate flora groups. Among postmenopausal women without HRT, only 46.3% (P < 0.001 with respect to fertile women) had normal flora, and as many as 47.5% (P < 0.001 with respect to fertile women) had intermediate flora, i.e., a partially pathological vaginal condition (Table 1).
In a more-refined analysis, the prevalence of score 4∗ (absence of lactobacilli, no Gardnerella-like species, and no Mobiluncus spp., i.e., disappearance of lactobacillus colonization that cannot be ascribed to the presence of BV-associated bacteria) (Fig. 1) was evaluated (Table 2). Fertile women had the lowest prevalence of score 4∗ (2.1%). A 5.4-fold-higher percentage of perimenopausal women than of fertile women had score 4∗ (P < 0.001). For all postmenopausal women together, the prevalence of score 4∗ was nearly 3-fold that among perimenopausal women (P < 0.001) and nearly 15-fold that among fertile women (P < 0.001). The prevalence of score 4∗ was 21-fold elevated in postmenopausal women who were not hormonally treated over that among women with regular menses (P < 0.001). The subgroups of postmenopausal women with and without HRT were markedly different in prevalence of physiological absence of lactobacilli (score 4∗): 6.9% versus 44.1% (P < 0.001). The prevalence of score 4∗ in women receiving HRT was close to that found in all premenopausal women over the age of 40 (the sum of women with regular and irregular menses) (6.9 versus 6.0%; P = 0.582).
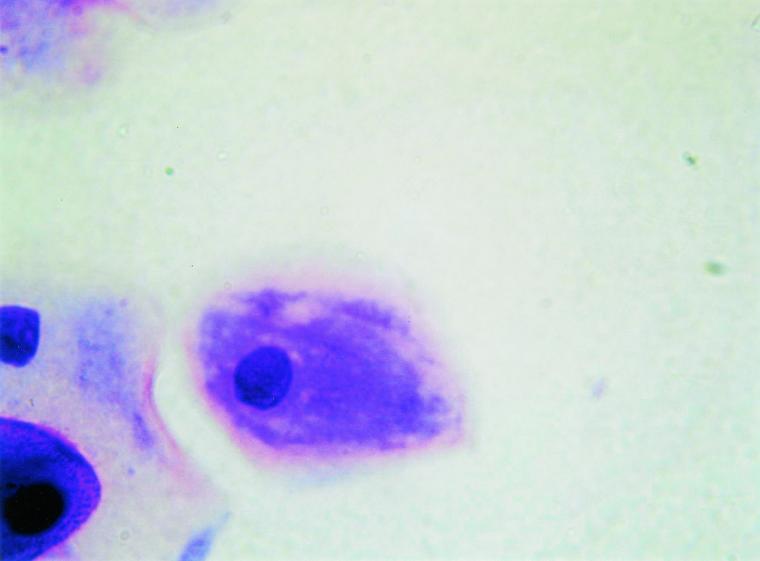
FIG. 1.
Gram-stained vaginal smear from a postmenopausal woman with a score of 4∗ (magnification, ×400). Epithelial cells are visualized; bacteria are absent.
TABLE 2.
Prevalence of women with score 4*a and redefined intermediate and normal flora groups in specific subgroups of women over the age of 40 years
| Group | % (no.) of women with:
|
||
|---|---|---|---|
| Score 4* | Intermediate flora*b | Normal flora*c | |
| All aged ≥40 yr | 21.3 (317) | 3.2 (48) | 89.2 (1,325) |
| Fertile | 2.1 (7) | 4.0 (13) | 86.3 (283) |
| Perimenopausal | 11.4 (27)d | 2.5 (6) | 86.5 (205) |
| All postmenopausal | 30.7 (283)d | 3.1 (29) | 90.9 (837)e |
| Postmenopausal without HRT | 44.1 (260)d | 3.4 (20) | 90.3 (533) |
| Postmenopausal with HRT | 6.9 (23)d | 2.7 (9) | 91.8 (304)e |
Absence of lactobacilli without BV-associated bacteria.
