Abstract
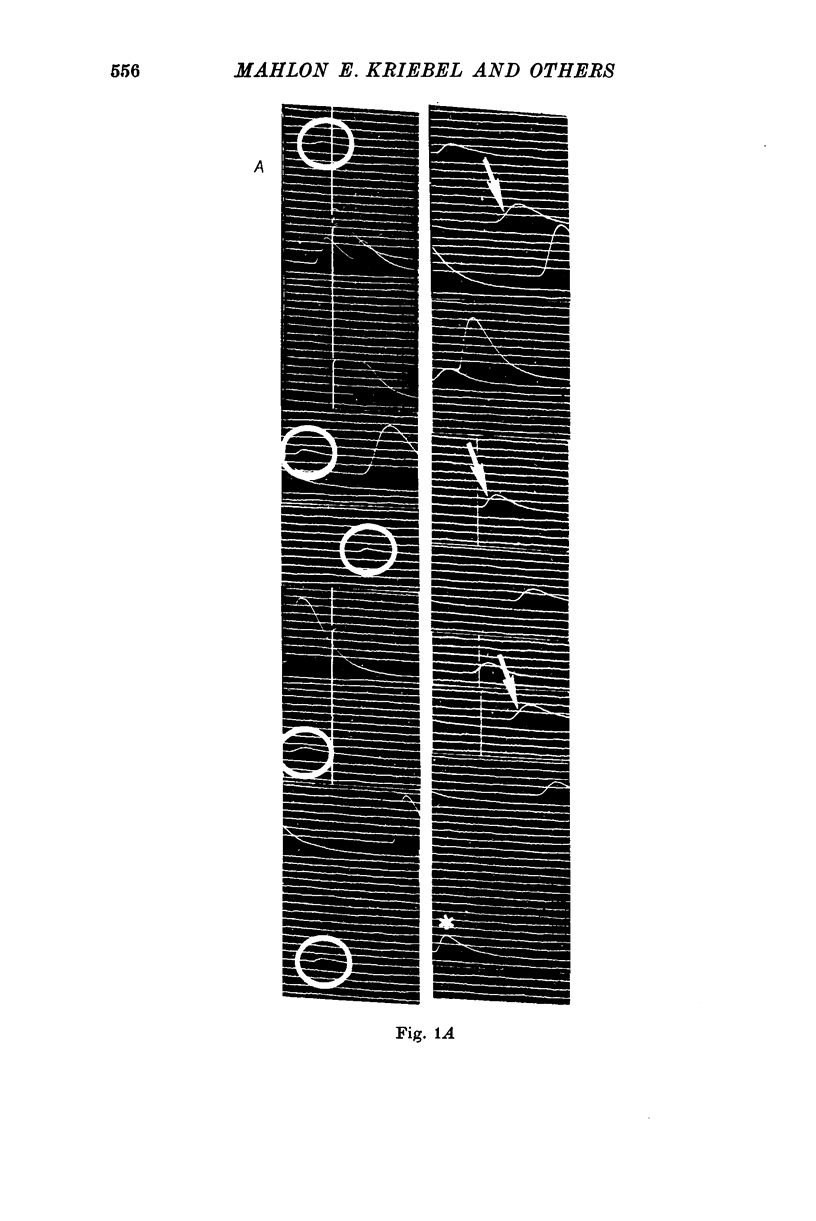
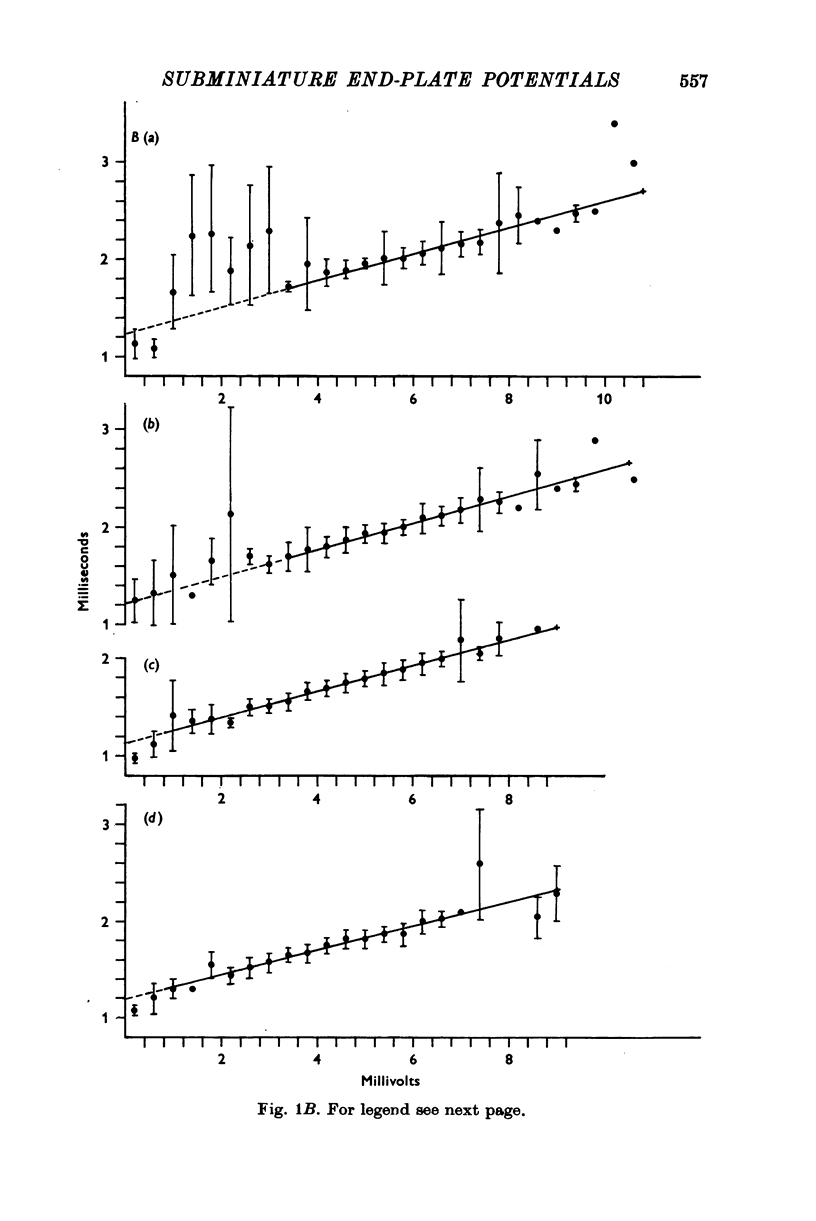
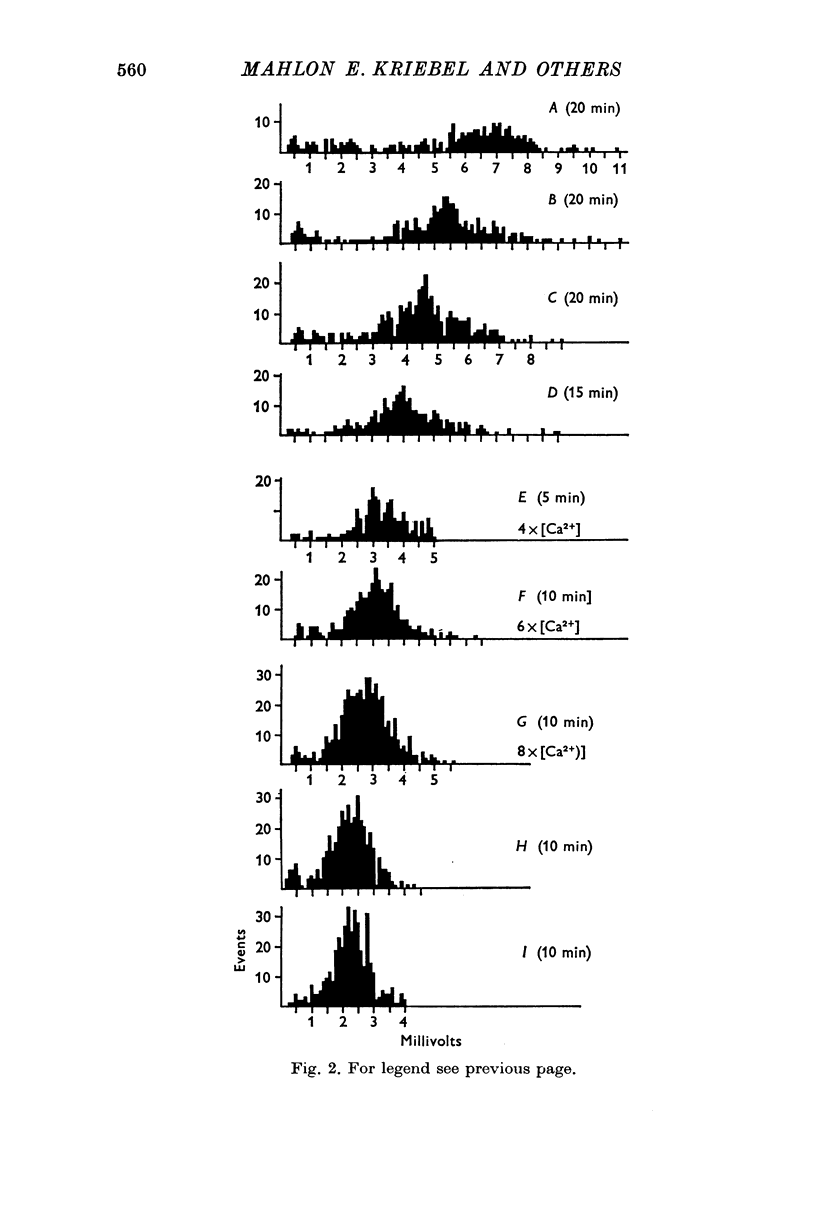
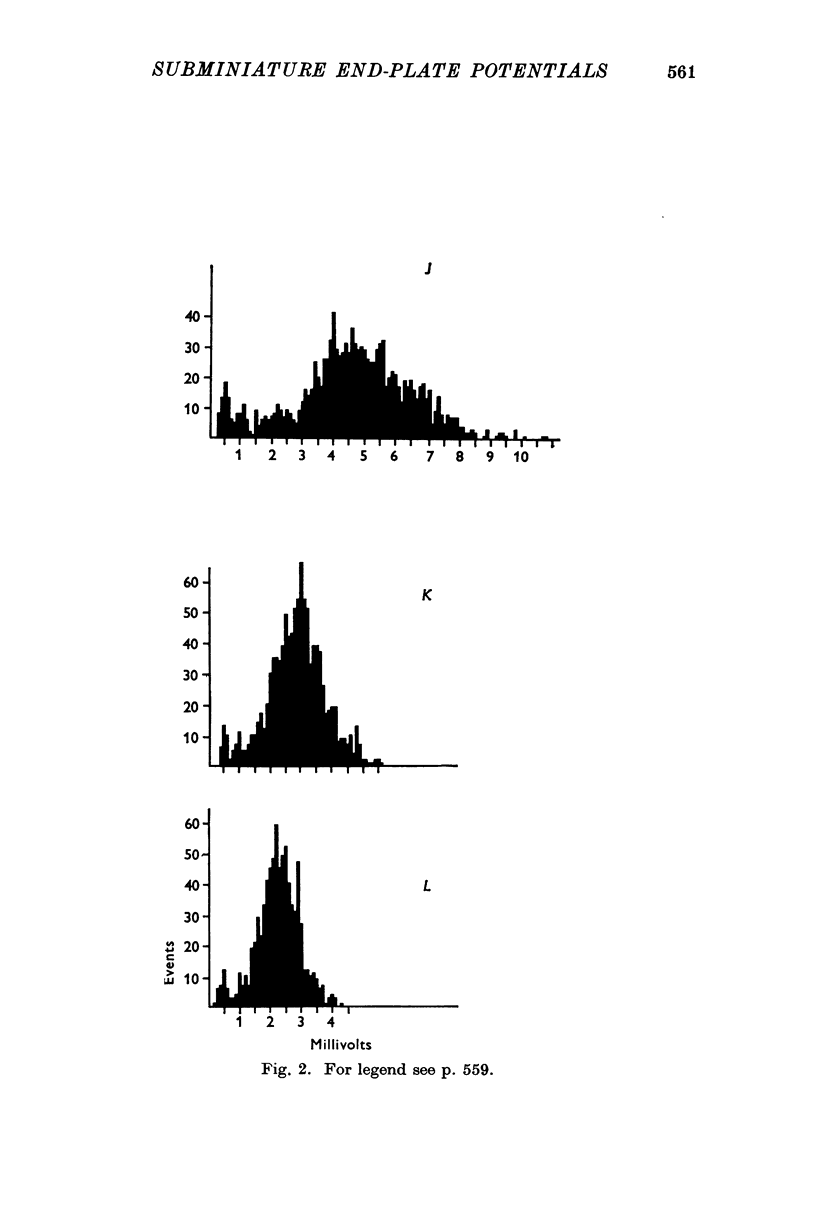
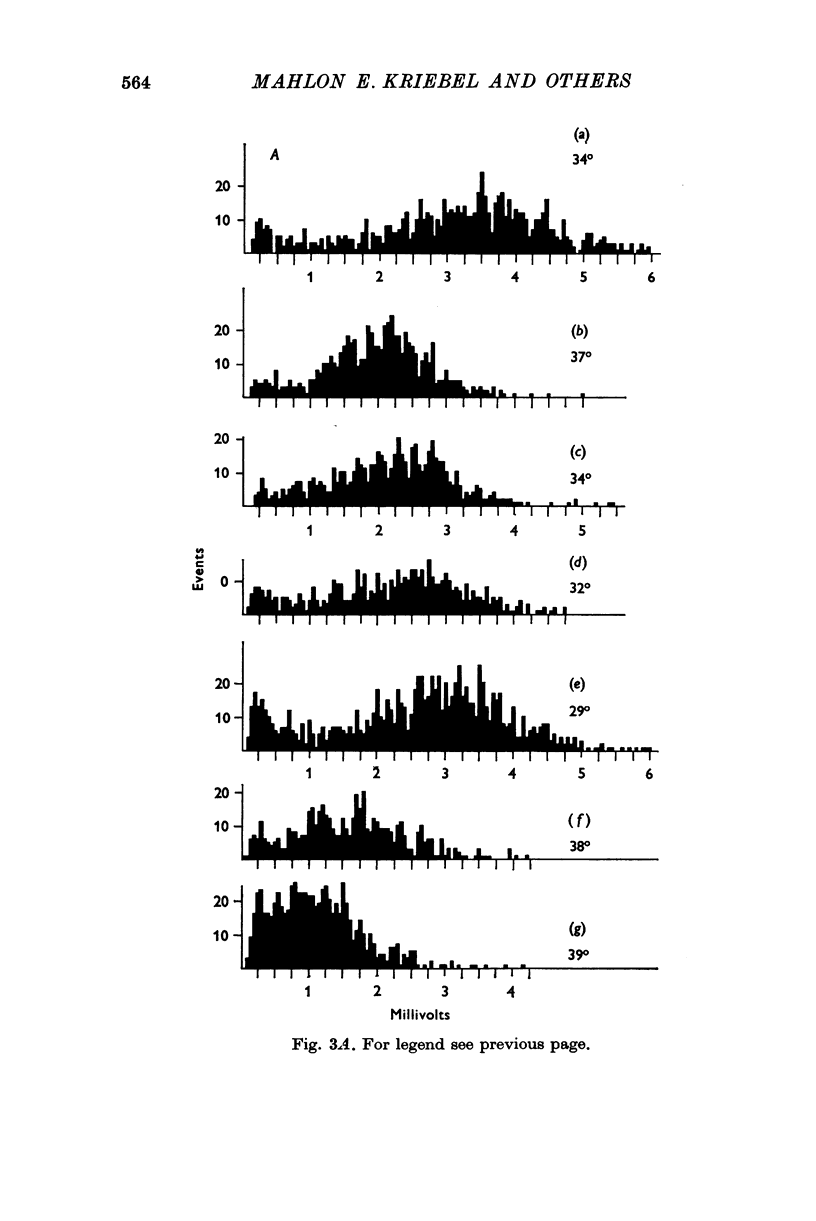
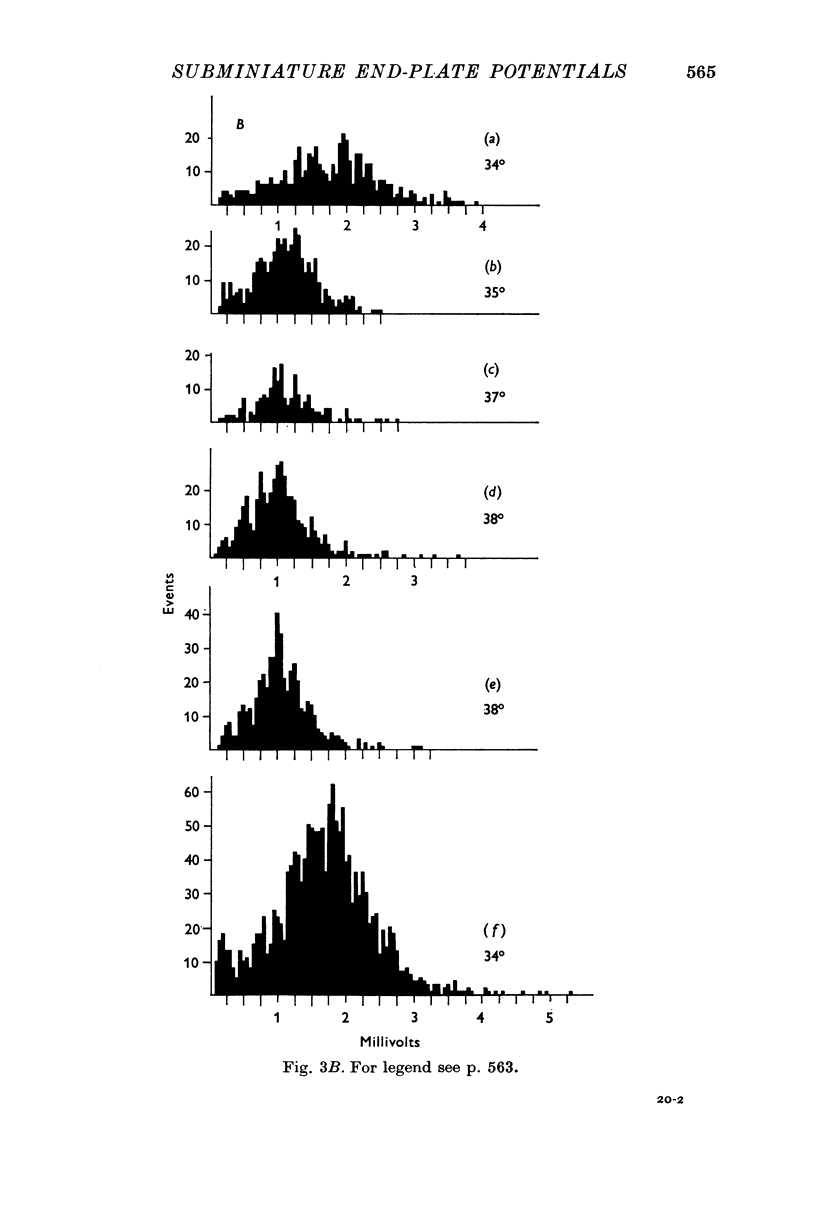
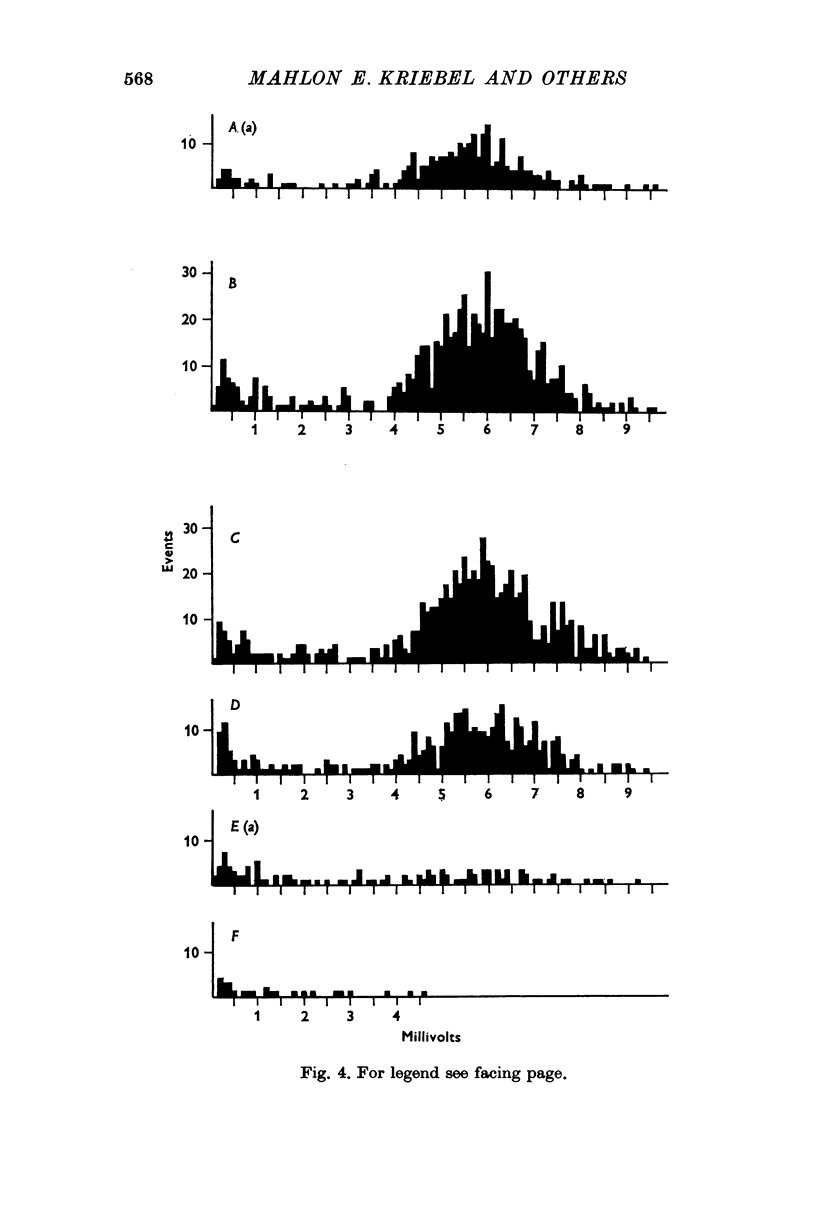
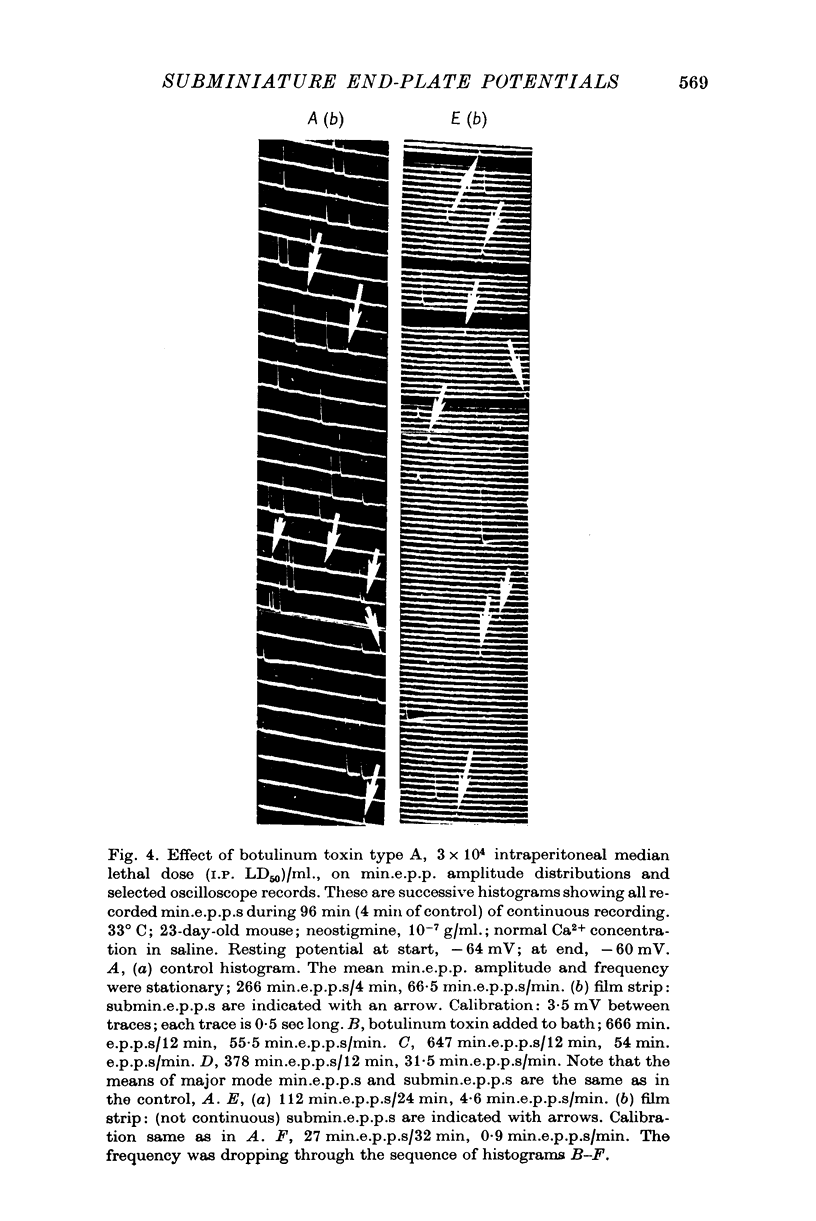
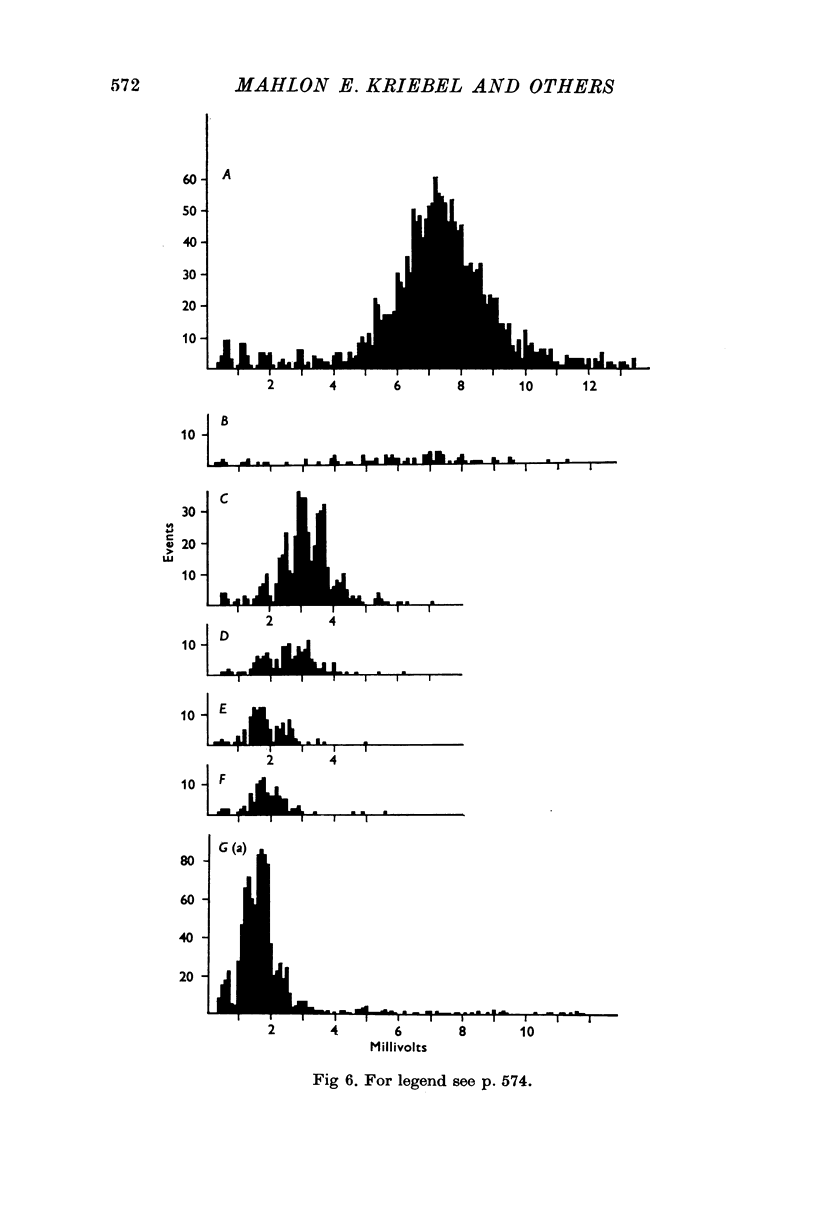
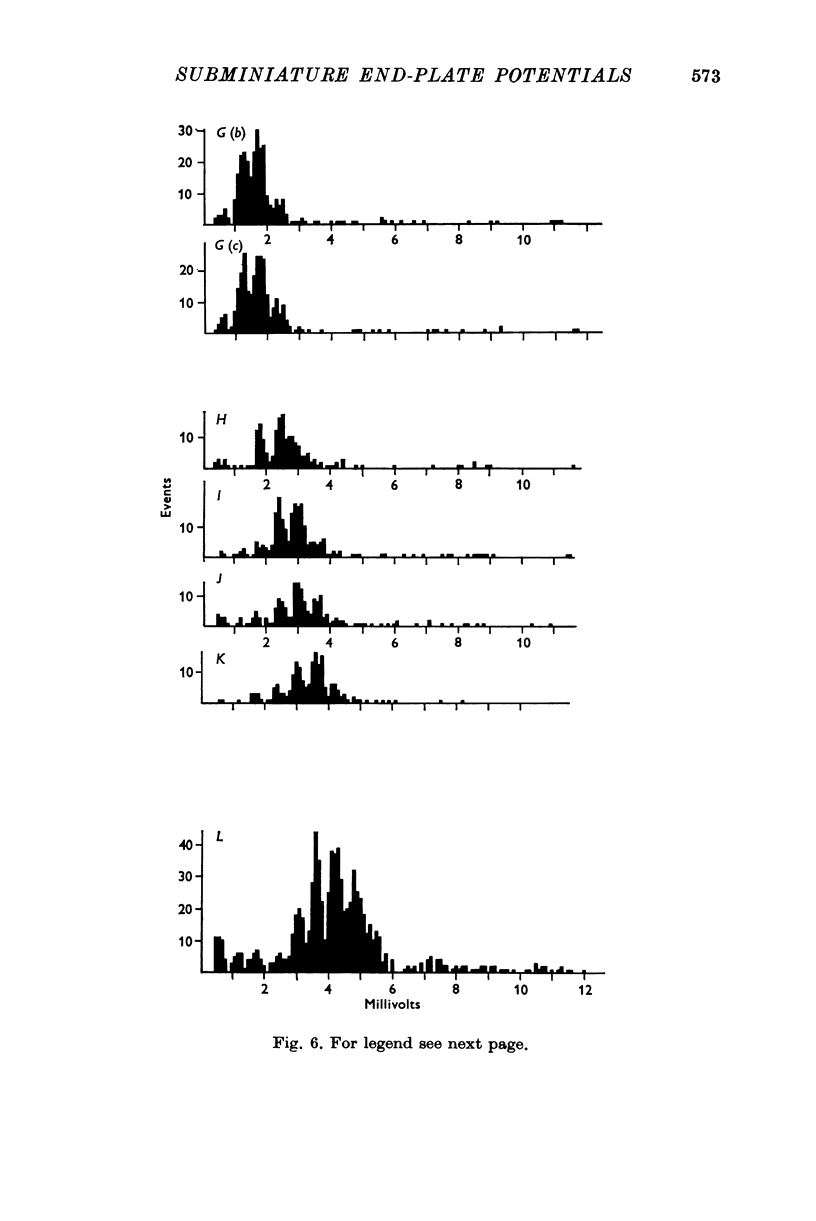
1. Miniature end-plate potentials (min.e.p.p.s) were recorded from small muscle cells of mouse diaphragms. Min.e.p.p. amplitude histograms showed successive peaks which were integral multiples of the smallest peak. The smallest potentials (submin.e.p.p.s) averaged 0-3-0-6mV and the mean of the larger min.e.p.p.s averaged 3-7 mV, depending on the muscle cell diameter. There was a positive correlation between time-to-peak and min.e.p.p. amplitude. Time-to-peak of the submin.e.p.p.s fell slightly below the regression line through the larger min.e.p.p.s. 2. Sometimes min.e.p.p. amplitude distributions changed spontaneously such that the mean of the major mode min.e.p.p.s decreased twofold during which time the mean of the submin.e.p.p.s did not change. Spontaneous decreases were most pronounced during low frequencies of release (10/min) achieved at 32 degrees C. 3. Small changes in temperature (2 degrees C steps in the range 32-40 degrees C) greatly altered the number of peaks of min.e.p.p. amplitude histograms without noticeably changing the position of the submin.e.p.p. peak. At 32 degrees C submin.e.p.p.s composed 5-20% of the histograms and the amplitude of the major mode peak was twelve to fifteen-times that of the submin.e.p.p.s. Over-all bell-shaped distributions were obtained at 37 degrees C which showed up to eight peaks with the major peak at the fourth to sixth peak. Temperatures slightly above 37 degrees C gave a flat distribution with the mean amplitude at the third peak. Min.e.p.p. amplitude histograms were initially skewed (mostly small min.e.p.p.s) after a 40 degrees C heat challenge. 