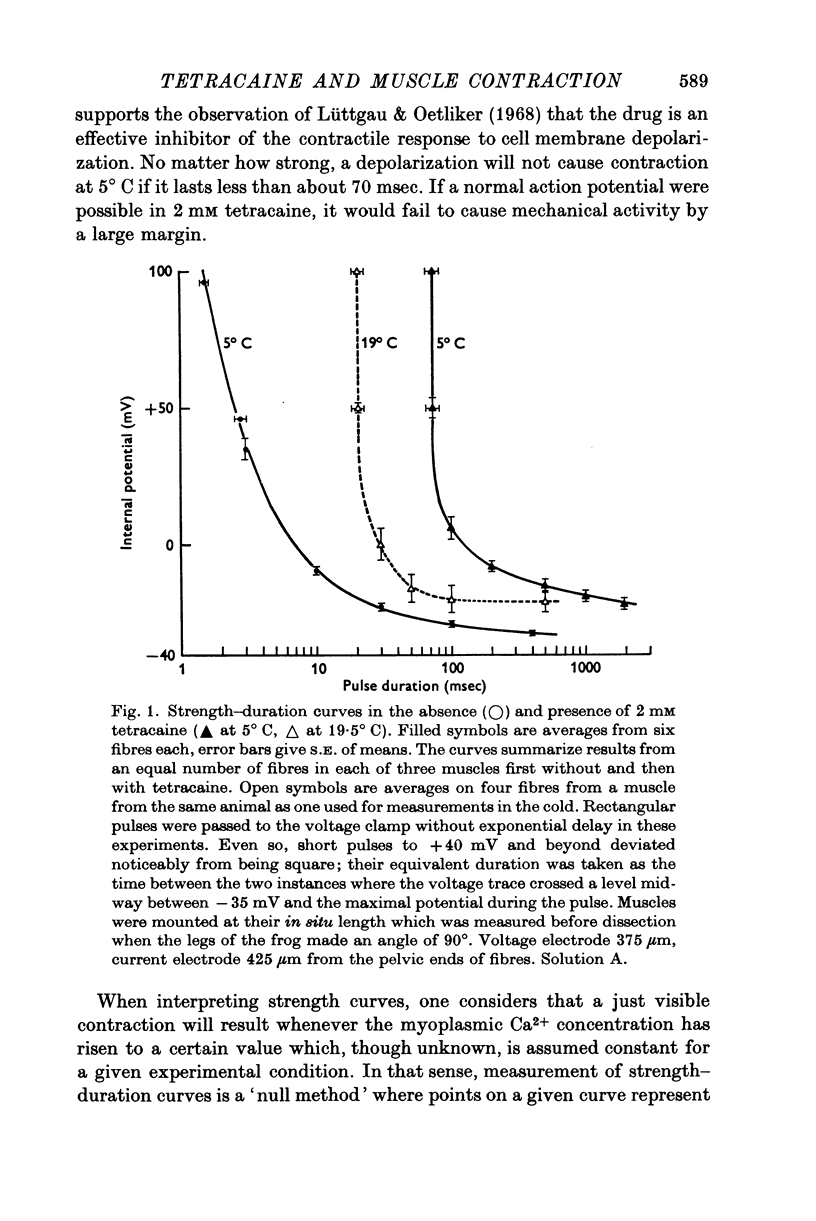
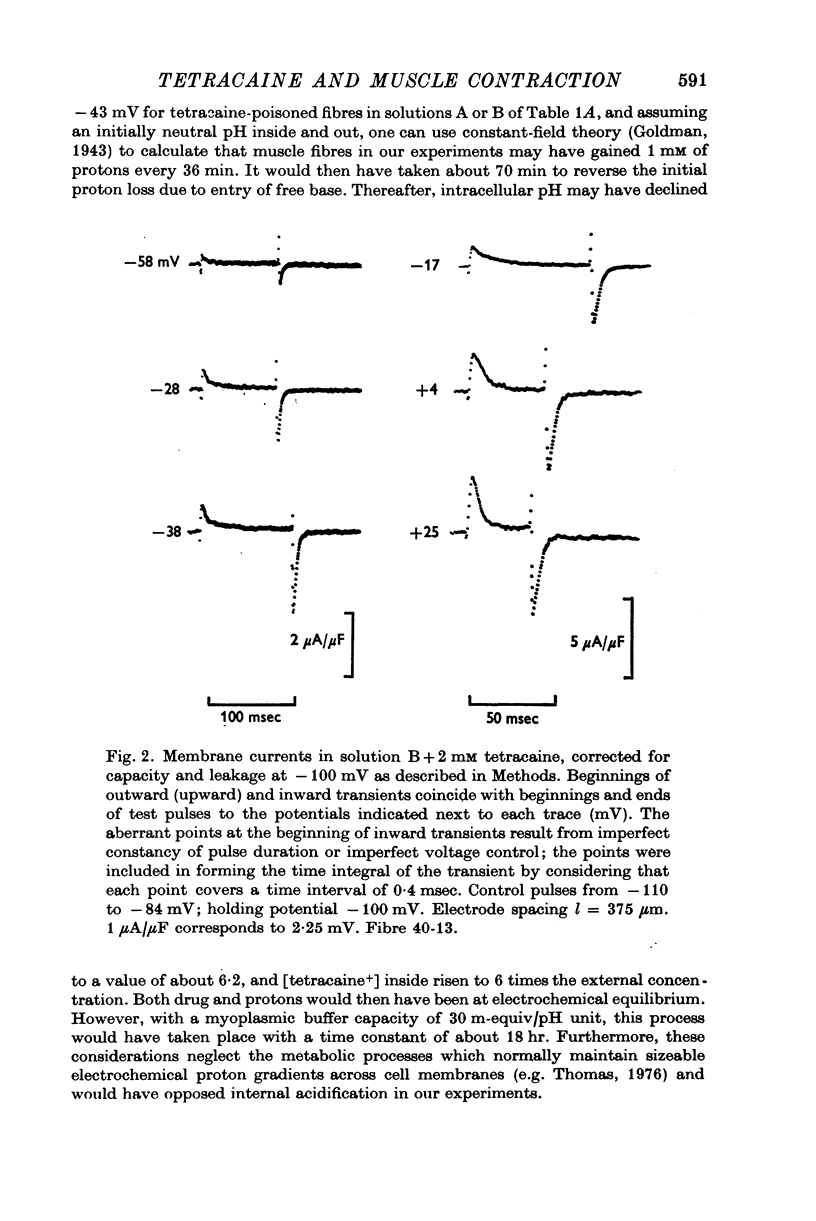
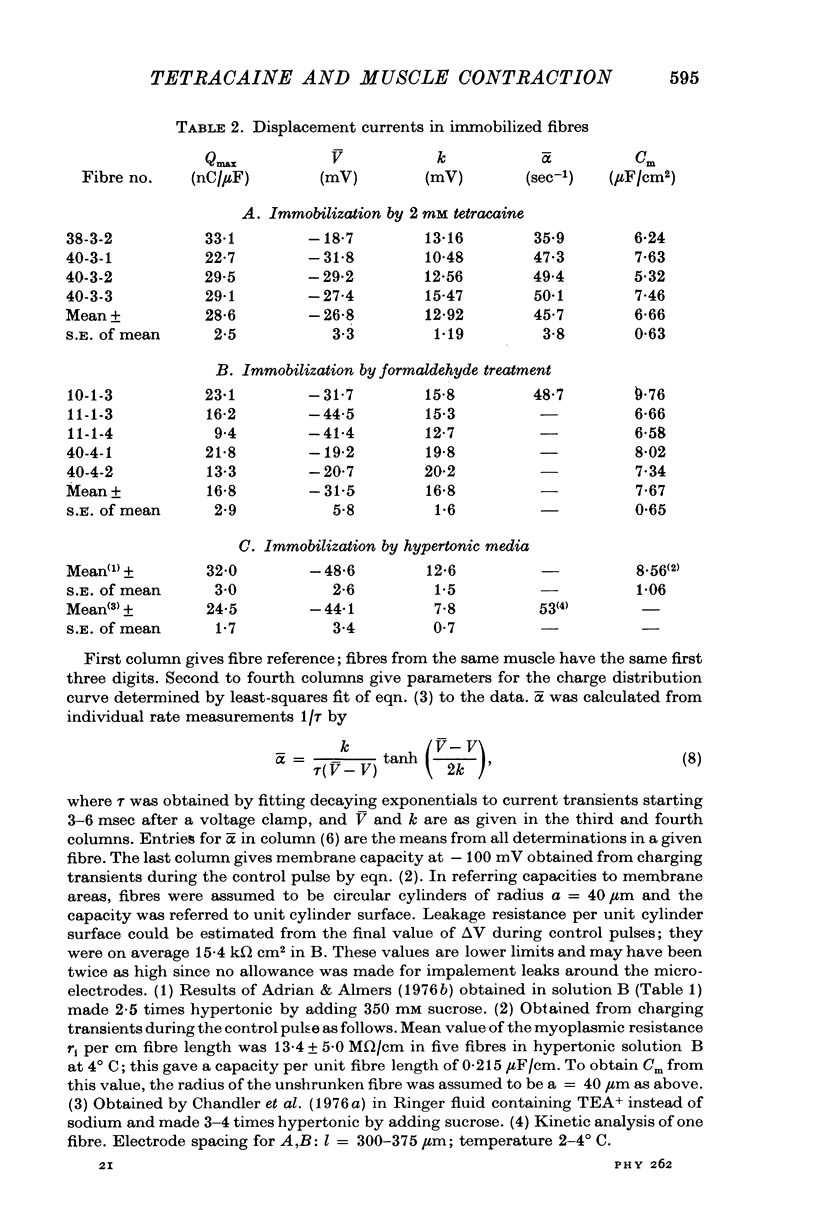
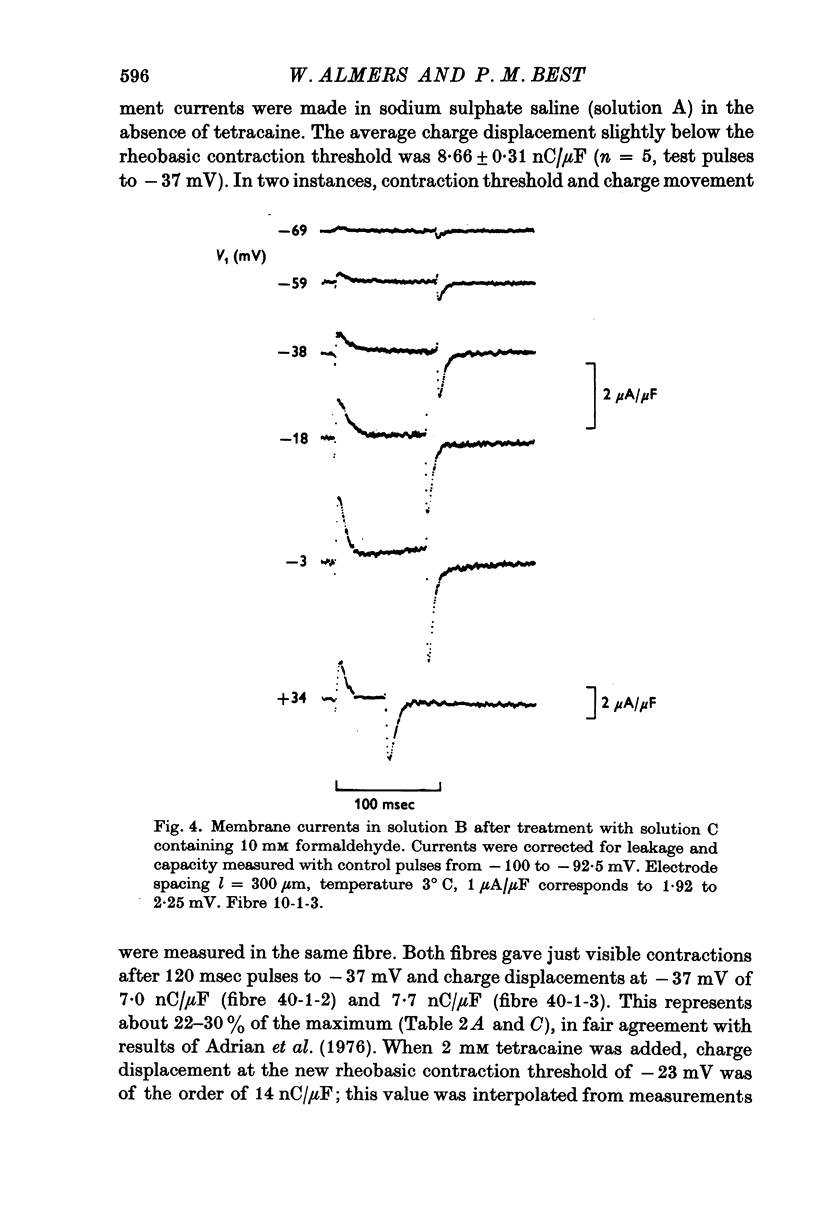
Abstract
The kinetics of mechanical activation of intact fibres were examined with a voltage-clamp technique. Tetracaine (2 mM) increases fifteen- to seventyfold the time required to produce a just visible contraction by cell membrane depolarization. 2. Displacement currents thought to be related to contractile activation remain in 2 mM tetracaine. Their characteristics are virtually identical to those found in the absence of the drug. Displacement currents also remain in fibres immobilized by treatment with 10 mM formaldehyde. 3. Despite its effect on contraction of intact fibres, tetracaine does not diminish contraction tension when Ca is applied directly to the contractile proteins of 'skinned' muscle fibres. The sensitivity of the myofilaments to Ca2+ also remains undiminished. 4. When acting on intact fibres the drug must therefore inhibit Ca2+-release from the sarcoplasmic reticulum. It is estimated that 2 mM tetracaine diminishes more than tenfold the capacity for Ca2+-release in response to cell membrane depolarization.5. If muscle displacement currents represent events linking depolarization to Ca2+-release, then tetracaine must be able to block the release without affecting the potential-sensing portion of the release regulating mechanism. 6. Further experiments on skinned fibres show that tetracaine blocks or greatly diminishes caffeine contractions, but that Cl-induced contractions of normal amplitude are still possible.