Nugent score of 4 to 6 minus score 4*.
Nugent score of 0 to 3 plus score 4*.
Significantly different from values obtained for the subgroup of fertile women (P ≤ 0.01).
Significantly different from values obtained for the subgroup of fertile women (P ≤ 0.05).
Since a decrease in lactobacillus levels in postmenopausal women is generally considered a normal physiologic change (3, 15, 23), women over the age of 40 with a score of 4∗ appear to be more properly ranked in the normal than in the intermediate vaginal flora group. Accordingly, the number of women with a score of 4∗ was subtracted from the number of women in the group with Nugent scores of 4 to 6, to obtain a redefined intermediate flora group, and was added to the number of women in the group with Nugent scores of 0 to 3 to create a redefined normal group (see Table 2). When score 4∗ was subtracted, the prevalence of intermediate flora in the fertile subgroup was not statistically different from those in all the other subgroups (Table 2). In addition, when score 4∗ was added, there were no statistically significant differences in the prevalence of normal flora between the fertile group, the perimenopausal group, and the group of postmenopausal women without HRT (Table 2).
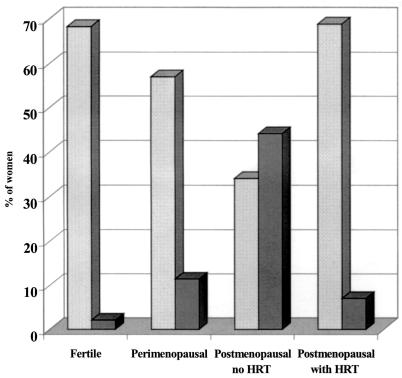
Figure 2 shows the profile of full lactobacillus colonization (see Materials and Methods) and of the physiological absence of vaginal lactobacilli (score 4∗) among the subgroups of women over the age of 40. The fertile women had a very high prevalence of full vaginal lactobacillus colonization (68.3%) in parallel with the lowest prevalence of score 4∗. Full lactobacillus colonization decreases from fertile to perimenopausal to postmenopausal women without HRT. Postmenopausal women without HRT had the lowest percentage of full lactobacillus colonization (34.1%) and the highest prevalence of score 4∗ (44.1%). Women with HRT showed a level of full lactobacillus colonization almost identical to that found in fertile women (68.9 versus 68.3%; P = 0.871). Overall, HRT causes lactobacillus colonization to revert to levels found in premenopausal women without provoking the restoration of conditions causing BV, as evidenced by the fact that BV prevalence in postmenopausal women with HRT is nearly half that found in all premenopausal women (5.4 versus 10.3%; P = 0.012).
FIG. 2.
Profile of full lactobacillus colonization (stippled bars) and of score 4∗ (physiological absence of lactobacilli) (dark shaded bars) in subgroups of women over the age of 40 years.
DISCUSSION
The present study differs from previously published studies on the vaginal flora of postmenopausal women in the larger number of subjects and in the direct comparison among fertile women, perimenopausal women, and postmenopausal women with and without HRT, all of whom belong to a homogeneous ethnic group. We examined women presenting for routine Pap smears in order to specifically assess the prevalence of BV in the general population, since these data are missing in the literature and very recently the necessity for such information has been highlighted (24). The mean age at menopause in the present investigation (49.9 years) is close to that recently reported for the general Italian population (50.9 years) (20).
We examined the vaginal flora by the objective and reproducible evaluation of Gram-stained smears (26), avoiding subjective clinical evaluations (1), because vaginal pH in women close to or in menopausal status is not exclusively a marker of anaerobic colonization (3). To determine the prevalence of BV, we applied the strict definition given by Nugent et al. (26), comprising scores from 7 to 10, but we also considered the enlarged group with abnormal anaerobic vaginal flora, comprising women with Nugent scores of ≥4 and with Gram-stained smears positive for clue cells, as this kind of classification has a sensitivity closer to that of the clinical method (6, 32).
