Abstract
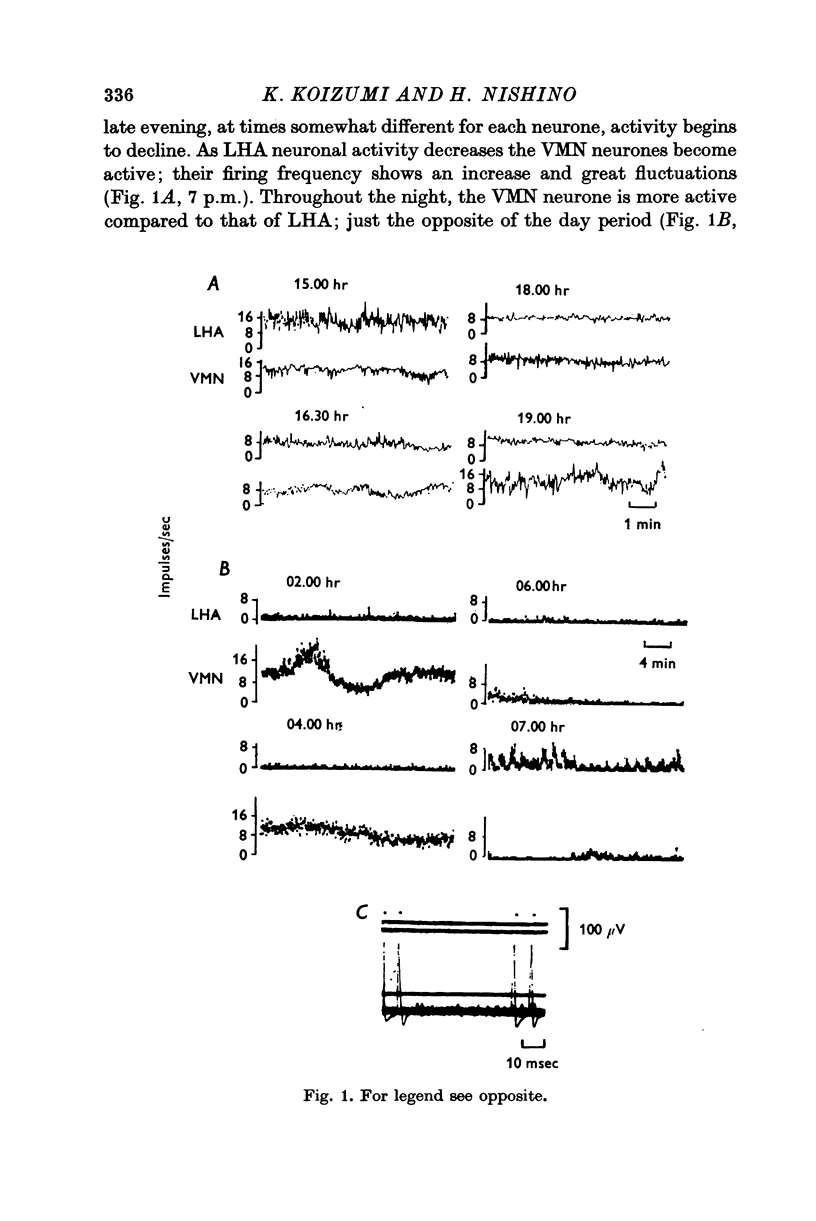
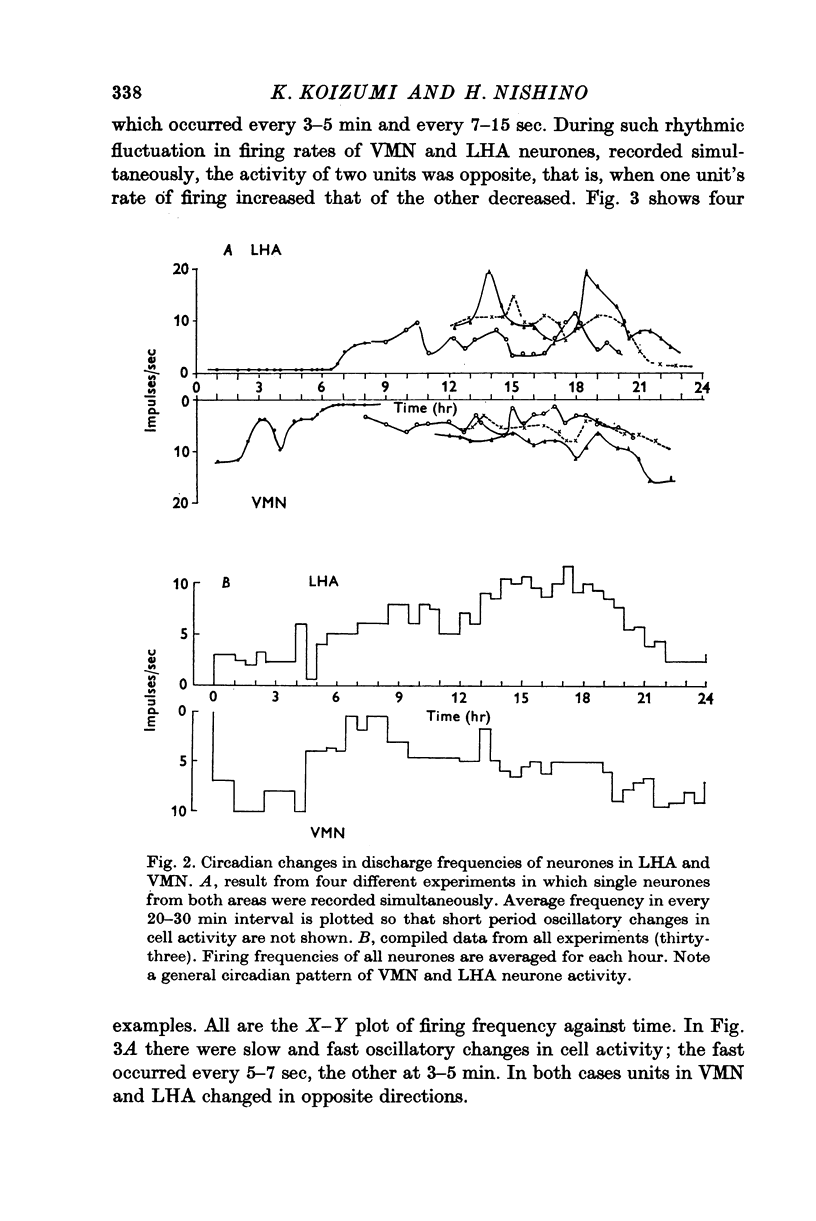
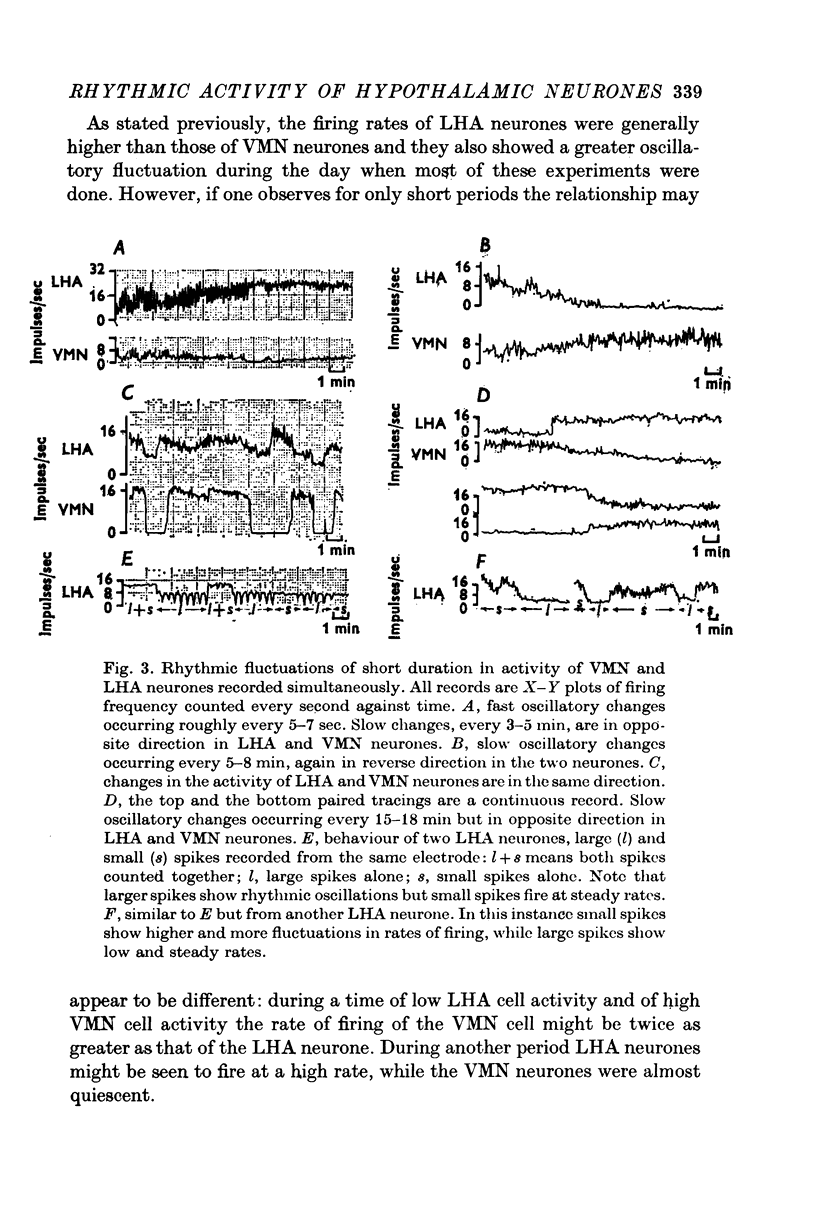
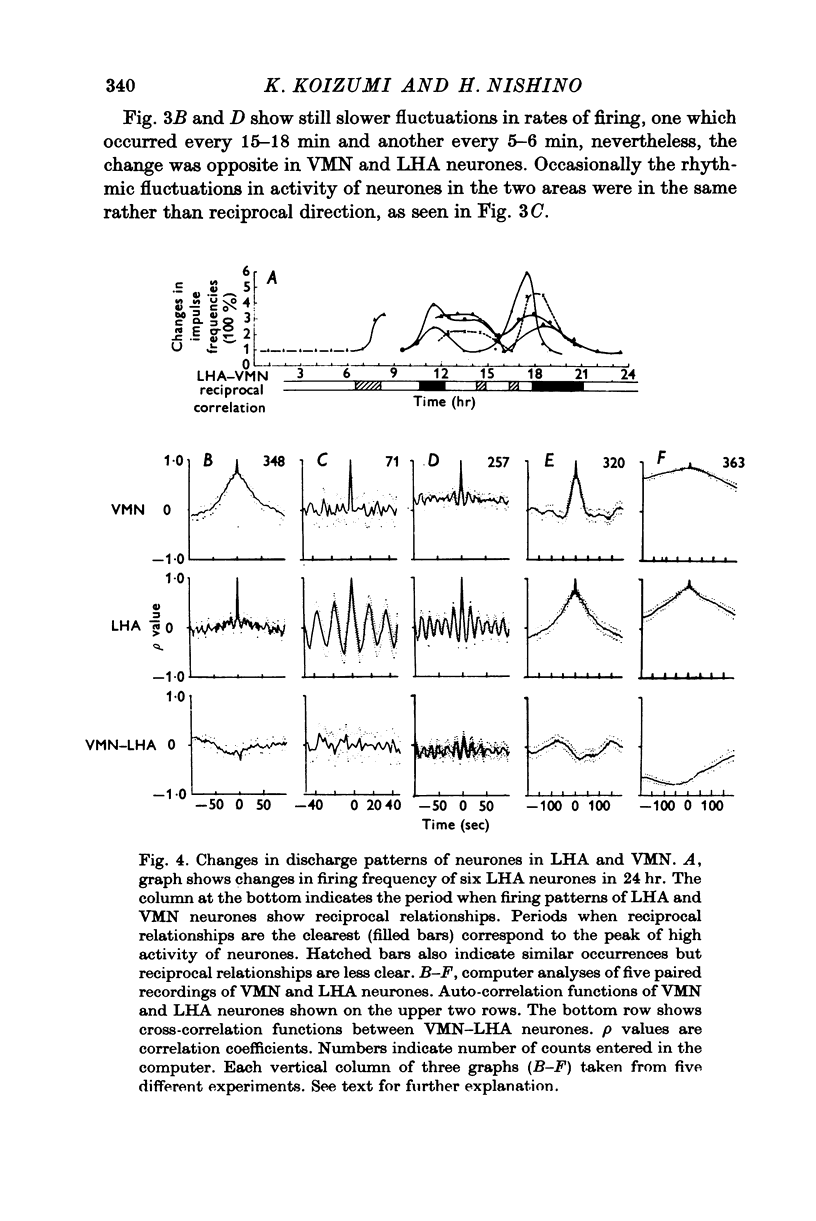