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Almers W. Charge movement in the membrane of striated muscle. J Physiol. 1976 Jan;254(2):339–360. doi: 10.1113/jphysiol.1976.sp011235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Almers W. Membrane capacity measurements on frog skeletal muscle in media of low ion content. J Physiol. 1974 Mar;237(3):573–605. doi: 10.1113/jphysiol.1974.sp010499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Almers W. The voltage dependence of membrane capacity. J Physiol. 1976 Jan;254(2):317–338. doi: 10.1113/jphysiol.1976.sp011234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. The kinetics of mechanical activation in frog muscle. J Physiol. 1969 Sep;204(1):207–230. doi: 10.1113/jphysiol.1969.sp008909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Rakowski R. F. Charge movement and mechanical repriming in skeletal muscle. J Physiol. 1976 Jan;254(2):361–388. doi: 10.1113/jphysiol.1976.sp011236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W. Some dielectric properties of muscle membrane and their possible importance for excitation-contraction coupling. Ann N Y Acad Sci. 1975 Dec 30;264:278–292. doi: 10.1111/j.1749-6632.1975.tb31489.x. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J Gen Physiol. 1974 May;63(5):533–552. doi: 10.1085/jgp.63.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi C. P., Bolton T. C. Action of local anesthetics on coupling systems in muscle. J Pharmacol Exp Ther. 1967 Aug;157(2):388–405. [PubMed] [Google Scholar]
- Caputo C. The effect of caffeine and tetracaine on the time course of potassium contractures of single muscle fibres. J Physiol. 1976 Feb;255(1):191–207. doi: 10.1113/jphysiol.1976.sp011275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. A non-linear voltage dependent charge movement in frog skeletal muscle. J Physiol. 