4. Two to eight-times the normal concentration of Ca2+ in the saline reversibly increased the min.e.p.p. frequency and also decreased the mean of the major mode min.e.p.p.s (two to nine-times) without noticeably changing the mean of the submin.e.p.p.s. 5. Botulinum toxin A, 10(5) X intraperitoneal median lethal dose (10(5)I.P.LD50)/ml., almost abolished min.e.p.p.s in 30-90 min. The relative proportion of submin.e.p.p.s increased and the mean of the major mode min.e.p.p.s usually did not change during the initial decrease in frequency. Major mode min.e.p.p.s essentially ceased after 200-1000 were generated and remaining min.e.p.p.s of some cells showed skewed distributions with three small peaks that were integral multiples of the submin.e.p.p. peak. Smaller min.e.p.p.s were more resistant to block than the larger min.e.p.p.s and, although frequencies were low, small min.e.p.p.s were recorded after 4 hr of botulinum toxin incubation. 6. Colchicine (5 X 10(-4)M) within minutes reduced the major mode min.e.p.p.s by half (mean of major peak reduced to sixth or seventh peak). Additional colchicine (10(-3)M reduced the major mode min.e.p.p. amplitude to a fifth of that of control (mean of major mode min.e.p.p.s at the third peak) with no change in position of the submin.e.p.p. peak. Min.e.p.p. amplitudes slowly recovered to half control values after washing. 7...
Full text
PDF








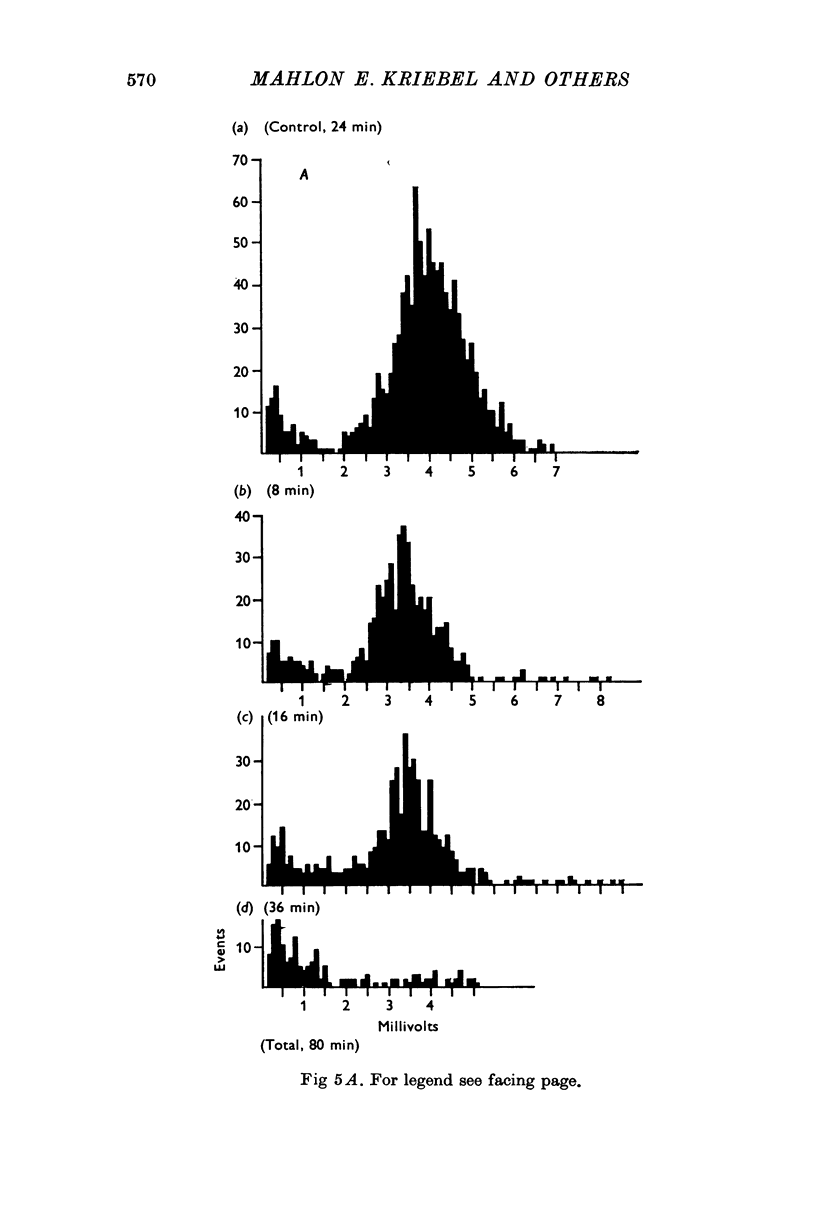
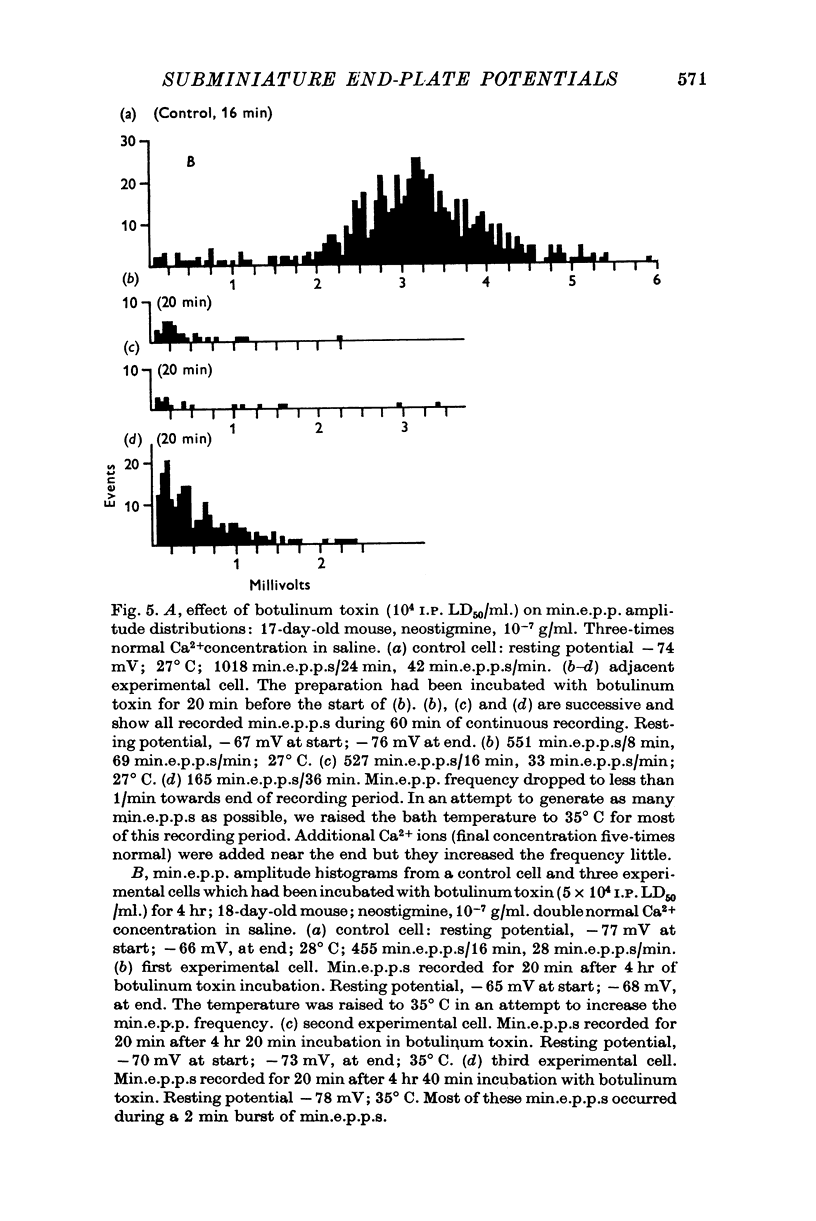








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., Warnick J. E., Tasse J. R., Sansone F. M. Effects of vinblastine and colchicine on neural regulation of the fast and slow skeletal muscles of the rat. Exp Neurol. 1972 Dec;37(3):607–634. doi: 10.1016/0014-4886(72)90103-3. [DOI] [PubMed] [Google Scholar]
