Abstract
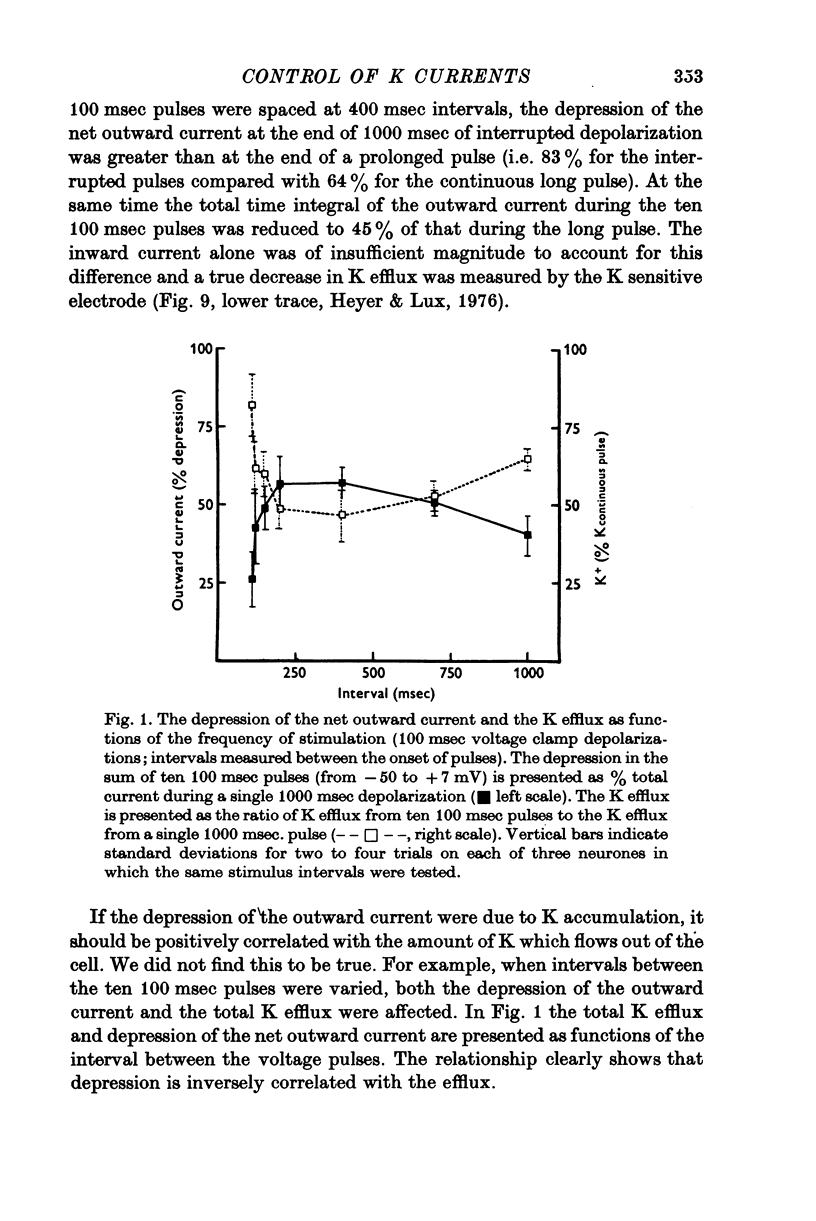
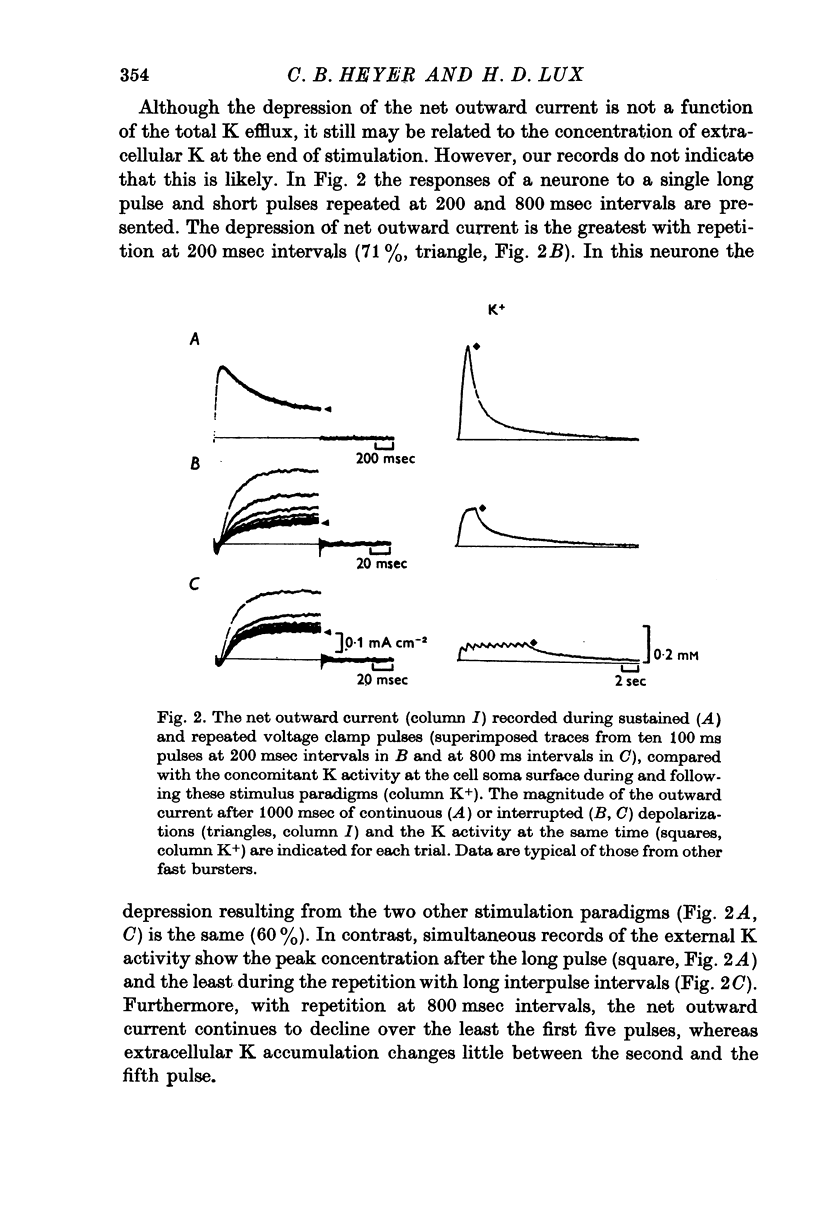
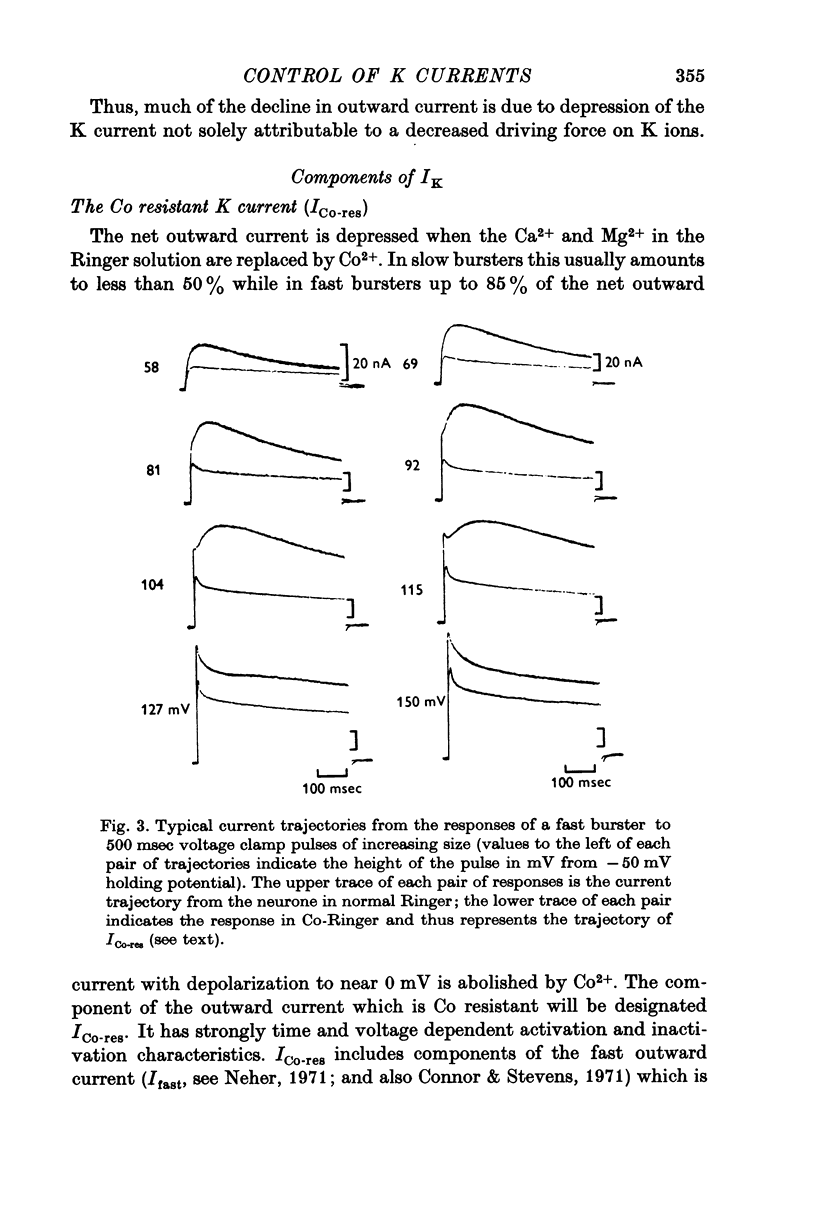
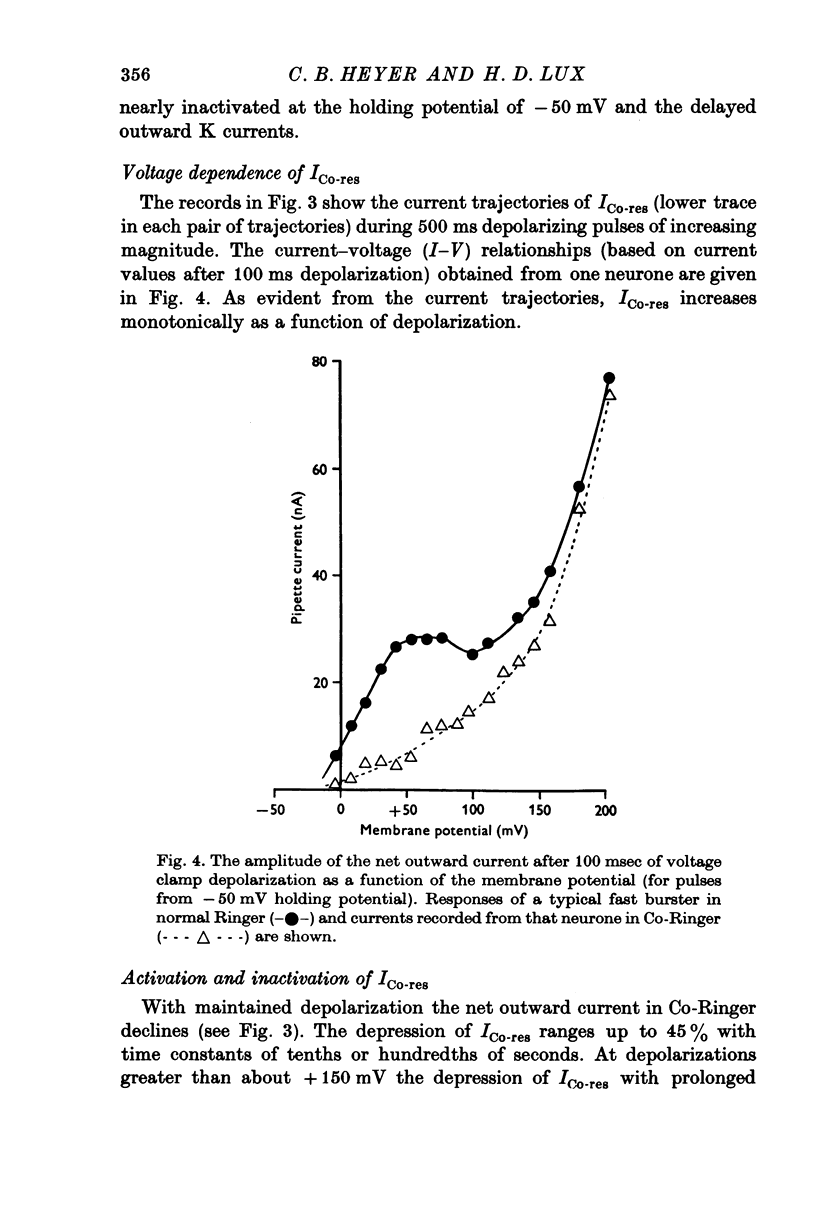
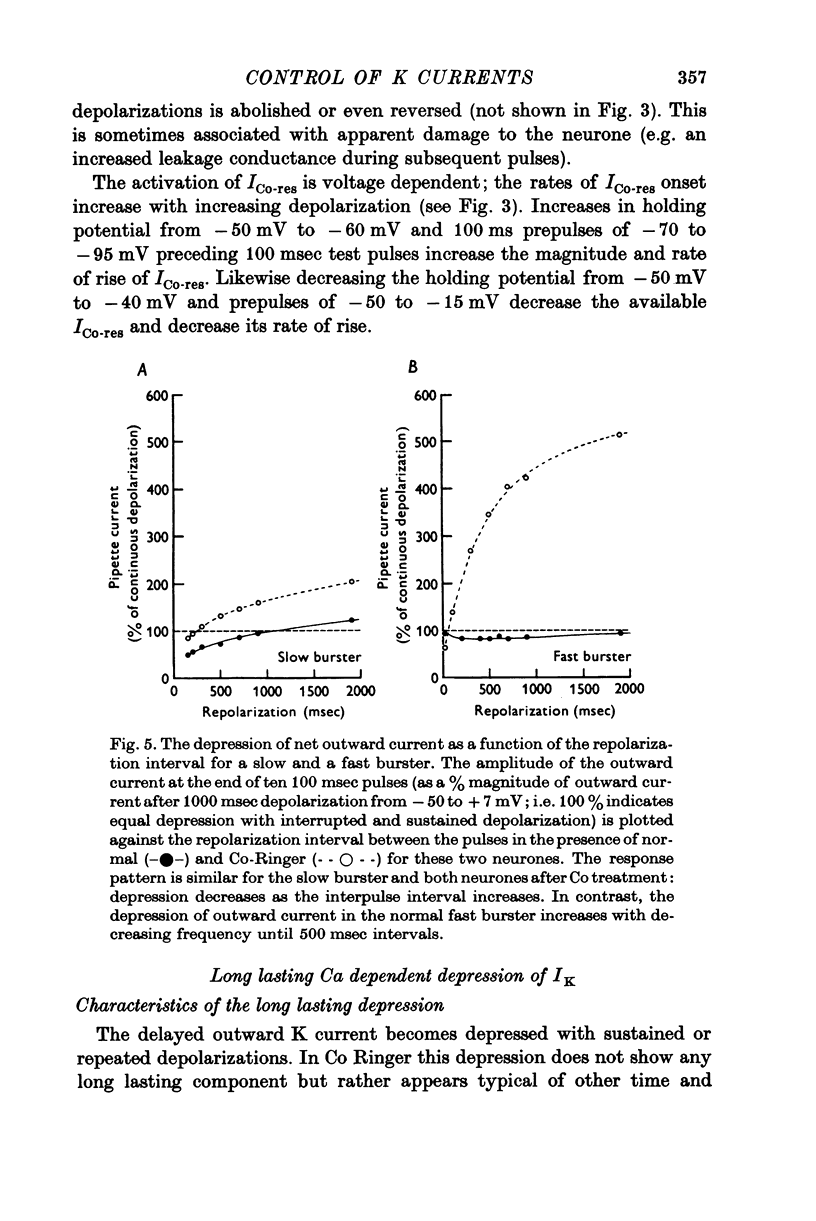
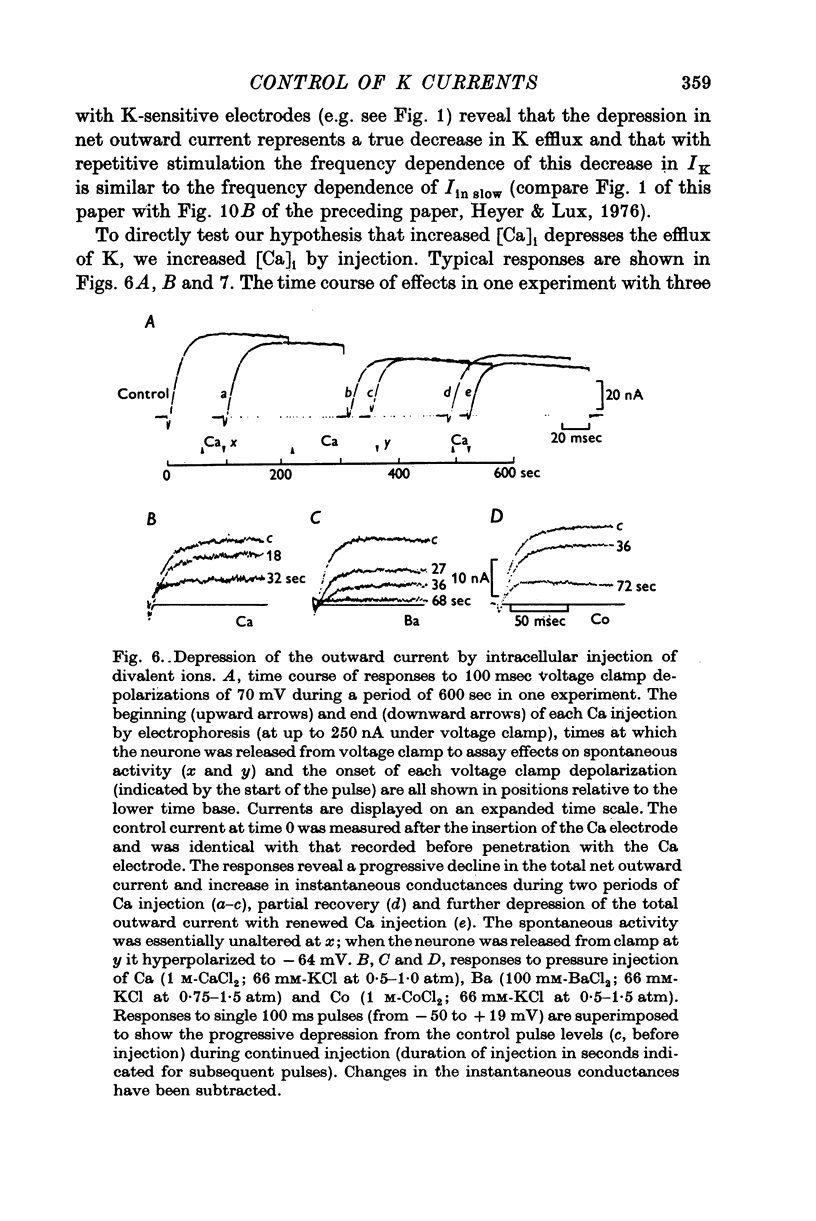
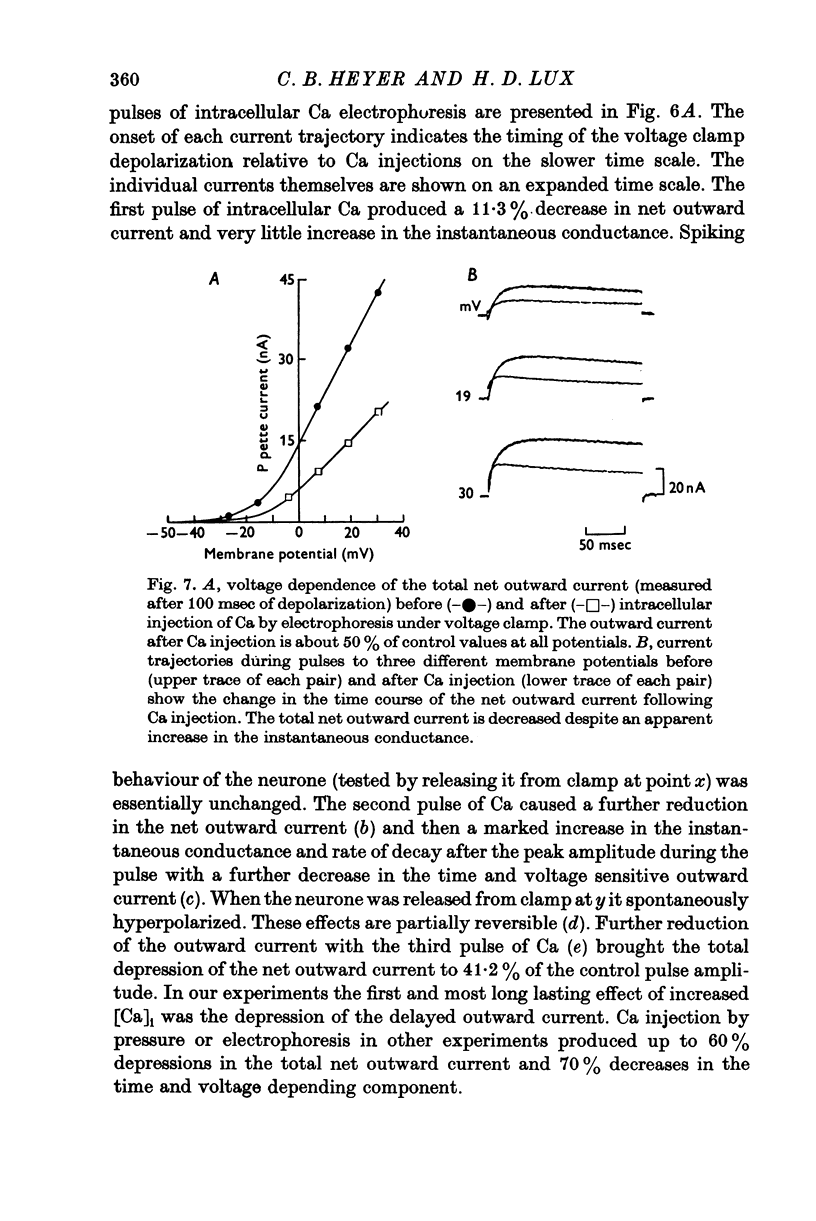
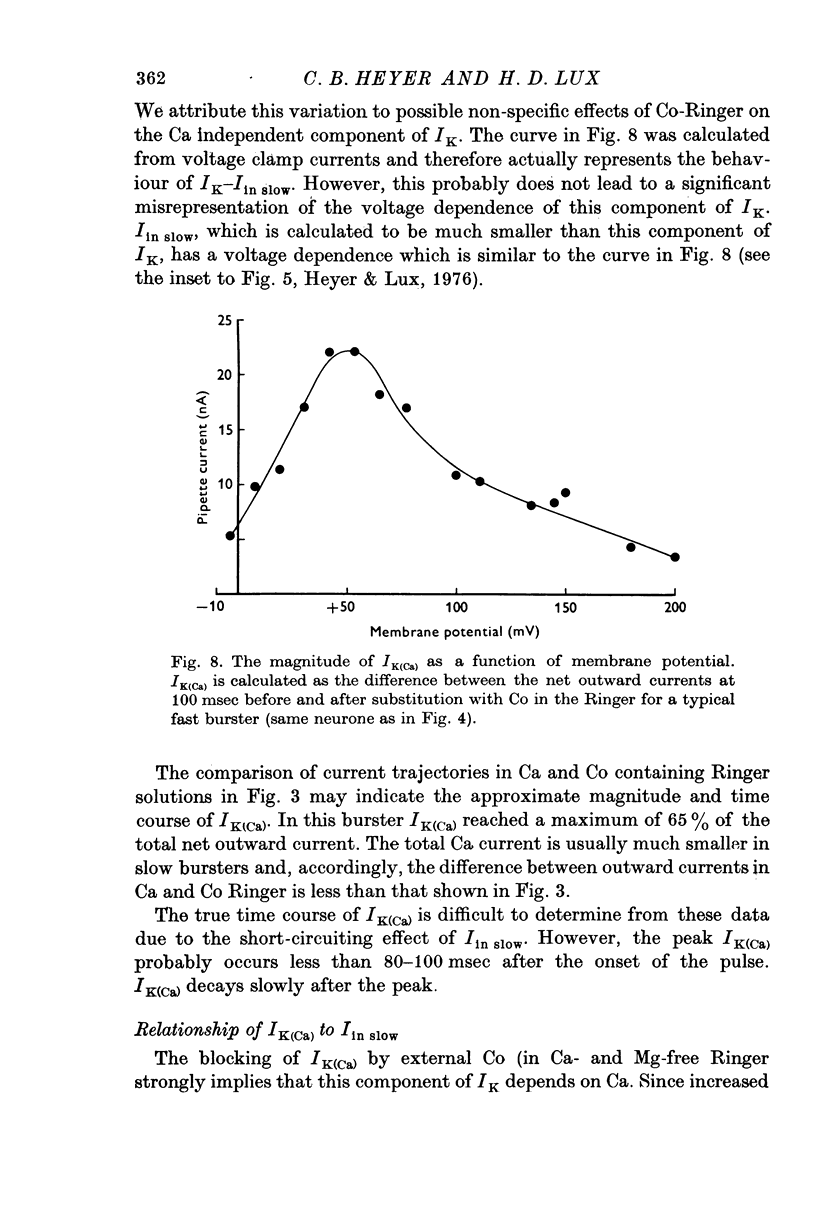
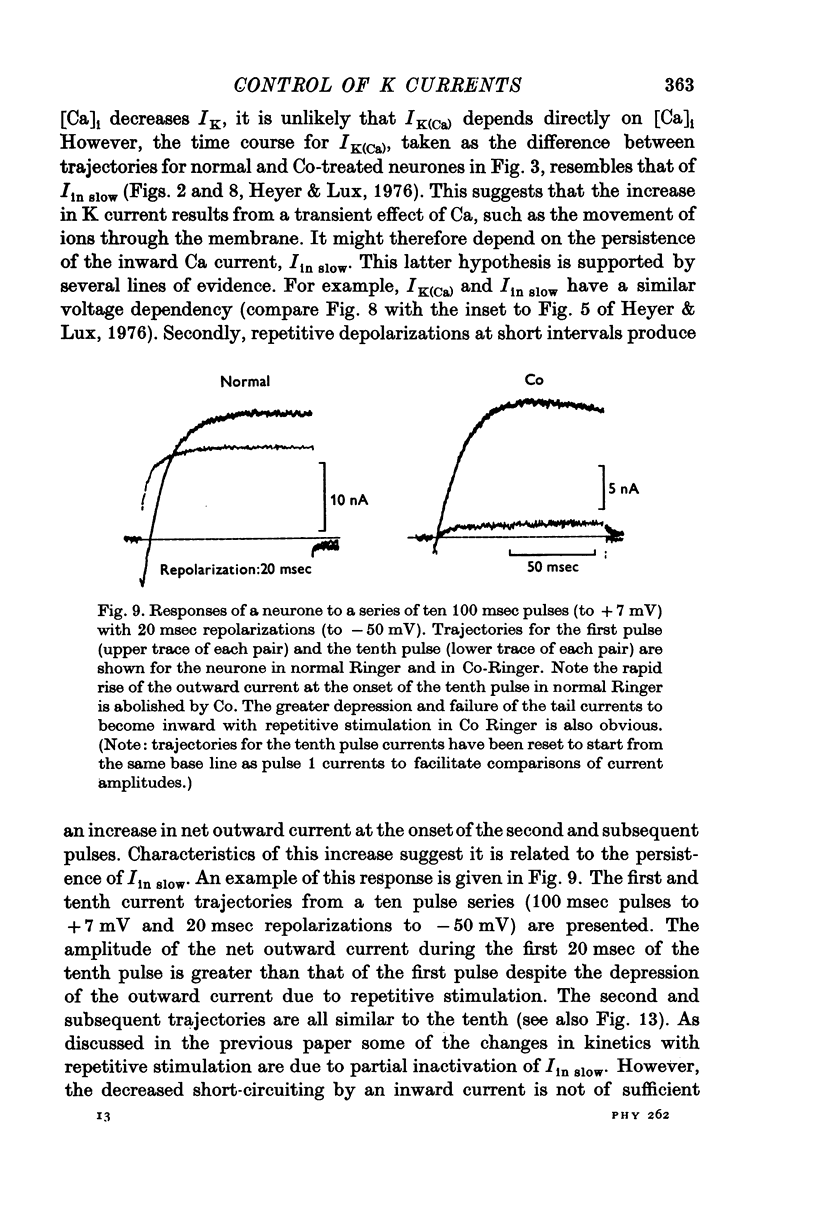
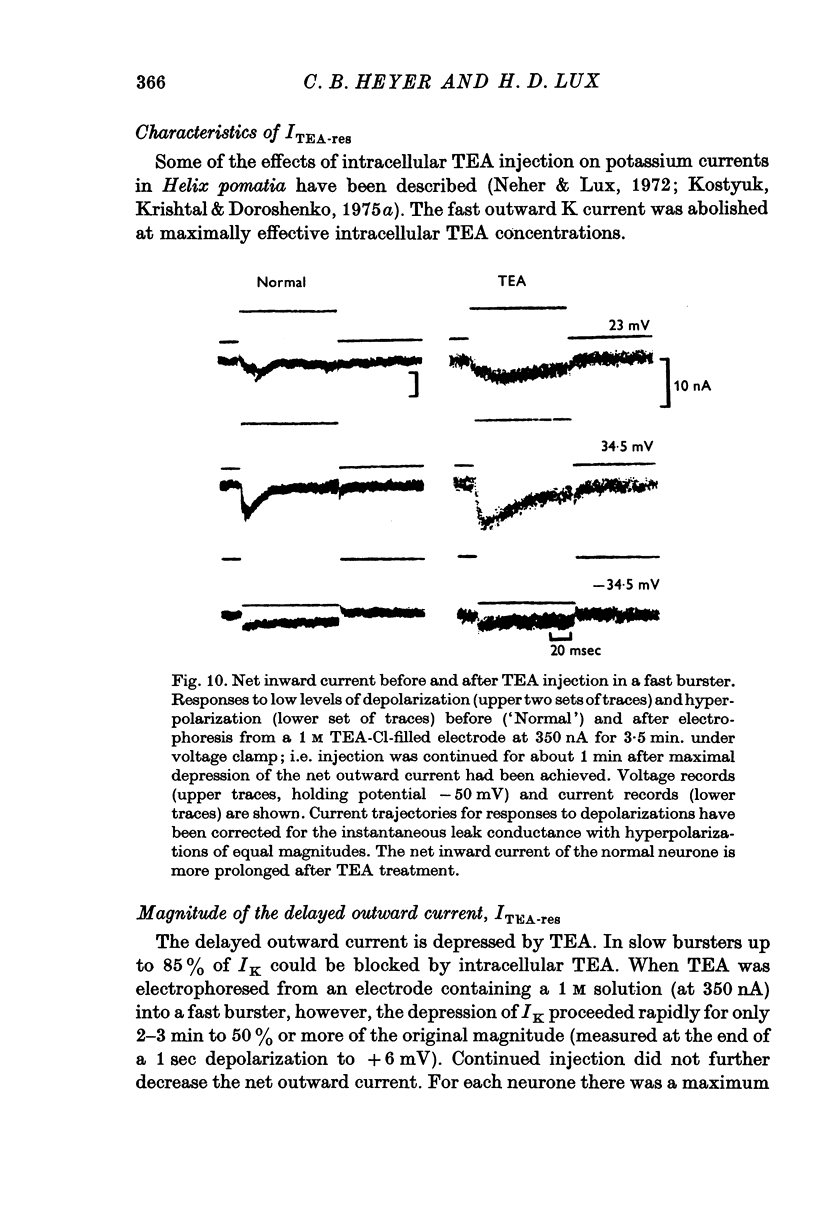
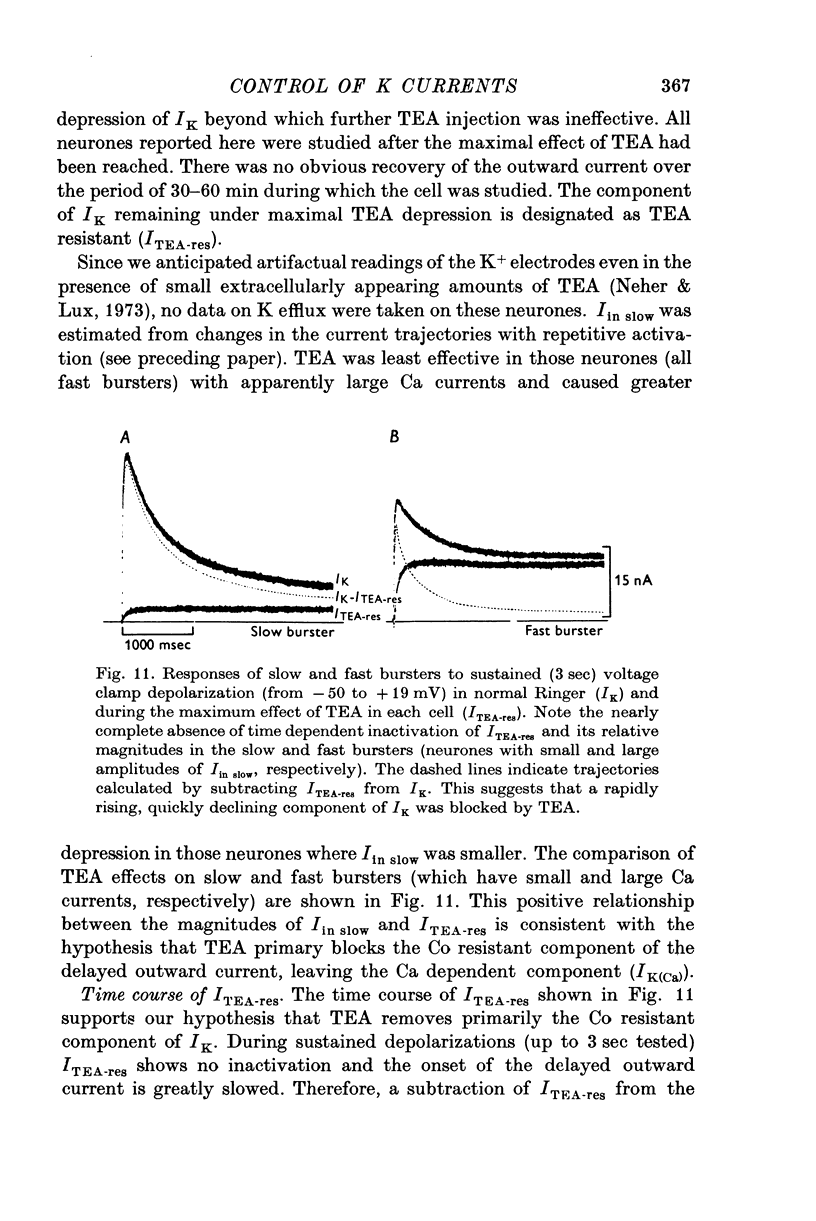
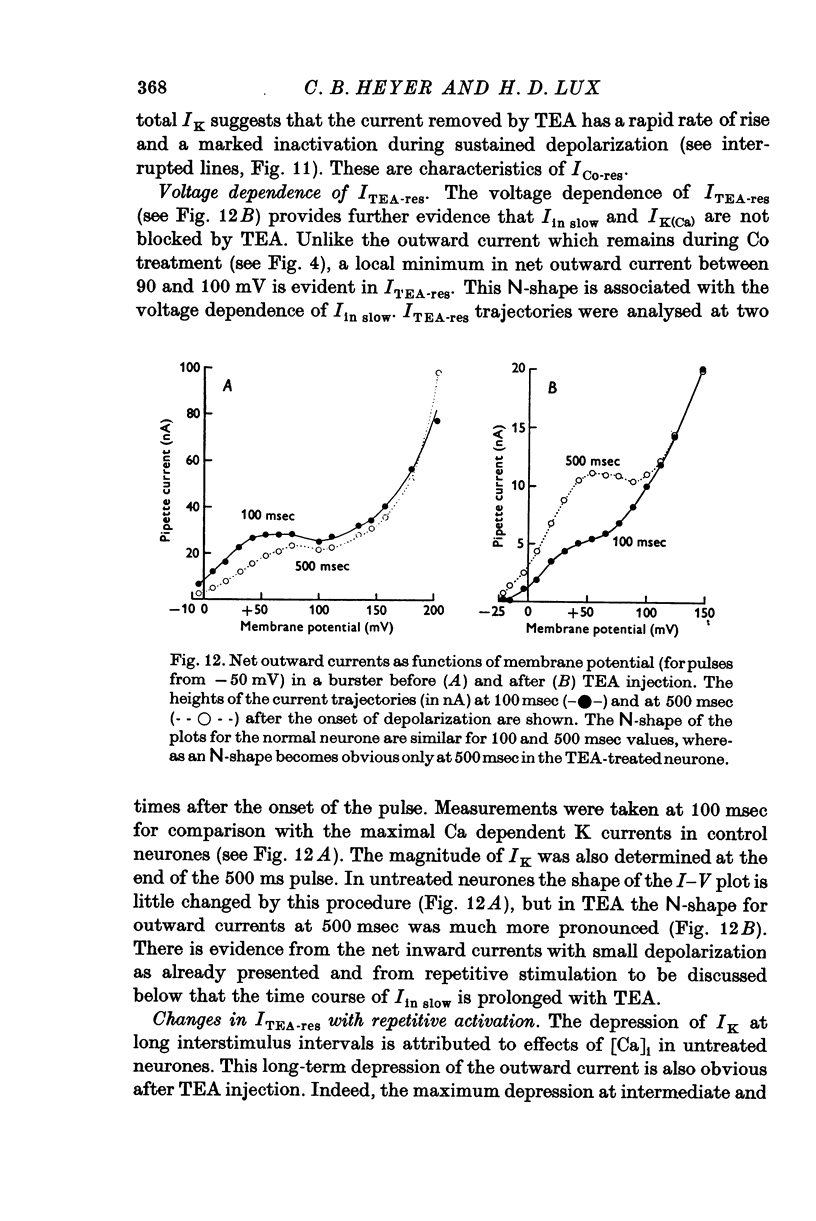
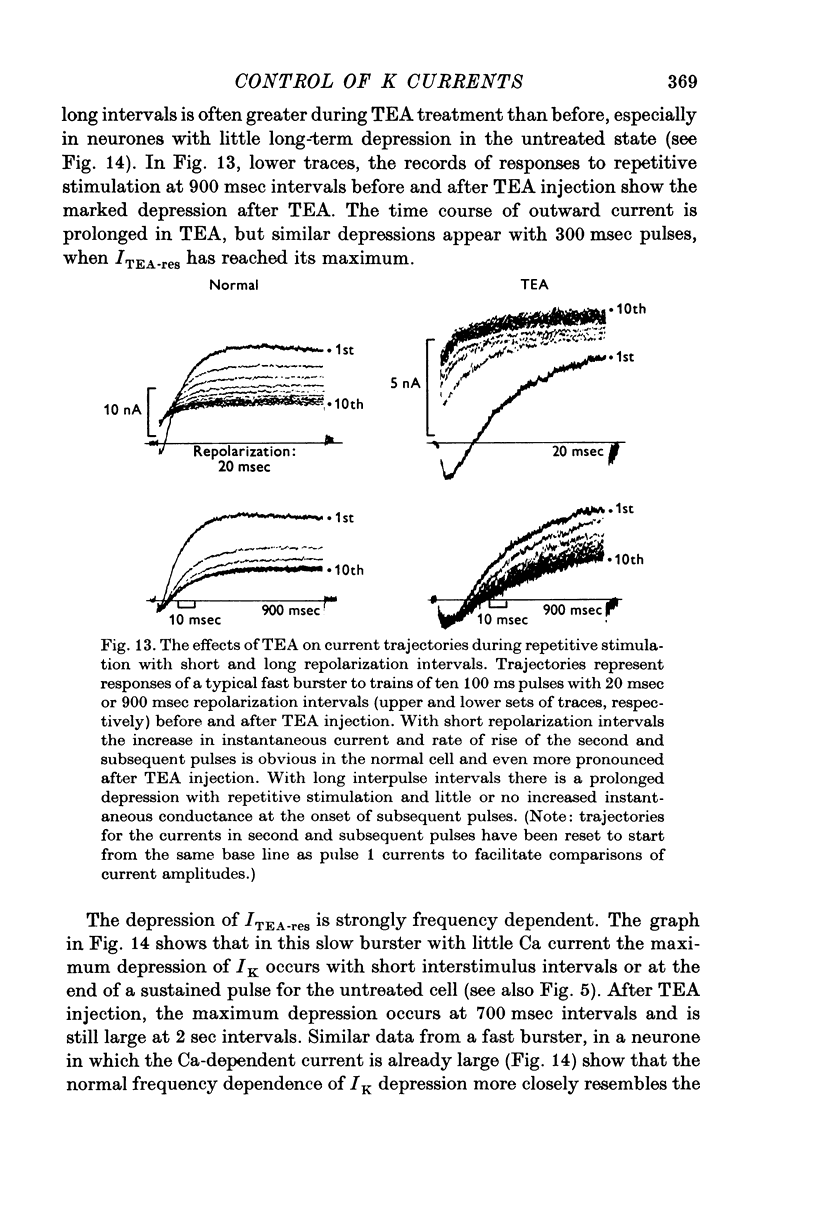