1976 Jan;254(2):245–283. doi: 10.1113/jphysiol.1976.sp011232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. Effects of glycerol treatment and maintained depolarization on charge movement in skeletal muscle. J Physiol. 1976 Jan;254(2):285–316. doi: 10.1113/jphysiol.1976.sp011233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantin L. L. Contractile activation in frog skeletal muscle. J Gen Physiol. 1974 Jun;63(6):657–674. doi: 10.1085/jgp.63.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson S. K., Kerrick W. G. Characterization of the effects of Mg2+ on Ca2+- and Sr2+-activated tension generation of skinned skeletal muscle fibers. J Gen Physiol. 1975 Oct;66(4):427–444. doi: 10.1085/jgp.66.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- FEINSTEIN M. B. INHIBITION OF CAFFEINE RIGOR AND RADIOCALCIUM MOVEMENTS BY LOCAL ANESTHETICS IN FROG SARTORIUS MUSCLE. J Gen Physiol. 1963 Sep;47:151–172. doi: 10.1085/jgp.47.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Calcium uptake and force development by skinned muscle fibres in EGTA buffered solutions. J Physiol. 1972 May;223(1):1–19. doi: 10.1113/jphysiol.1972.sp009830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Intracellular calcium movements in skinned muscle fibres. J Physiol. 1972 May;223(1):21–33. doi: 10.1113/jphysiol.1972.sp009831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C. STUDIES OF THE TRIAD : I. Structure of the Junction in Frog Twitch Fibers. J Cell Biol. 1970 Nov 1;47(2):488–499. doi: 10.1083/jcb.47.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa K., Kerridge P. M. The hydrogen ion concentration of the muscles of the cat. J Physiol. 1927 Jun 7;63(1):33–41. doi: 10.1113/jphysiol.1927.sp002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutknecht J., Tosteson D. C. Diffusion of weak acids across lipid bilayer membranes: effects of chemical reactions in the unstirred layers. Science. 1973 Dec 21;182(4118):1258–1261. doi: 10.1126/science.182.4118.1258. [DOI] [PubMed] [Google Scholar]