A number of trends emerged from the present study. We found that in the group of all premenopausal women over the age of 40, the prevalence of BV was 10.3% and that of abnormal anaerobic flora was 12.7%. The presence of regular (fertile women) or irregular (perimenopausal women) menses did not statistically affect the percentages of full BV and abnormal anaerobic flora. In the group of all postmenopausal women, the prevalence of BV was 6.0% and that of abnormal anaerobic flora was 8.1%, both statistically lower than those found in premenopausal women. Estroprogestinic treatment of postmenopausal women had no statistically significant effect on either full BV (prevalence, 5.4%) or abnormal anaerobic flora (prevalence, 7.6%). To the best of our knowledge, no previous study has evaluated the effects of HRT on the prevalence of BV or abnormal anaerobic flora.
The absence of lactobacilli without detectable BV-associated microorganisms is a rather rare event in women over the age of 40 who still have regular menses (2.1%), whereas this condition increases markedly in perimenopausal women (11.4%) and is present in as many as 44.1% of postmenopausal women without HRT. We defined this condition as score 4∗ because it constitutes a subset of women with a Nugent score of 4. It appears that this distinction has to be made especially for peri- and postmenopausal women, because the absence of lactobacillus colonization in these women appears to be a normal physiologic condition rather than an intermediate abnormal flora. In fact, in healthy fertile women, full vaginal colonization by lactobacilli is properly considered the normal physiologic condition. However, the mere absence of lactobacillus colonization (without any evidence of Gardnerella-like or Mobiluncus microorganisms) should be distinguished from other conditions resulting in a Nugent score of 4, which derive from a partial decrease in lactobacillus colonization and a limited increase in growth of BV-associated bacteria. This kind of mixed flora, in our opinion, should be more properly defined as intermediate abnormal flora. On this basis, we propose a new scoring system, a refinement of the Nugent score method, that distinguishes score 4∗ among women having a Nugent score of 4. We consider it advisable that our method be adopted for all women regardless of hormonal status.
The large increase in the prevalence of score 4∗ among postmenopausal women without HRT is paralleled by a large decrease in the percentage of women with full lactobacillus colonization (34.1% in postmenopausal women versus 68.3% in fertile women and 57.0% in perimenopausal women). The overall pronounced decrease in lactobacillus levels that we found in postmenopausal women is in line with values obtained in previous studies carried out on more limited numbers of subjects (3, 15, 23).
In our study HRT restores the percentage of full lactobacillus colonization recorded for fertile women. Among postmenopausal women on estroprogestinic treatment, the prevalence of score 4∗ (6.9%) is sixfold reduced from that for postmenopausal women without HRT and is threefold higher than that for fertile women; however, it is close to that found in the group of all premenopausal women (the sum of the fertile and perimenopausal subgroups) over the age of 40 (6.0%). The positive influence of HRT on lactobacilli is a known phenomenon, although we were unable to find quantitative data in the published literature (12, 21, 22). The growth of lactobacilli observed in women receiving estroprogestinic treatment is very likely the consequence of the estrogen-induced increase in glycogen content in vaginal epithelial cells, since glucose derived from the metabolism of glycogen is believed to constitute the main nutritional factor for lactobacilli (which convert it to lactic acid, lowering the vaginal pH) (2, 11, 14). On the other hand, the availability of glucose potentially could also promote the growth of other microorganisms (18). In our study HRT did not increase the prevalence of full BV or of abnormal anaerobic flora. Such a finding highlights the fact that some irreversible changes occur in the postmenopausal vaginal environment. Among possible factors that are not significantly affected by estroprogestinic treatments, but that could be involved in the persistence of BV-associated bacteria in the vagina, are the availability of receptors for bacteria on host cells, variations in the biofilm lining the vaginal epithelium, thinning of the vaginal epithelium, changes in mucus abundance, viscosity, and glycoprotein content, availability of nutritional factors (other than glucose) such as metal ions, especially iron, and changes in the innate and/or adaptive immune response (4-6, 9, 27, 28). The paradox of the postmenopausal condition is that in spite of the impressive decrease in lactobacillus colonization, increase in vaginal pH, increase in levels of gram-positive cocci, and increase in coliform colonization (15), the prevalence of BV is significantly lower than those in perimenopausal and fertile women.