- BIRKS R., KATZ B., MILEDI R. Physiological and structural changes at the amphibian myoneural junction, in the course of nerve degeneration. J Physiol. 1960 Jan;150:145–168. doi: 10.1113/jphysiol.1960.sp006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACKMAN J. G., GINSBORG B. L., RAY C. Spontaneous synaptic activity in sympathetic ganglion cells of the frog. J Physiol. 1963 Jul;167:389–401. doi: 10.1113/jphysiol.1963.sp007157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. Spontaneous subthreshold activity at mammalian neural muscular junctions. J Physiol. 1956 Apr 27;132(1):61–73. doi: 10.1113/jphysiol.1956.sp005502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. The end-plate potential in mammalian muscle. J Physiol. 1956 Apr 27;132(1):74–91. doi: 10.1113/jphysiol.1956.sp005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGEN A. S. V., DICKENS F., ZATMAN L. J. The action of botulinum toxin on the neuro-muscular junction. J Physiol. 1949 Aug;109(1-2):10–24. doi: 10.1113/jphysiol.1949.sp004364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Florin T. A statistical analysis of the release of acetylcholine at newly formed synapses in striated muscle. J Physiol. 1974 Apr;238(1):93–107. doi: 10.1113/jphysiol.1974.sp010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Florin T., Woog R. The formation of synapses in regenerating mammalian striated muscle. J Physiol. 1974 Apr;238(1):79–92. doi: 10.1113/jphysiol.1974.sp010511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., McLachlan E. M., Taylor R. S. The formation of synapses in reinnervated mammalian striated muscle. J Physiol. 