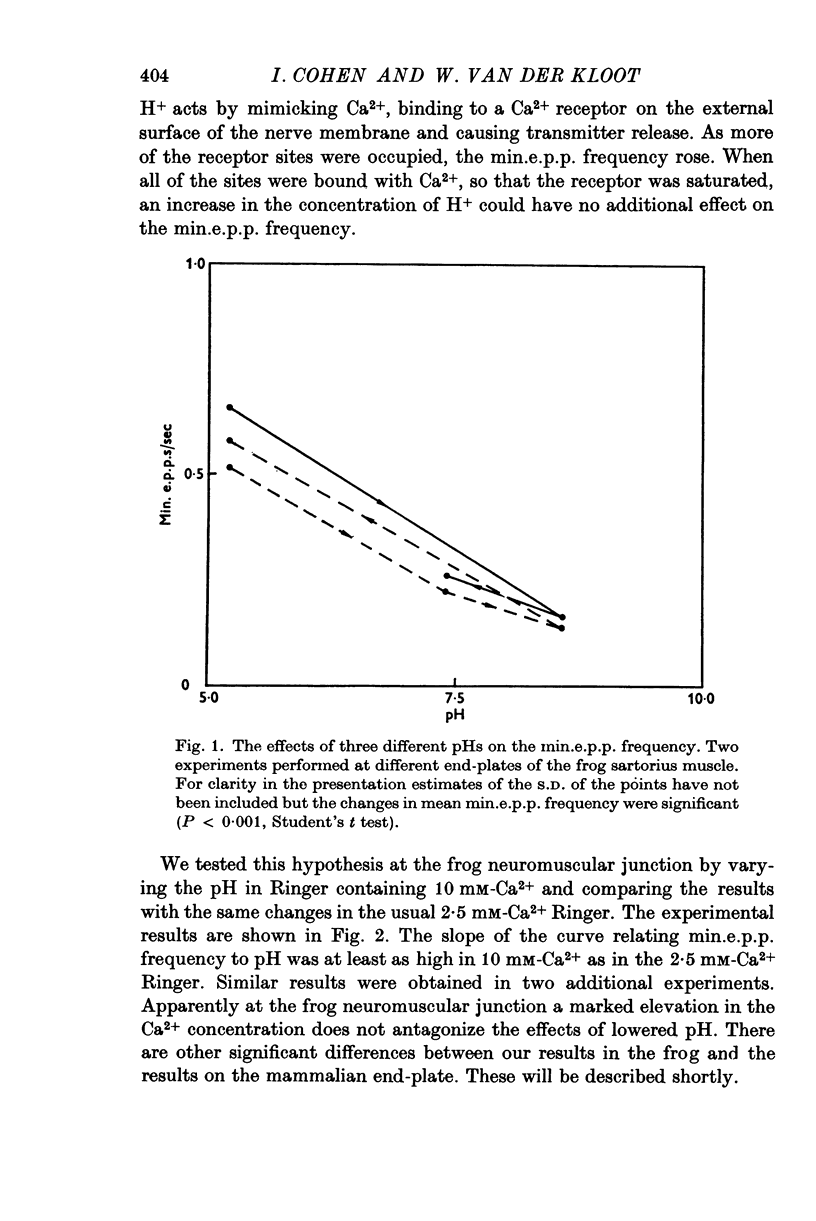
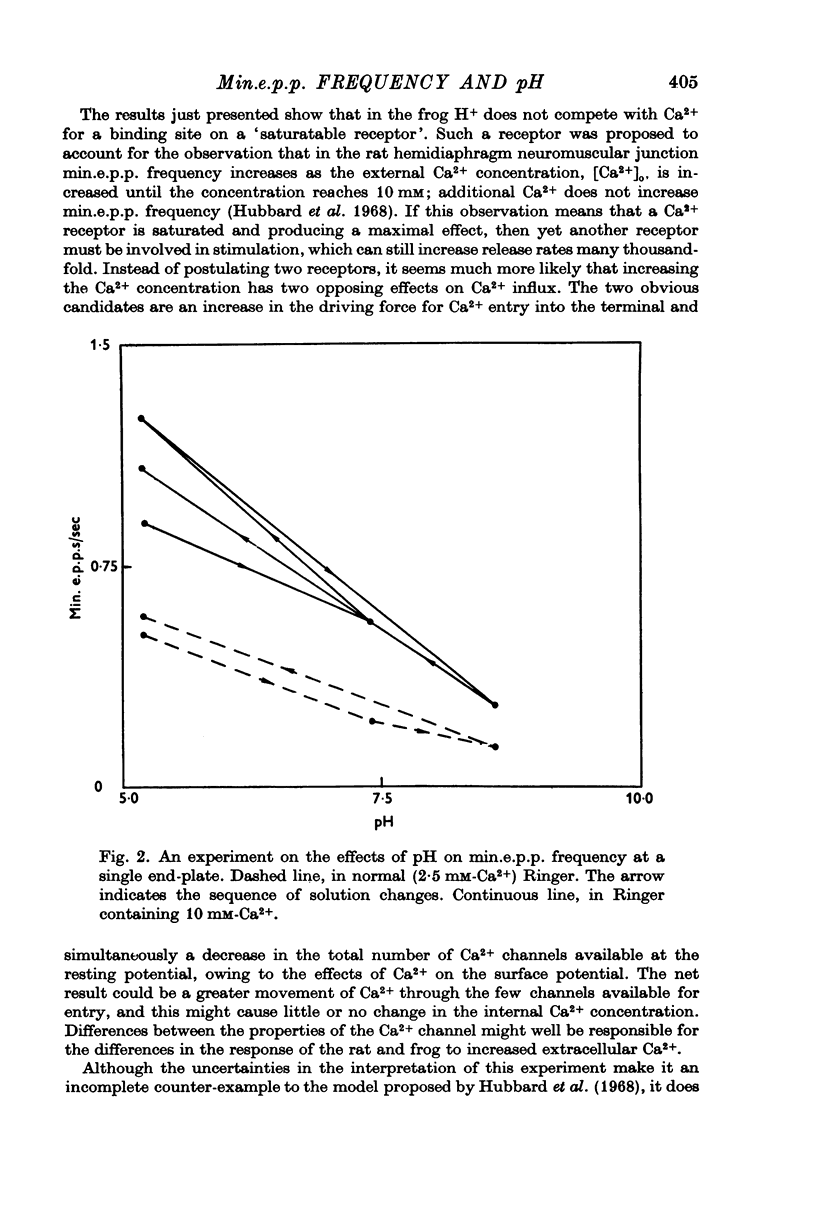
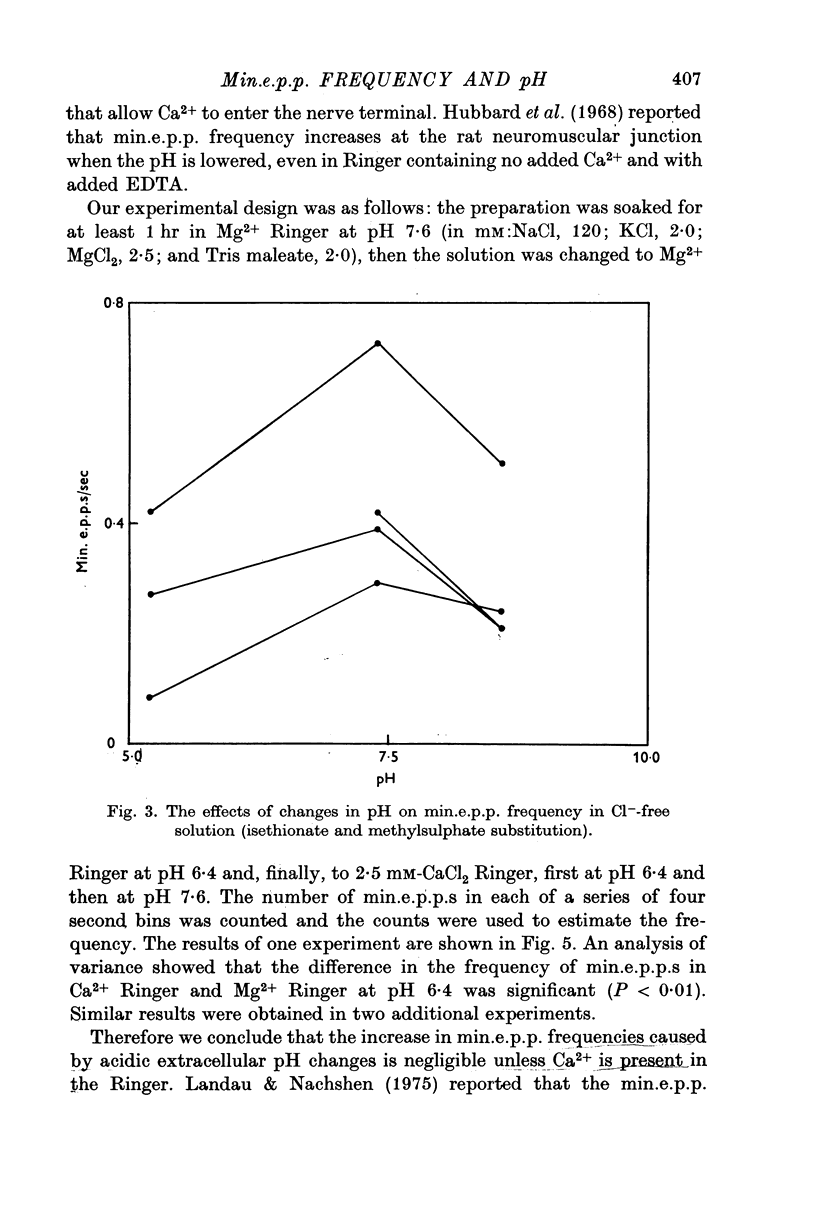
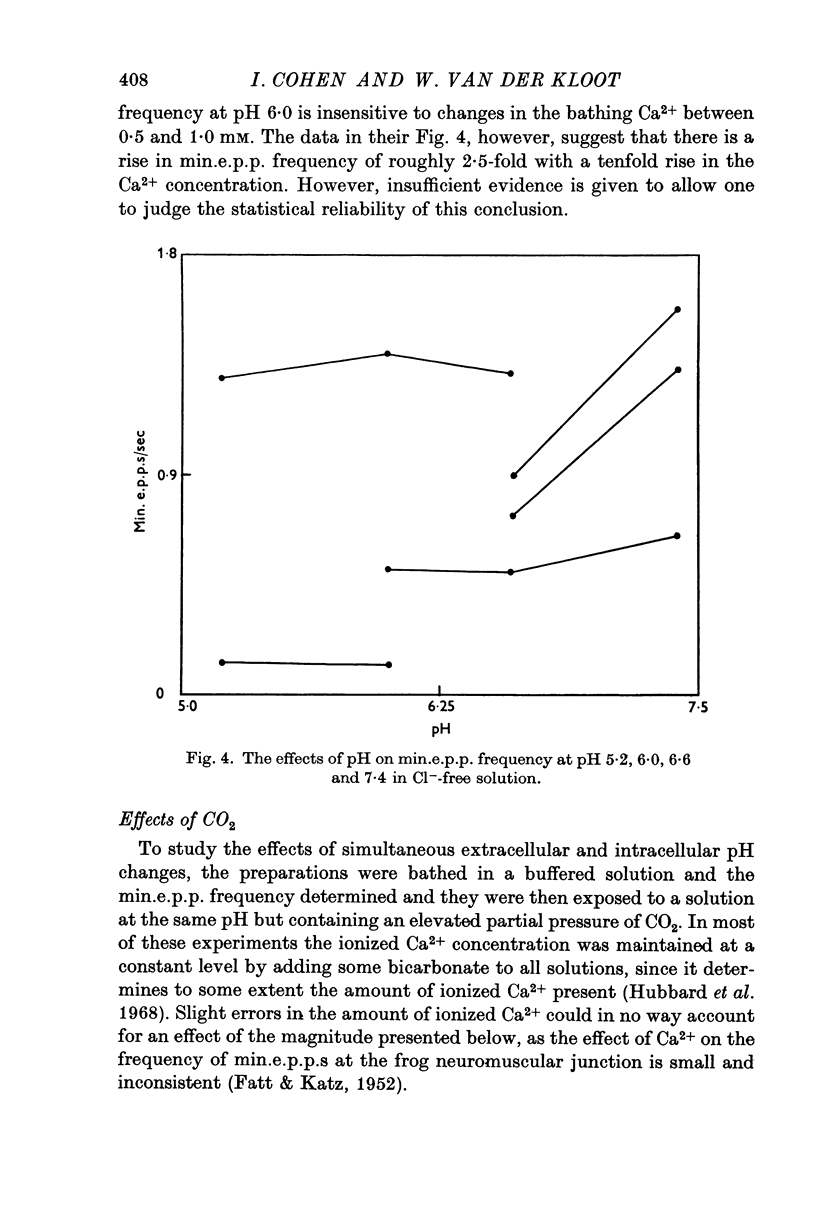
Abstract
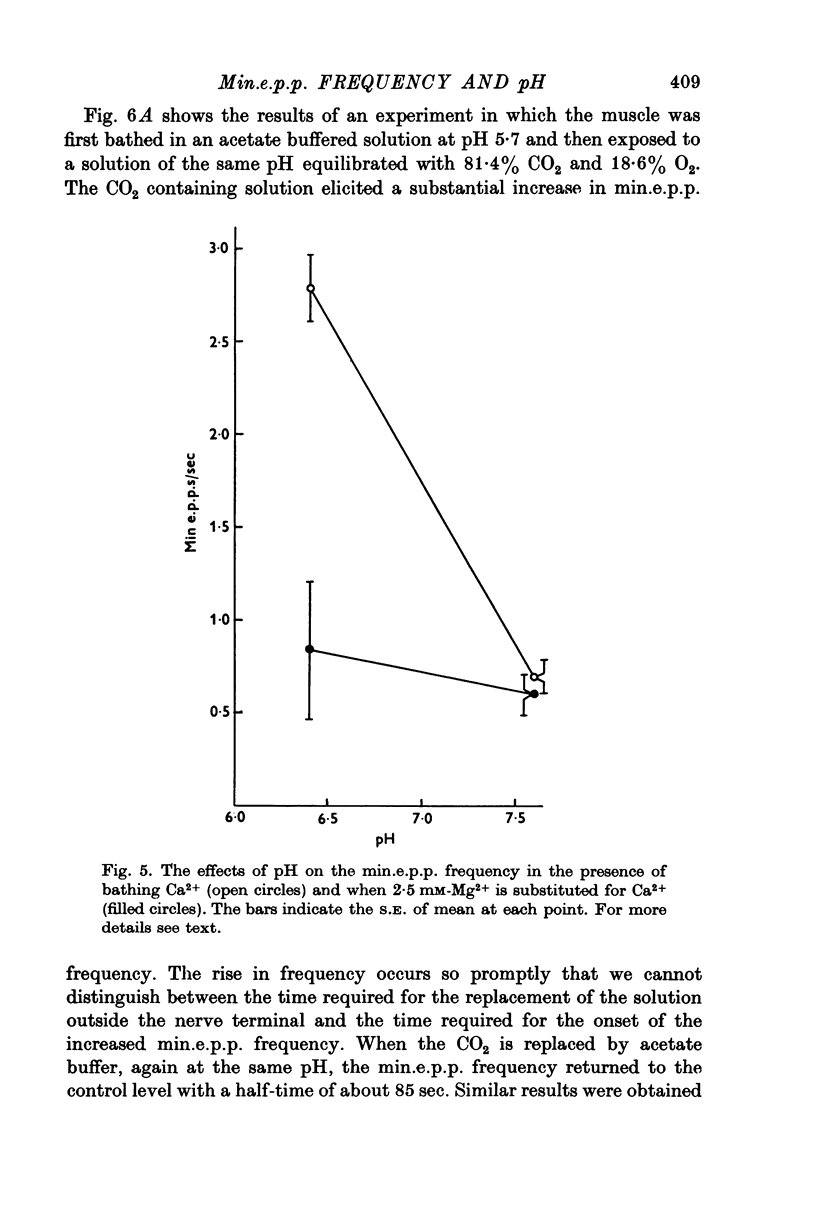
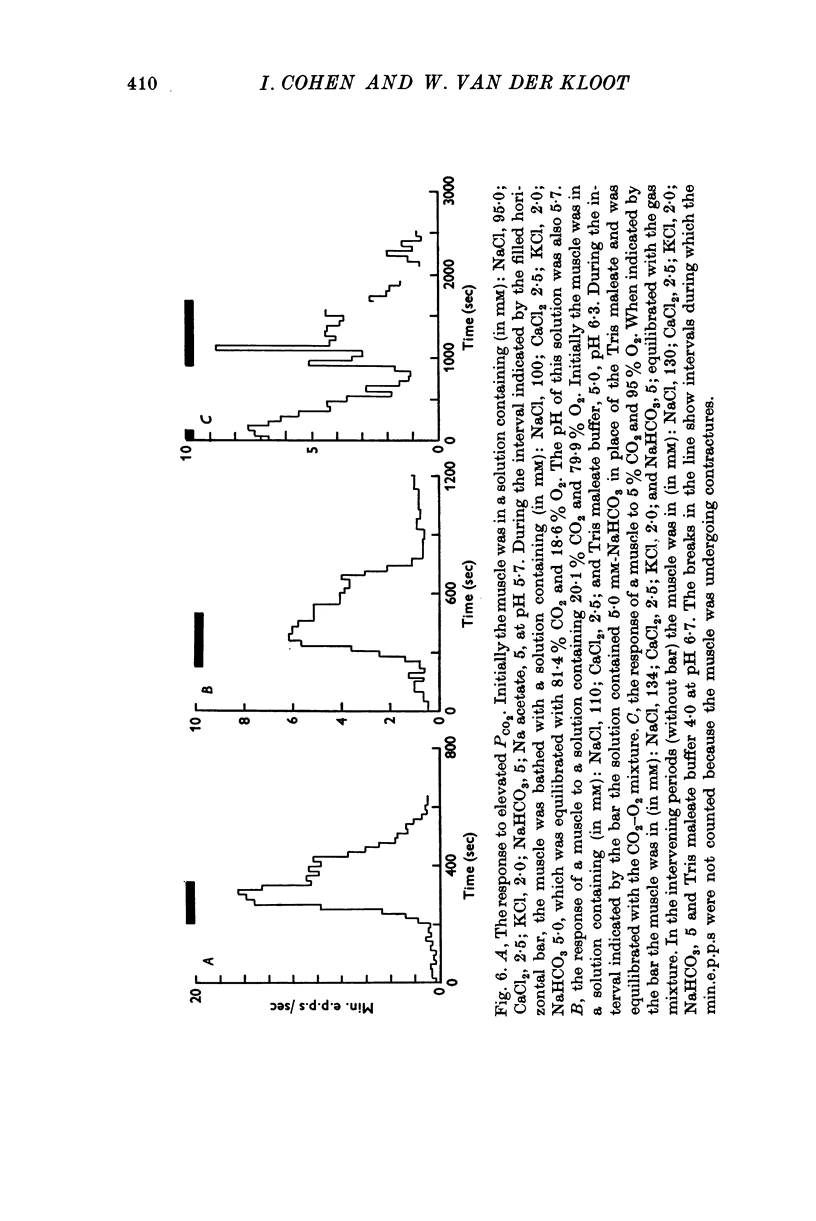
As reported by Landau & Nachshen (1975), a decrease in extracellular pH at the frog neuromuscular junction leads to an increase in min.e.p.p. frequency. 2. Decreasing the extracellular pH still increases the min.e.p.p. frequency when the bathing Ringer contains 10 mM-Ca2+, in place of the usual 2-5 mM. At the mammalian neuromuscular junction, the elevated Ca2+ blocks the effect of the pH change on the min.e.p.p. frequency (Hubbard, Jones & Landau, 1968). 3. In Cl--free solution (isethionate or methylsulphate substitution) min.e.p.p. frequency is no longer a monotonic function of decreasing pH. Instead there is an optimum pH for spontaneous release between pH 6-6 and 8-6. 