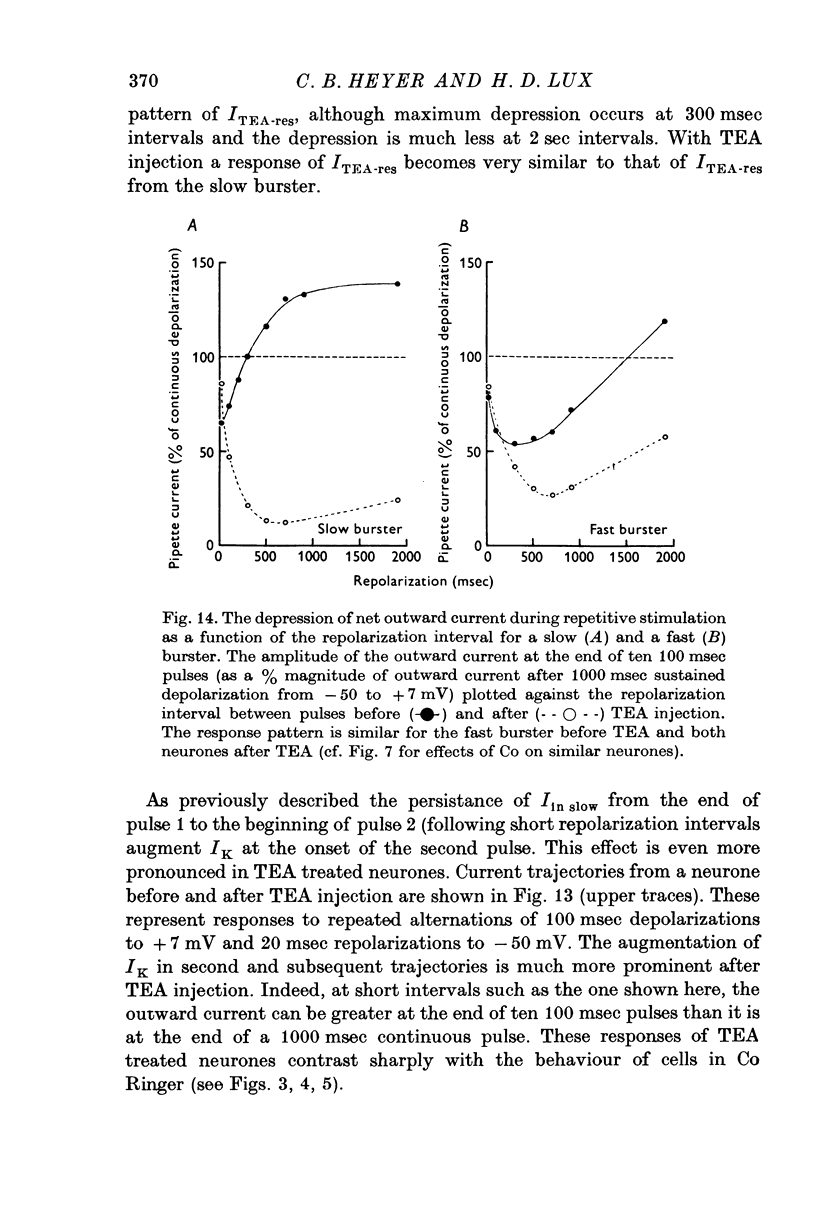
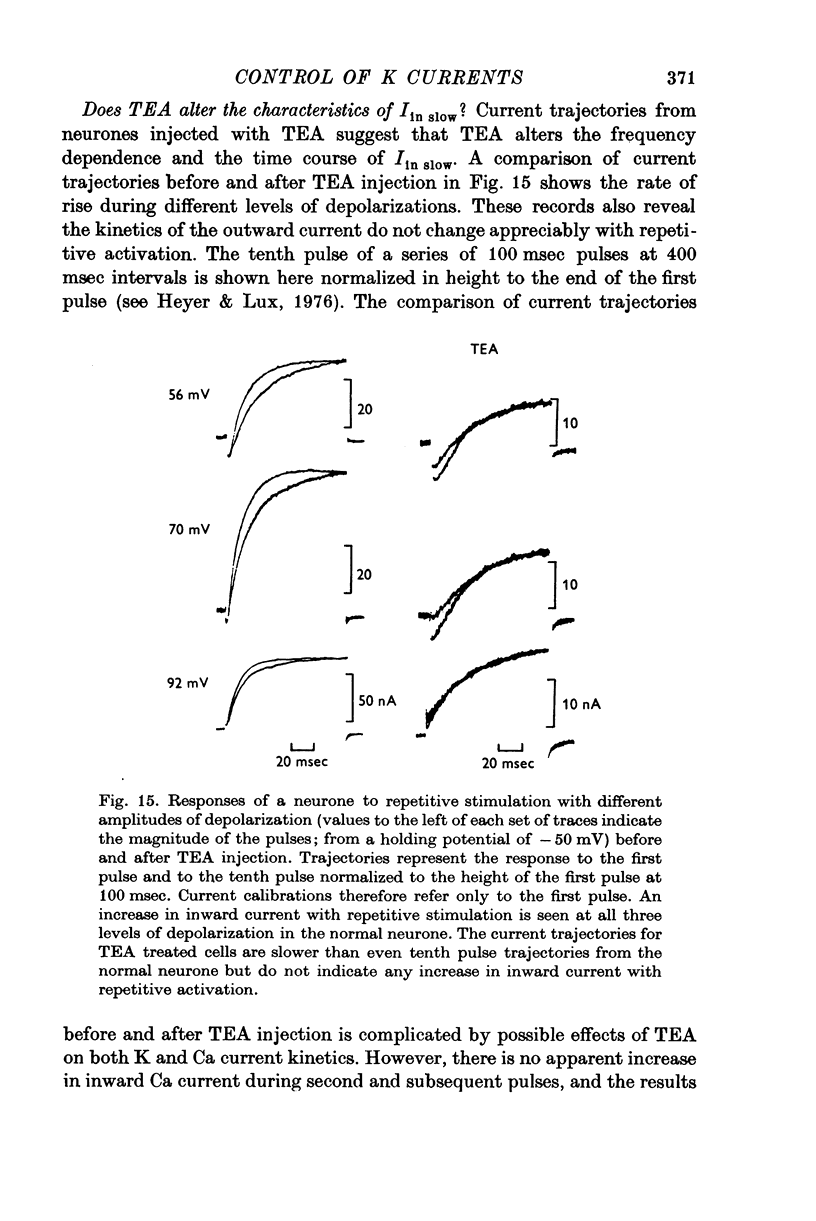
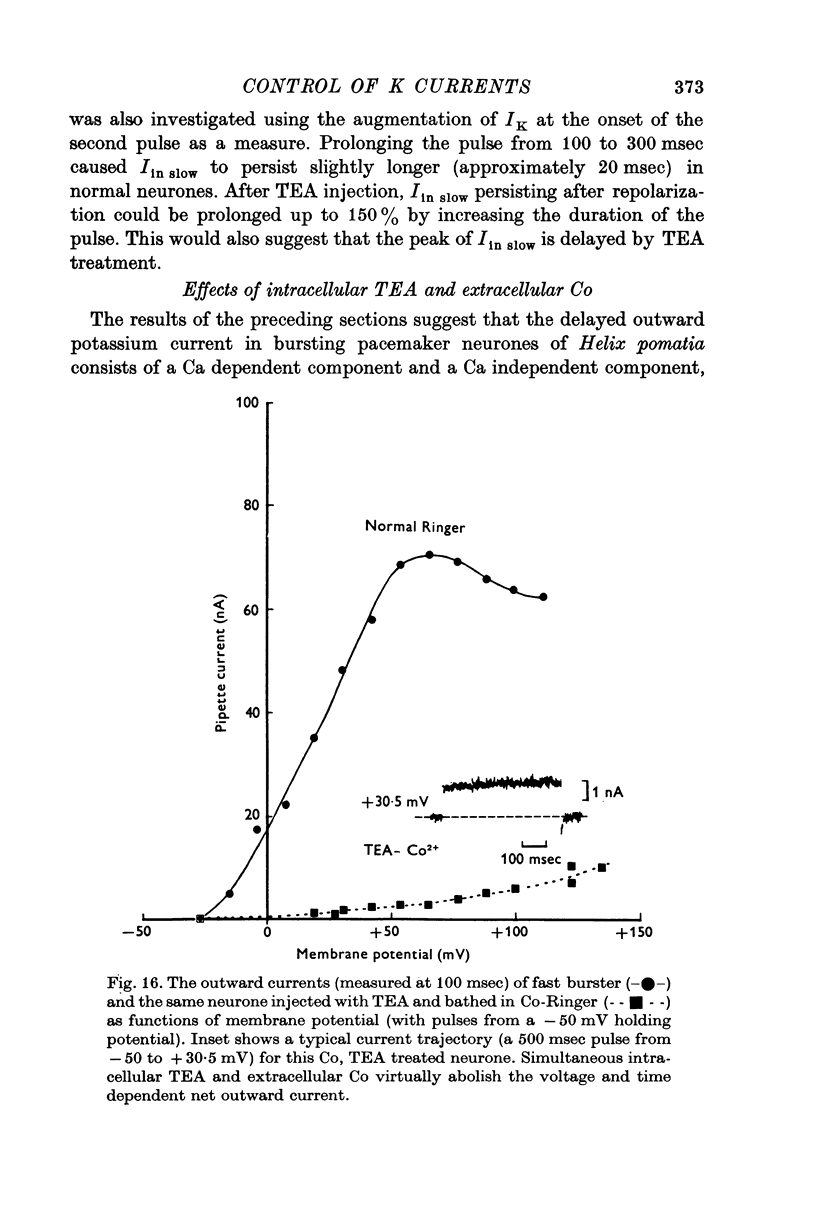
The net outward current in bursting pace-maker neurones of the snail (Helix pomatia) during sustained and repeated voltage clamp pulses was studied. The properties of currents remaining in cobalt-Ringer or after TEA injection were compared with those in untreated cells. 2. With sustained voltage clamp depolarizations the net outward current first increases to a maximum at 150 msec and