- HILL A. V. The abrupt transition from rest to activity in muscle. Proc R Soc Lond B Biol Sci. 1949 Oct;136(884):399–420. doi: 10.1098/rspb.1949.0033. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The effect of sudden changes in ionic concentrations on the membrane potential of single muscle fibres. J Physiol. 1960 Sep;153:370–385. doi: 10.1113/jphysiol.1960.sp006540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellam D. C., Podolsky R. J. Force measurements in skinned muscle fibres. J Physiol. 1969 Feb;200(3):807–819. doi: 10.1113/jphysiol.1969.sp008723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. V. The Combinations of Haemoglobin with Oxygen and with Carbon Monoxide. I. Biochem J. 1913 Oct;7(5):471–480. doi: 10.1042/bj0070471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Nakajima S. The effect of diameter on the electrical constants of frog skeletal muscle fibres. J Physiol. 1972 Feb;221(1):105–120. doi: 10.1113/jphysiol.1972.sp009742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter O. F. Potassium conductance of skeletal muscle treated with formaldehyde. Nature. 1969 Dec 20;224(5225):1215–1217. doi: 10.1038/2241215a0. [DOI] [PubMed] [Google Scholar]
- Johnson P. N., Inesi G. The effect of methylxanthines and local anesthetics on fragmented sarcoplasmic reticulum. J Pharmacol Exp Ther. 1969 Oct;169(2):308–314. [PubMed] [Google Scholar]
- Kerrick W. G., Best P. M. Calcium ion release in mechanically disrupted heart cells. Science. 1974 Feb 1;183(4123):435–437. doi: 10.1126/science.183.4123.435. [DOI] [PubMed] [Google Scholar]