1973 Sep;233(3):481–500. doi: 10.1113/jphysiol.1973.sp010319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G. The formation of synapses in amphibian striated muscle during development. J Physiol. 1975 Oct;252(1):203–239. doi: 10.1113/jphysiol.1975.sp011141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G. The formation of synapses in reinnervated and cross-reinnervated striated muscle during development. J Physiol. 1974 Sep;241(2):547–573. doi: 10.1113/jphysiol.1974.sp010671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G. The formation of synapses in striated muscle during development. J Physiol. 1974 Sep;241(2):515–545. doi: 10.1113/jphysiol.1974.sp010670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroff D. A., del Castillo J., Evoy W. H., Steinhardt R. A. Observations on the action of type A botulinum toxin on frog neuromuscular junctions. J Physiol. 1974 Jul;240(2):227–253. doi: 10.1113/jphysiol.1974.sp010608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray J. J., Harris A. J. Dissociation between nerve-muscle transmission and nerve trophic effects on rat diaphragm using type D botulinum toxin. J Physiol. 1975 Dec;253(1):53–77. doi: 10.1113/jphysiol.1975.sp011179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Kita H., Van Der Kloot W. The intervals between miniature end-plate potentials in the frog are unlikely to be independently or exponentially distributed. J Physiol. 1974 Jan;236(2):327–339. doi: 10.1113/jphysiol.1974.sp010437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Kita H., Van Der Kloot W. The stochastic properties of spontaneous quantal release of transmitter at the frog neuromuscular junction. J Physiol. 1974 Jan;236(2):341–361. doi: 10.1113/jphysiol.1974.sp010438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Quastel D. M. Transmitter release by mammalian motor nerve terminals in response to focal polarization. J Physiol. 