then declines to 60% or less of its peak intensity. This depression, which is greater during repetition of short pulses (e.g. 100 msec pulses at 0-5 sec intervals), represents a true decrease in the outward flow of K (designated IK) and is not due to a decreased driving force resulting from extracellular K accumulation. The steady-state current-voltage (I-V) relationship for IK is N-shaped (Heyer & Lux, 1976). 3. A component of IK persists when Ca and Mg in the medium are replaced by Co (ICo-res). With voltage clamp depolarizations ICo-res increases rapidly to a maximum and then partially inactivates with voltage dependent time constants of hundredths or tenths of seconds. Repolarization removes the inactivation. Thus, repeated stimulation with short pulses does not increase the depression of ICo-res-ICo-res (e.g. measured during voltage steps from holding potentials of -50 to near 0 mV) is smaller in test pulses preceded by depolarization and larger in pulses preceded by hyperpolarization. The steady state I-V relationship is not N-shaped. ICo-res is blocked by intracellular injection of tetraethylammonium (TEA). 4. Repeated voltage clamp depolarization to near 0 mV with 100 msec pulses for neurones with large Ca currents in normal Ringer produces a long-term depression which is maximal with 300-400 msec repolarizations (to -50 mV) between pulses. This corresponds with stimulus parameters for the maximum Ca current (Heyer & Lux, 1976). Intracellular injection of Ca2+ (also Ba2+ and Co2+) likewise reduces the total net outward current and especially the delayed outward current under voltage clamp. 5. The component of IK which is removed by Co is identified as Ca dependent and designated IK(Ca). With single voltage clamp pulses IK(Ca) follows the approximate time course and voltage dependence of the slow inward Ca current (Iin slow; Heyer & Lux, 1976). Several lines of evidence suggest that Ca ions moving through the membrane activate IK(Ca). 6. Part of IK cannot be blocked by intracellular TEA injection. In different neurones the magnitude of the IK component resistant to TEA (ITEA-res) is approximately proportional to the relative magnitudes of Iin slow.ITEA-res does not inactivate with sustained depolarization and shows pronounced long-term depression with repetitive stimulation at intermediate intervals and an increased outward current at the onset of the second and subsequent pulses following short repolarizations. The steady-state I-V relationship is N-shaped. ITEA-res is abolished by extracellular Co. 7. A net inward current with low depolarizations can be measured after TEA injection...