- Lüttgau H. C., Oetliker H. The action of caffeine on the activation of the contractile mechanism in straited muscle fibres. J Physiol. 1968 Jan;194(1):51–74. doi: 10.1113/jphysiol.1968.sp008394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y., Endo M. Release of calcium induced by 'depolarisation' of the sarcoplasmic reticulum membrane. Nat New Biol. 1973 Dec 19;246(155):216–218. doi: 10.1038/newbio246216a0. [DOI] [PubMed] [Google Scholar]
- OHNISHI T., EBASHI S. SPECTROPHOTOMETRICAL MEASUREMENT OF INSTANTANEOUS CALCIUM BINDING OF THE RELAXING FACTOR OF MUSCLE. J Biochem. 1963 Dec;54:506–511. doi: 10.1093/oxfordjournals.jbchem.a127823. [DOI] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. The effect of carbon dioxide on the intracellular pH and buffering power of snail neurones. J Physiol. 1976 Mar;255(3):715–735. doi: 10.1113/jphysiol.1976.sp011305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdiosera R., Clausen C., Eisenberg R. S. Impedance of frog skeletal muscle fibers in various solutions. J Gen Physiol. 1974 Apr;63(4):460–491. doi: 10.1085/jgp.63.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdiosera R., Clausen C., Eisenberg R. S. Measurement of the impedance of frog skeletal muscle fibers. Biophys J. 1974 Apr;14(4):295–315. doi: 10.1016/S0006-3495(74)85917-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A. Regulatory mechanisms of the calcium transport system of fragmented rabbit sarcoplasmic rticulum. I. The effect of accumulated calcium on transport and adenosine triphosphate hydrolysis. J Gen Physiol. 1971 Jan;57(1):50–63. doi: 10.1085/jgp.57.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]