1973 Jan;228(2):377–405. doi: 10.1113/jphysiol.1973.sp010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Harris A. J., Kuffler S. W. Synaptic transmission and its duplication by focally applied acetylcholine in parasympathetic neurons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):509–539. doi: 10.1098/rspb.1971.0045. [DOI] [PubMed] [Google Scholar]
- Dennis M. J., Miledi R. Characteristics of transmitter release at regenerating frog neuromuscular junctions. J Physiol. 1974 Jun;239(3):571–594. doi: 10.1113/jphysiol.1974.sp010583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen L. W., Tonge D. A. The effects of tetanus toxin on neuromuscular transmission and on the morphology of motor end-plates in slow and fast skeletal muscle of the mouse. J Physiol. 1973 Jan;228(1):157–172. doi: 10.1113/jphysiol.1973.sp010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Spontaneous activity at a mammalian neuromuscular junction in tetrodotoxin. Acta Physiol Scand. 1965 Aug;64(4):475–476. doi: 10.1111/j.1748-1716.1965.tb04206.x. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. Effects of membrane potential, temperature and neostigmine on the conductance change caused by a quantum or acetylcholine at the toad neuromuscular junction. J Physiol. 1975 Jan;244(2):385–407. doi: 10.1113/jphysiol.1975.sp010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I. The effect of calcium and magnesium on the spontaneous release of transmitter from mammalian motor nerve endings. J Physiol. 1961 Dec;159:507–517. doi: 10.1113/jphysiol.1961.sp006824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. J., Miledi R. The effect of type D botulinum toxin on frog neuromuscular junctions. J Physiol. 1971 Sep;217(2):497–515. doi: 10.1113/jphysiol.1971.sp009582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Yoshikami D. Post-synaptic potentiation: interaction between quanta of acetylcholine at the skeletal neuromuscular synapse. J Physiol. 