To our knowledge, no detailed data on vaginal flora in perimenopausal women have been reported previously. In our study, perimenopausal women show a grade of lactobacilli which is intermediate between that of women who still have regular cycles and that of postmenopausal women without HRT. Perimenopausal women have almost the same BV prevalence as fertile women, which is roughly twice that of postmenopausal women, indicating that those factors preventing BV-associated microorganism colonization in postmenopausal women have not yet developed. From our findings it follows that, in dealing with the vaginal flora of women over the age of 40, perimenopausal women should be considered as a distinct group of subjects.
Among the limitations of the present investigation is the lack of information on body mass, sexual habits (frequency of sexual intercourse, number of partners over the life span, age of sexual debut), and hygienic, alimentary, and smoking habits. No serum samples were available on which to perform measurements of follicule-stimulating hormone, luteinizing hormone, and estradiol levels.
In summary, our data reveal that postmenopausal status involves major vaginal changes that are not confined to the glycogen content of epithelial cells, which can be modulated by HRT. Other, still unclarified subtle differences occur in the vaginas of postmenopausal women, and their future identification could contribute to understanding of the general mechanisms of colonization by BV-associated microorganisms. Our data show that the absence of lactobacilli by itself is not related to an elevated prevalence of BV, as observed in peri- and postmenopausal women. This finding suggests that the decrease in lactobacillus colonization is more likely a consequence than a leading cause of the anaerobic alteration of the vaginal ecology.
Acknowledgments
This work was supported by Cofin 1998 and Cofin 2000 grants from the “Ministero Istruzione, Università, Ricerca” of Italy, a year 2000 Regione Friuli Venezia Giulia grant, the University of Udine, the University of Trieste, the University of Bologna, and the IRCCS Burlo Garofolo Hospital, Trieste, Italy.
We thank Sandra Flatow, New Haven, Conn., for reading the manuscript, Nunziata Verdolina, Azienda Servizi Sanitari n. 4, Udine, Italy, for nursing assistance, and Marina Cudin, Dipartimento di Scienze e Tecnologie Biomediche, Udine, Italy, for laboratory technical help.
REFERENCES
- 1.Amsel, R., P. A. Totten, C. A. Spiegel, K. C. Chen, D. Eschenbach, and K. K. Holmes. 1983. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 74:14-22. [DOI] [PubMed] [Google Scholar]
- 2.Boskey, E. R., K. M. Telsch, K. J. Whaley, T. R. Moench, and R. A. Cone. 1999. Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infect. Immun. 67:5170-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caillouette, J. C., C. F. Sharp, G. J. Zimmerman, and R. Subir. 1997. Vaginal pH as a marker for bacterial pathogens and menopausal status. Am. J. Obstet. Gynecol. 176:1270-1277. [DOI] [PubMed] [Google Scholar]
- 4.Cauci, S. 1999. Mucosal immune response and microbial factors in bacterial vaginosis. Old Herborn Univ. Semin. Monogr. 12:27-37. [Google Scholar]