4. This suggests that in Cl- containing Ringer min.e.p.p. frequency increases with increasing extracellular acidity because there is a change in the PCl of the nerve terminal leading to a depolarization. In agreement with this idea,in low Ca2+ Ringer, acid pH has little effect on the min.e.p.p. frequency. 5. Decreasing the intracellular pH by raising PCO2 produces substantial increases in the min.e.p.p. frequency. The effects are much greater than the effects of equal changes of H+ in the extracellular solution. 6. Possible explanations for the effects of increased PCO2 are discussed. Although release of Ca2+ from mitochondria or other unknown effects of intracellular pH change or molecular CO2 are possible, the results do give some support to the hypothesis that an important step in transmitter release involves an electrostatic repulsion between fixed membrane surface charges on the transmitter containing vesicles and the inner face of the nerve terminal. The surface charge density would be decreased by a lower pH in the axoplasm, and this would increase the rate of spontaneous transmitter release, in agreement with the observations.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blioch Z. L., Glagoleva I. M., Liberman E. A., Nenashev V. A. A study of the mechanism of quantal transmitter release at a chemical synapse. J Physiol. 1968 Nov;199(1):11–35. doi: 10.1113/jphysiol.1968.sp008637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., NELSON T. E., Jr, SANCHEZ V. Mechanism of the increased acetylcholine sensitivity of skeletal muscle in low pH solutions. J Cell Comp Physiol. 1962 Feb;59:35–44. doi: 10.1002/jcp.1030590105. [DOI] [PubMed] [Google Scholar]