1975 Oct;251(2):427–463. doi: 10.1113/jphysiol.1975.sp011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E. Proceedings: A possible origin of the 'giant' spontaneous potentials that occur after prolonged transmitter release at frog neuromuscular junctions. J Physiol. 1974 Jun;239(2):106P–108P. doi: 10.1113/jphysiol.1974.sp010593. [DOI] [PubMed] [Google Scholar]
- Highstein S. M., Bennett M. V. Fatigue and recovery of transmission at the Mauthner fiber-giant fiber synapse of the hatchetfish. Brain Res. 1975 Nov 14;98(2):229–242. doi: 10.1016/0006-8993(75)90003-7. [DOI] [PubMed] [Google Scholar]
- Hofmann W. W., Thesleff S. Studies on the trophic influence of nerve on skeletal muscle. Eur J Pharmacol. 1972 Dec;20(3):256–260. doi: 10.1016/0014-2999(72)90182-3. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the spontaneous release of transmitter from mammalian motor nerve terminals. J Physiol. 1968 Feb;194(2):355–380. doi: 10.1113/jphysiol.1968.sp008413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F. Spontaneous quantal transmitter release: a statistical analysis and some implications. J Physiol. 1973 Jul;232(1):1–21. doi: 10.1113/jphysiol.1973.sp010254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz N. L. The effects on frog neuromuscular transmission of agents which act upon microtubules and microfilaments. Eur J Pharmacol. 1972 Jul;19(1):88–93. doi: 10.1016/0014-2999(72)90080-5. [DOI] [PubMed] [Google Scholar]
- Kriebel M. E., Gross C. E. Multimodal distribution of frog miniature endplate potentials in adult denervated and tadpole leg muscle. J Gen Physiol. 1974 Jul;64(1):85–103. doi: 10.1085/jgp.64.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebel M. E., Stolper D. R. Non-Poisson distribution in time of small- and large-mode miniature end-plate potentials. Am J Physiol. 1975 Nov;229(5):1321–1329. doi: 10.1152/ajplegacy.1975.229.5.1321. [DOI] [PubMed] [Google Scholar]