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG C. M., BINSTOCK L. ANOMALOUS RECTIFICATION IN THE SQUID GIANT AXON INJECTED WITH TETRAETHYLAMMONIUM CHLORIDE. J Gen Physiol. 1965 May;48:859–872. doi: 10.1085/jgp.48.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman W. J., Jr, Palti Y., Senft J. P. Potassium ion accumulation in a periaxonal space and its effect on the measurement of membrane potassium ion conductance. J Membr Biol. 1973 Nov 8;13(4):387–410. doi: 10.1007/BF01868237. [DOI] [PubMed] [Google Scholar]
- Alving B. O. Differences between pacemaker and nonpacemaker neurons of Aplysia on voltage clamping. J Gen Physiol. 1969 Oct;54(4):512–531. doi: 10.1085/jgp.54.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Glitsch H. G. Voltage-dependent changes in the permeability of nerve membranes to calcium and other divalent cations. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):389–409. doi: 10.1098/rstb.1975.0018. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu G., Frank G. B., Inoue F. Tetraethylammonium-induced contractions of frog's skeletal muscle. II. Effects on intramuscular nerve endings. Can J Physiol Pharmacol. 1967 Sep;45(5):833–844. doi: 10.1139/y67-098. [DOI] [PubMed] [Google Scholar]
- Begenisich T., Lynch C. Effects of internal divalent cations on voltage-clamped squid axons. J Gen Physiol. 1974 Jun;63(6):675–689. doi: 10.1085/jgp.63.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clusin W., Spray D. C., Bennett M. V. Activation of a voltage-insensitive conductance by inward calcium current. Nature. 1975 Jul 31;256(5516):425–427. doi: 10.1038/256425a0. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton D. C. Potassium ion accumulation near a pace-making cell of Aplysia. J Physiol. 1972 Jul;224(2):421–440. doi: 10.1113/jphysiol.1972.sp009903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Lux H. D. A non-inactivating inward current recorded during small depolarizing voltage steps in snail pacemaker neurons. Brain Res. 1975 Jan 17;83(3):486–489. doi: 10.1016/0006-8993(75)90840-9. [DOI] [PubMed] [Google Scholar]
- Eckert R., Lux H. D. A voltage-sensitive persistent calcium conductance in neuronal somata of Helix. J Physiol. 1976 Jan;254(1):129–151. doi: 10.1113/jphysiol.1976.sp011225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Fukuda J., Eaton D. C. Membrane currents carried by Ca, Sr, and Ba in barnacle muscle fiber during voltage clamp. J Gen Physiol. 1974 May;63(5):564–578. doi: 10.1085/jgp.63.5.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer C. B., Lux H. D. Properties of a facilitating calcium current in pace-maker neurones of the snail, Helix pomatia. J Physiol. 1976 Nov;262(2):319–348. doi: 10.1113/jphysiol.1976.sp011598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOKETSU K., CERF J. A., NISHI S. Effect of quaternary ammonium ions on electrical activity of spinal ganglion cells in frogs. J Neurophysiol. 1959 Mar;22(2):177–194. doi: 10.1152/jn.1959.22.2.177. [DOI] [PubMed] [Google Scholar]
- Kao C. Y., Stanfield P. R. Actions of some cations on the electrical properties and mechanical threshold of frog sartorius muscle fibers. J Gen Physiol. 1970 May;55(5):620–639. doi: 10.1085/jgp.55.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Tsien R. W. Multiple effects of calcium antagonists on plateau currents in cardiac Purkinje fibers. J Gen Physiol. 1975 Aug;66(2):169–192. doi: 10.1085/jgp.66.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E., Taylor R. E., Vergara J. Calcium and potassium systems of a giant barnacle muscle fibre under membrane potential control. J Physiol. 1973 Mar;229(2):409–455. doi: 10.1113/jphysiol.1973.sp010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C. T. Excitation and contraction in bovine tracheal smooth muscle. J Physiol. 1975 Jan;244(2):263–281. doi: 10.1113/jphysiol.1975.sp010796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhaus A. L., Prichard J. W. Calcium dependent action potentials produced in leech Retzius cells by tetraethylammonium chloride. J Physiol. 1975 Mar;246(2):351–369. doi: 10.1113/jphysiol.1975.sp010894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregenow F. M., Hoffman J. F. Some kinetic and metabolic characteristics of calcium-induced potassium transport in human red cells. J Gen Physiol. 1972 Oct;60(4):406–429. doi: 10.1085/jgp.60.4.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Lisiewicz A. Injections of calcium ions into spinal motoneurones. J Physiol. 1972 Sep;225(2):363–390. doi: 10.1113/jphysiol.1972.sp009945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Nicholson C. Calcium role in depolarization-secretion coupling: an aequorin study in squid giant synapse. Proc Natl Acad Sci U S A. 1975 Jan;72(1):187–190. doi: 10.1073/pnas.72.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux H. D., Eckert R. Inferred slow inward current in snail neurones. Nature. 1974 Aug 16;250(467):574–576. doi: 10.1038/250574a0. [DOI] [PubMed] [Google Scholar]
- Meech R. W. Intracellular calcium injection causes increased potassium conductance in Aplysia nerve cells. Comp Biochem Physiol A Comp Physiol. 1972 Jun 1;42(2):493–499. doi: 10.1016/0300-9629(72)90128-4. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. The sensitivity of Helix aspersa neurones to injected calcium ions. J Physiol. 1974 Mar;237(2):259–277. doi: 10.1113/jphysiol.1974.sp010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minota S. Calcium ions and the post-tetanic hyperpolarization of bullfrog sympathetic ganglion cells. Jpn J Physiol. 1974 Oct;24(5):501–512. doi: 10.2170/jjphysiol.24.501. [DOI] [PubMed] [Google Scholar]
- Mounier Y., Vassort G. Evidence for a transient potassium membrane current dependent on calcium influx in crab muscle fibre. J Physiol. 1975 Oct;251(3):609–625. doi: 10.1113/jphysiol.1975.sp011111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier Y., Vassort G. Initial and delayed membrane currents in crab muscle fibre under voltage-clamp conditions. J Physiol. 1975 Oct;251(3):589–608. doi: 10.1113/jphysiol.1975.sp011110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHI S., SOEDA H. HYPERPOLARIZATION OF A NEURONE MEMBRANE BY BARIUM. Nature. 1964 Nov 21;204:761–764. doi: 10.1038/204761a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Lux H. D. Differential action of TEA + on two K + -current componentss of a molluscan neurone. Pflugers Arch. 1972;336(2):87–100. doi: 10.1007/BF00592924. [DOI] [PubMed] [Google Scholar]
- Neher E., Lux H. D. Rapid changes of potassium concentration at the outer surface of exposed single neurons during membrane current flow. J Gen Physiol. 1973 Mar;61(3):385–399. doi: 10.1085/jgp.61.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi R., Nishiye H. Effect of intracellular tetraethylammonium ion on action potential in the guinea-pig's myocardium. Pflugers Arch. 1974 May 6;348(4):305–316. doi: 10.1007/BF00589220. [DOI] [PubMed] [Google Scholar]
- Osa T. Effects of tetraethylammonium on the electrical activity of pregnant mouse myometrium and the interaction with manganese and cadmium. Jpn J Physiol. 1974 Feb;24(1):119–133. doi: 10.2170/jjphysiol.24.119. [DOI] [PubMed] [Google Scholar]
- Reuter H. Divalent cations as charge carriers in excitable membranes. Prog Biophys Mol Biol. 1973;26:1–43. doi: 10.1016/0079-6107(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Sperelakis N., Schneider M. F., Harris E. J. Decreased K+ conductance produced by Ba++ in frog sartorius fibers. J Gen Physiol. 1967 Jul;50(6):1565–1583. doi: 10.1085/jgp.50.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinnakre J., Tauc L. Calcium influx in active Aplysia neurones detected by injected aequorin. Nat New Biol. 1973 Mar 28;242(117):113–115. doi: 10.1038/newbio242113b0. [DOI] [PubMed] [Google Scholar]
- Vassort G. Voltage-clamp analysis of transmembrane ionic currents in guinea-pig myometrium: evidence for an initial potassium activation triggered by calcium influx. J Physiol. 1975 Nov;252(3):713–734. doi: 10.1113/jphysiol.1975.sp011167. [DOI] [PMC free article] [PubMed] [Google Scholar]