- Erlij D., Van der Kloot W. G. Depolarization of frog muscle by low temperatures and by chloride-free solutions. Am J Physiol. 1967 Nov;213(5):1290–1294. doi: 10.1152/ajplegacy.1967.213.5.1290. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the spontaneous release of transmitter from mammalian motor nerve terminals. J Physiol. 1968 Feb;194(2):355–380. doi: 10.1113/jphysiol.1968.sp008413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbut W. P., Longenecker H. B., Jr, Mauro A. Effects of calcium and magnesium on the frequency of miniature end-plate potentials during prolonged tetanization. J Physiol. 1971 Dec;219(1):17–38. doi: 10.1113/jphysiol.1971.sp009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter O. F., Warner A. E. The pH sensitivity of the chloride conductance of frog skeletal muscle. J Physiol. 1967 Apr;189(3):403–425. doi: 10.1113/jphysiol.1967.sp008176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H., Van der Kloot W. Action of Co and Ni at the frog neuromuscular junction. Nat New Biol. 1973 Sep 12;245(141):52–53. doi: 10.1038/newbio245052a0. [DOI] [PubMed] [Google Scholar]
- Kita H., Van der Kloot W. The effects of changing the osmolarity of the Ringer on acetylcholine release at the frog neuromuscular junction. Life Sci I. 1971 Dec 15;10(24):1423–1432. doi: 10.1016/0024-3205(71)90272-4. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol. 1956 Nov 28;134(2):427–443. doi: 10.1113/jphysiol.1956.sp005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau E. M., Nachshen D. A. The interaction of pH and divalent cations at the neuromuscular junction. J Physiol. 1975 Oct;251(3):775–790. doi: 10.1113/jphysiol.1975.sp011121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. G., Hinke J. A. Optical density changes of single muscle fibres in sodium-free solutions. Can J Physiol Pharmacol. 1968 Mar;46(2):247–260. doi: 10.1139/y68-041. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Intracellular pH of snail neurones measured with a new pH-sensitive glass mirco-electrode. J Physiol. 1974 Apr;238(1):159–180. doi: 10.1113/jphysiol.1974.sp010516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kloot W., Kita H. The possible role of fixed membrane surface charges in acetylcholine release at the frog neuromuscular junction. J Membr Biol. 1973;14(4):365–382. doi: 10.1007/BF01868085. [DOI] [PubMed] [Google Scholar]
- Walker J. L., Jr, Brown A. M. Unified account of the variable effects of carbon dioxide on nerve cells. Science. 1970 Mar 13;167(3924):1502–1504. doi: 10.1126/science.167.3924.1502. [DOI] [PubMed] [Google Scholar]
- van Breemen C., Farinas B. R., Casteels R., Gerba P., Wuytack F., Deth R. Factors controlling cytoplasmic Ca 2+ concentration. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):57–71. doi: 10.1098/rstb.1973.0009. [DOI] [PubMed] [Google Scholar]