- Kuno M., Turkanis S. A., Weakly J. N. Correlation between nerve terminal size and transmitter release at the neuromuscular junction of the frog. J Physiol. 1971 Mar;213(3):545–556. doi: 10.1113/jphysiol.1971.sp009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. Spontaneous release of transmitter substance in multiquantal units. J Physiol. 1957 May 23;136(3):595–605. doi: 10.1113/jphysiol.1957.sp005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. QUANTAL COMPONENTS OF THE SYNAPTIC POTENTIAL IN THE CILIARY GANGLION OF THE CHICK. J Physiol. 1964 Dec;175:1–16. doi: 10.1113/jphysiol.1964.sp007499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. Properties of regenerating neuromuscular synapses in the frog. J Physiol. 1960 Nov;154:190–205. doi: 10.1113/jphysiol.1960.sp006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellanby J., Thompson P. A. The effect of tetanus toxin at the neuromuscular junction in the goldfish. J Physiol. 1972 Jul;224(2):407–419. doi: 10.1113/jphysiol.1972.sp009902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. On the degeneration of rat neuromuscular junctions after nerve section. J Physiol. 1970 Apr;207(2):507–528. doi: 10.1113/jphysiol.1970.sp009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M. D., Volle R. L. Enhancement by carbachol of transmitter release from motor nerve terminals. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1489–1492. doi: 10.1073/pnas.71.4.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrete J., Del Castillo J., Escobar I., Yankelevich G. Correlation between amplitudes and rise times of the miniature endplate potentials in frog muscle. Int J Neurosci. 1972 Jul;4(1):1–10. doi: 10.3109/00207457209147158. [DOI] [PubMed] [Google Scholar]