- 5.Cauci, S., F. Scrimin, S. Driussi, S. Ceccone, R. Monte, L. Fant, and F. Quadrifoglio. 1996. Specific immune response against Gardnerella vaginalis hemolysin in patients with bacterial vaginosis. Am. J. Obstet. Gynecol. 175:1601-1605. [DOI] [PubMed] [Google Scholar]
- 6.Cauci, S., R. Monte, S. Driussi, P. Lanzafame, and F. Quadrifoglio. 1998. Impairment of the mucosal immune system: IgA and IgM cleavage detected in vaginal washings of a subgroup of patients with bacterial vaginosis. J. Infect. Dis. 178:1698-1706. [DOI] [PubMed] [Google Scholar]
- 7.Cu-Uvin, S., J. W. Hogan, D. Warren, R. S. Klein, J. Peipert, P. Schuman, S. Holmberg, J. Anderson, E. Schoenbaum, D. Vlahov, K. H. Mayer, et al. 1999. Prevalence of lower genital tract infections among human immunodeficiency virus (HIV)-seropositive and high-risk HIV-seronegative women. Clin. Infect. Dis. 29:1145-1150. [DOI] [PubMed] [Google Scholar]
- 8.de Aloysio, D., P. Altieri, P. Penacchioni, M. Mauloni, and F. Bottiglioni. 1996. Premenopause-dependent changes. Gynecol. Obstet. Investig. 42:120-127. [DOI] [PubMed] [Google Scholar]
- 9.Donders, G. G., E. Bosmans, A. Dekeermaecker, A. Vereecken, B. Van Bulck, and B. Spitz. 2000. Pathogenesis of abnormal vaginal bacterial flora. Am. J. Obstet. Gynecol. 182:872-878. [DOI] [PubMed] [Google Scholar]
- 10.Eschenbach, D. A. 1993. History and review of bacterial vaginosis. Am. J. Obstet. Gynecol. 169:441-445. [DOI] [PubMed] [Google Scholar]
- 11.Eschenbach, D. A., S. S. Thwin, D. L. Patton, T. M. Hooton, A. E. Stapleton, K. Agnew, C. Winter, A. Meier, and W. E. Stamm. 2000. Influence of the normal menstrual cycle on vaginal tissue, discharge, and microflora. Clin. Infect. Dis. 30:901-907. [DOI] [PubMed] [Google Scholar]
- 12.Ginkel, P. D., D. E. Soper, R. C. Bump, and H. P. Dalton. 1993. The vaginal flora in post-menopausal women: the effect of estrogen replacement. Infect. Dis. Obstet. Gynecol. 1:94-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harmanli, O. H., G. Y. Cheng, P. Nyirjesy, A. Chatwani, and J. P. Gaughan. 2000. Urinary tract infections in women with bacterial vaginosis. Obstet. Gynecol. 95:710-712. [DOI] [PubMed] [Google Scholar]
- 14.Hillier, S. L., M. A. Krohn, L. K. Rabe, S. J. Klebanoff, and D. A. Eschenbach. 1993. The normal vaginal flora, H2O2-producing lactobacilli, and bacterial vaginosis in pregnant women. Clin. Infect. Dis. 16:S273-S281. [DOI] [PubMed]
- 15.Hillier, S. L., and R. J. Lau. 1997. Vaginal microflora in postmenopausal women who have not received estrogen replacement therapy. Clin. Infect. Dis. 25(Suppl. 2):S123-S126. [DOI] [PubMed]
- 16.Hillier, S. L., R. P. Nugent, D. A. Eschenbach, M. A. Krohn, R. S. Gibbs, D. H. Martin, M. F. Cotch, R. Edelman, J. G. Pastorek, A. V. Rao, D. McNellis, J. A. Regan, J. C. Carey, and M. A. Klebanoff. 1995. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. N. Engl. J. Med. 333:1737-1742. [DOI] [PubMed] [Google Scholar]
- 17.Lamont, R. F., D. J. Morgan, S. D. Wilden, and D. Taylor-Robinson. 2000. Prevalence of bacterial vaginosis in women attending one of the three general practices for routine cervical cytology. Int. J. STD AIDS. 11:495-498. [DOI] [PubMed] [Google Scholar]
- 18.Mårdh, P. A. 1991. The vaginal ecosystem. Am. J. Obstet. Gynecol. 165:1163-1168. [DOI] [PubMed] [Google Scholar]