- Negrette J., Del Castillo J., Escobar I., Yankelevich G. Spreading activation of end-plate receptors by single transmitter quanta. Nat New Biol. 1972 Feb 2;235(57):158–159. doi: 10.1038/newbio235158a0. [DOI] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer N. Miniature end-plate potentials at mammalian neuromuscular junctions poisoned by botulinum toxin. Nat New Biol. 1972 May 3;237(70):26–27. doi: 10.1038/newbio237026a0. [DOI] [PubMed] [Google Scholar]
- Spoor R. P., Ferguson F. C., Jr Colchicine. IV. Neuromuscular transmission in isolated frog and rat tissues. J Pharm Sci. 1965 May;54(5):779–780. doi: 10.1002/jps.2600540524. [DOI] [PubMed] [Google Scholar]
- THESLEFF S. Supersensitivity of skeletal muscle produced by botulinum toxin. J Physiol. 1960 Jun;151:598–607. doi: 10.1113/jphysiol.1960.sp006463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonge D. A. Chronic effects of botulinum toxin on neuromuscular transmission and sensitivity to acetylcholine in slow and fast skeletal muscle of the mouse. J Physiol. 1974 Aug;241(1):127–139. doi: 10.1113/jphysiol.1974.sp010644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonge D. A. Physiological characteristics of re-innervation of skeletal muscle in the mouse. J Physiol. 1974 Aug;241(1):141–153. doi: 10.1113/jphysiol.1974.sp010645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkanis S. A. Some effects of vinblastine and colchicine on neuromuscular transmission. Brain Res. 1973 May 17;54:324–329. doi: 10.1016/0006-8993(73)90055-3. [DOI] [PubMed] [Google Scholar]
- Wernig A. Estimates of statistical release parameters from crayfish and frog neuromuscular junctions. J Physiol. 1975 Jan;244(1):207–221. doi: 10.1113/jphysiol.1975.sp010792. [DOI] [PMC free article] [PubMed] [Google Scholar]