- 19.McGregor, J. A., and J. I. French. 2000. Bacterial vaginosis in pregnancy. Obstet. Gynecol. Surv. 55:S1-S19. [DOI] [PubMed]
- 20.Meschia, M., F. Pansini, A. B. Modena, D. de Aloysio, M. Gambacciani, F. Parazzini, C. Campagnoli, G. Maiocchi, and E. Peruzzi. 2000. Determinants of age at menopause in Italy: results from a large cross-sectional study. ICARUS Study Group. Italian Climacteric Research Group Study. Maturitas 34:119-125. [DOI] [PubMed] [Google Scholar]
- 21.Miller, L., D. L. Patton, A. Meier, S. S. Thwin, T. M. Hooton, and D. A. Eschenbach. 2000. Depomedroxyprogesterone-induced hypoestrogenism and changes in vaginal flora and epithelium. Obstet. Gynecol. 96:431-439. [DOI] [PubMed] [Google Scholar]
- 22.Milson, I., L. A. Nilsson, A. Brandberg, P. Ekelund, D. Mellström, and O. Eriksson. 1991. Vaginal immunoglobulin A (IgA) levels in post-menopausal women: influence of oestriol therapy. Maturitas 13:129-135. [DOI] [PubMed] [Google Scholar]
- 23.Milson, I., L. Arvidsson, P. Ekelund, U. Molander, and O. Eriksson. 1993. Factors influencing vaginal cytology, pH and bacterial flora in elderly women. Acta Obstet. Gynecol. Scand. 72:286-291. [DOI] [PubMed] [Google Scholar]
- 24.Morris, M., A. Nicoll, I. Simms, J. Wilson, and M. Catchpole. 2001. Bacterial vaginosis: a public health review. Br. J. Obstet. Gynaecol. 108:439-450. [DOI] [PubMed] [Google Scholar]
- 25.Ness, R. B., D. E. Soper, R. L. Holley, J. Peipert, H. Randall, R. L. Sweet, S. J. Sondheimer, S. L. Hendrix, S. L. Hillier, A. Amortegui, G. Trucco, and D. C. Bass. 2001. Douching and endometritis: results from the PID evaluation and clinical health (PEACH) study. Sex. Transm. Dis. 28:240-245. [DOI] [PubMed] [Google Scholar]
- 26.Nugent, R. P., M. A. Krohn, and S. L. Hillier. 1991. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J. Clin. Microbiol. 29:297-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajan, N., Q. Cao, B. E. Anderson, D. L. Pruden, J. Sensibar, J. L. Duncan, and A. J. Schaeffer. 1999. Roles of glycoproteins and oligosaccharides found in human vaginal fluid in bacterial adherence. Infect. Immun. 67:5027-5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reid, G., F. Soboh, A. W. Bruce, and M. Mittelman. 1998. Effect of nutrient composition on the in vitro growth of urogenital lactobacilli and uropathogens. Can. J. Microbiol. 44:866-871. [DOI] [PubMed] [Google Scholar]
- 29.Sewankambo, N., R. H. Gray, M. J. Wawer, L. Paxton, D. McNaim, F. Wabwire-Mangen, D. Serwadda, C. Li, N. Kiwanuka, S. L. Hillier, L. Rabe, C. A. Gaydos, T. C. Quinn, and J. Konde-Lule. 1997. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet 350:546-550. [DOI] [PubMed] [Google Scholar]
- 30.Soper, D. E. 1999. Gynecologic complications of bacterial vaginosis: fact or fiction? Curr. Infect. Dis. Rep. 1:393-397. [DOI] [PubMed] [Google Scholar]
- 31.Sweet, R. L. 2000. Gynecologic conditions and bacterial vaginosis: implications for the non-pregnant patient. Infect. Dis. Obstet. Gynecol. 8:184-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomason, J. L., S. M. Gelbart, R. J. Anderson, A. K. Walt, P. J. Osypowski, and F. F. Broekhuizen. 1990. Statistical evaluation of diagnostic criteria for bacterial vaginosis. Am. J. Ostet. Gynecol. 162:155-160. [DOI] [PubMed] [Google Scholar]