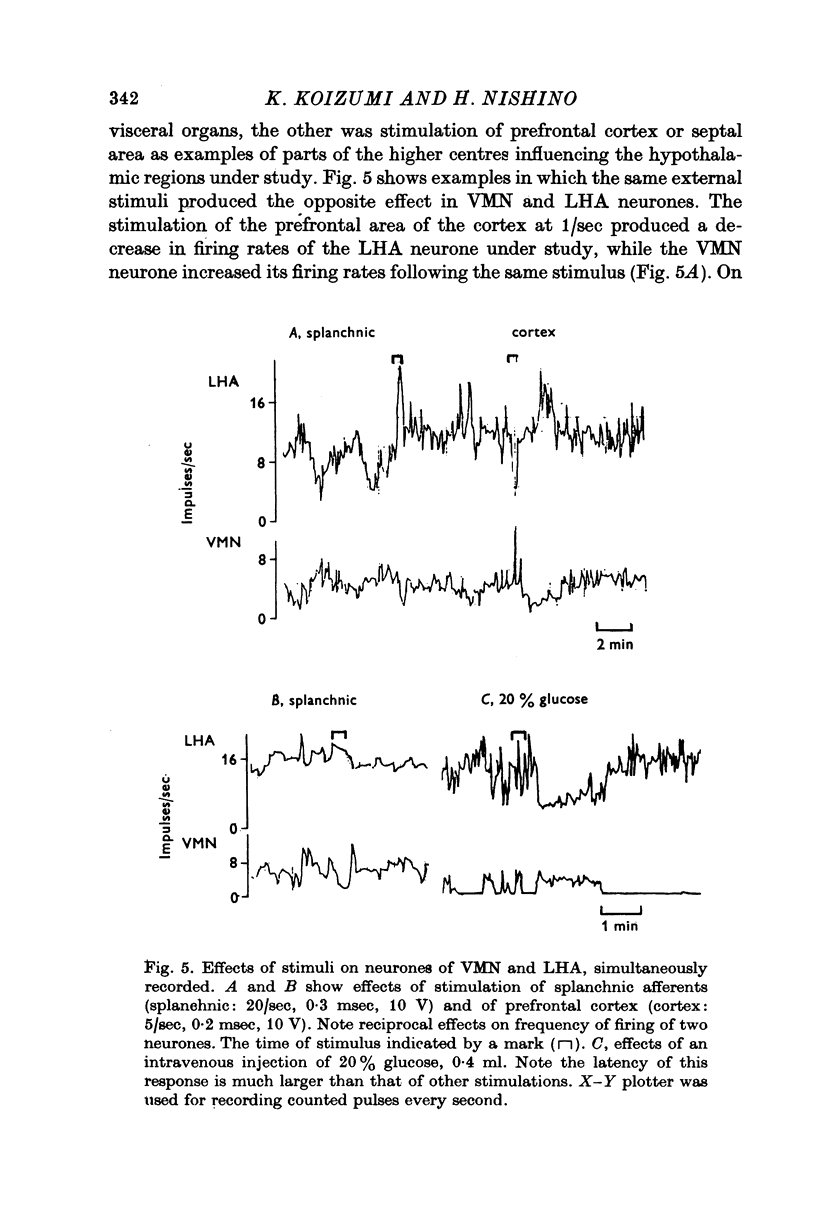
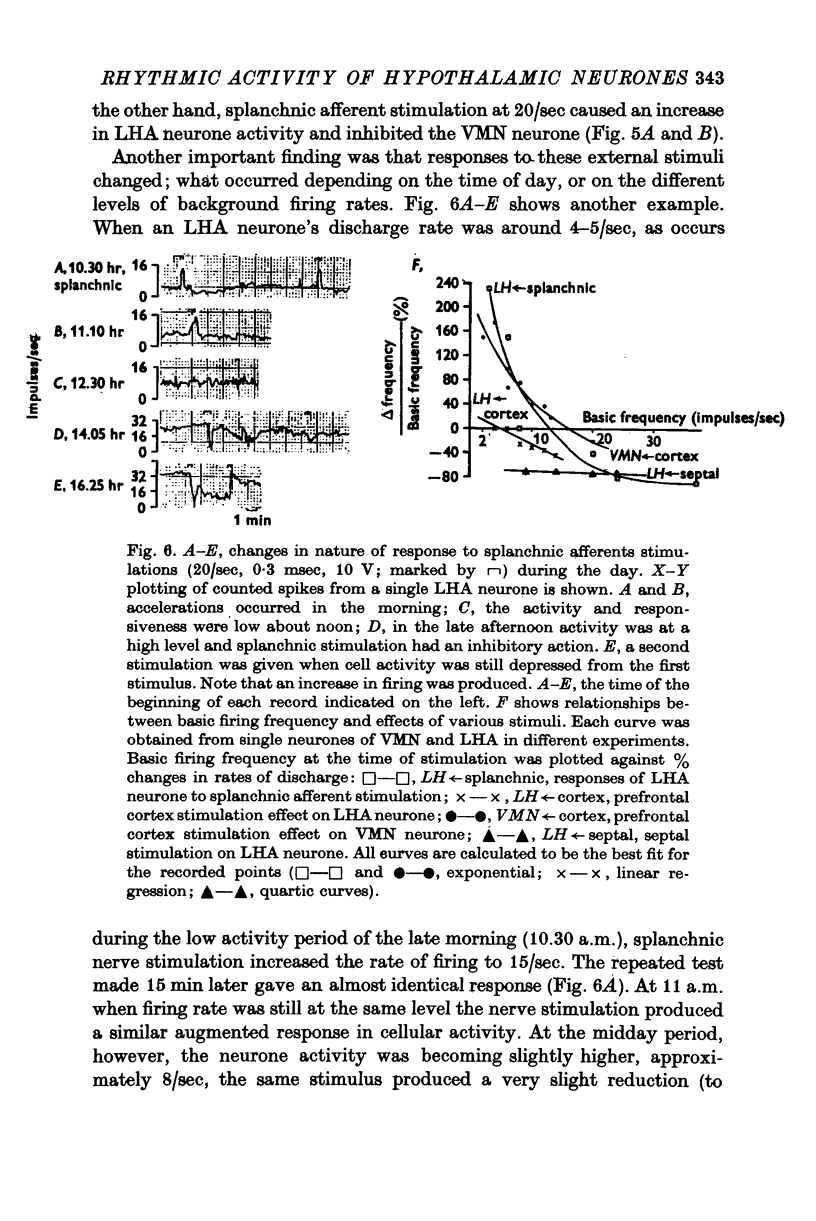
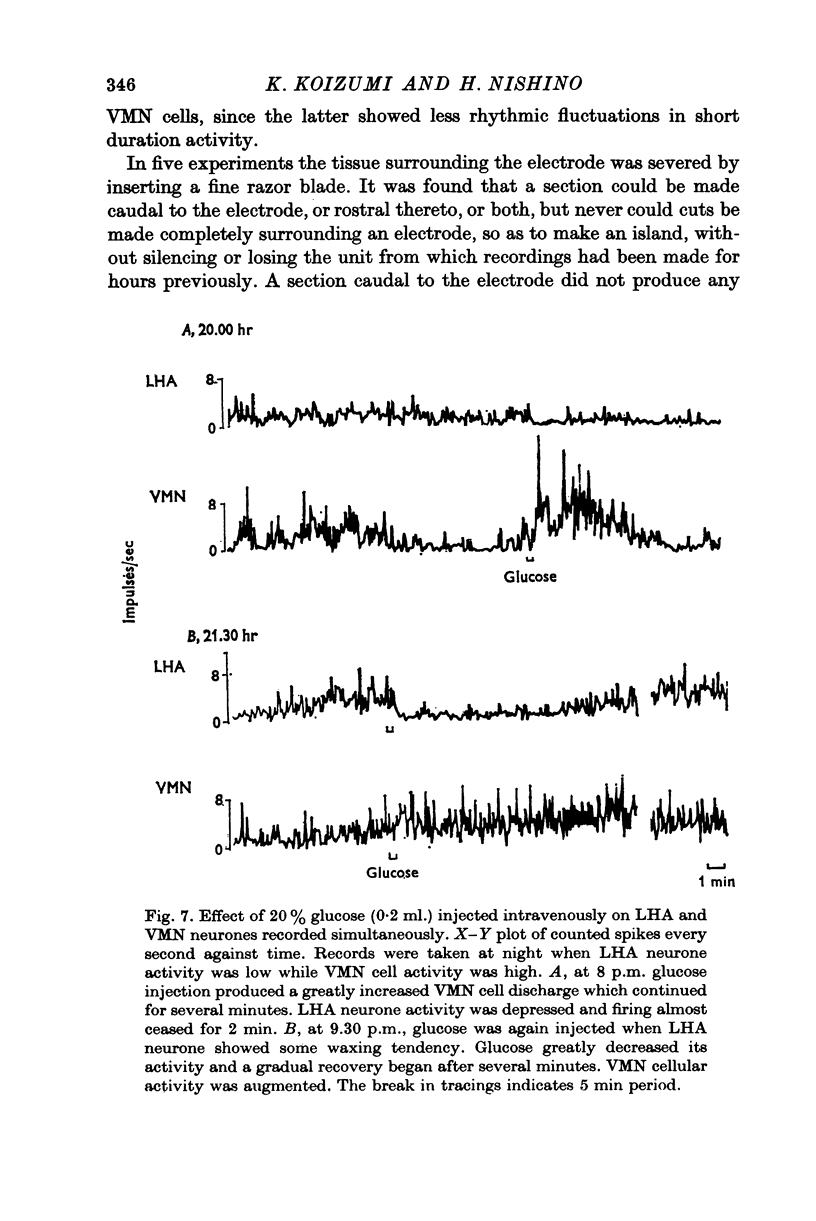
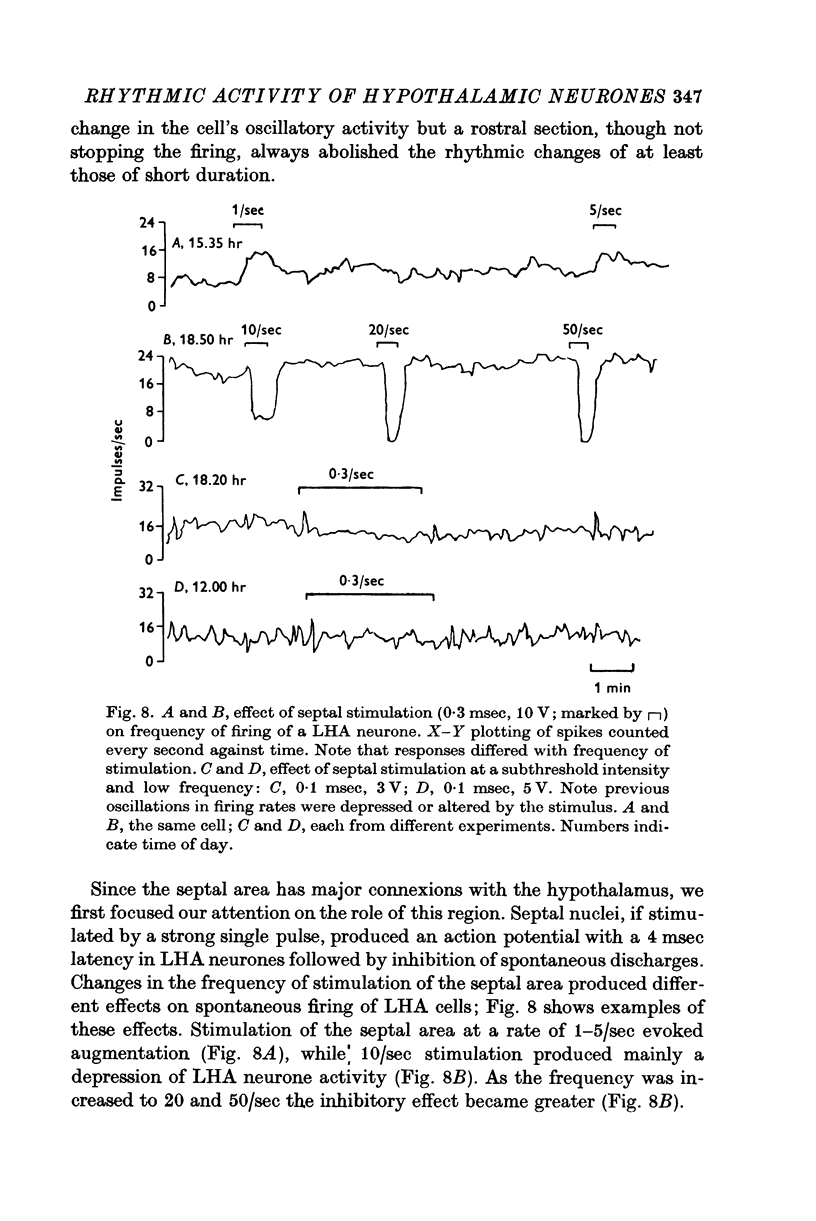
1. The frequency of firing was simultaneously recorded from single neurones of the ventromedial nuclei (VMN) and the lateral hypothalamic area (LHA) in urethane anaesthetized rats for many hours. 2. There were circadian changes of VMN and LHA neurone activity. The pattern of this circadian rhythm is as follows: throughout the day LHA neurones show higher activity than that of VMN, as indicated by higher frequency and more fluctuations in their rates of firing. In late afternoon the discharge rate of LHA neurones increases further, showing oscillations of short duration. In the early evening hours LHA neurone activity gradually goes down, as the VMN neurones become active. Throughout the night, VMN neurones are more active than those of LHA, just the opposite of the day period. In early morning hours VMN neurones gradually become quiet, while LHA neurones begin to show activity. 3. Superimposed on the circadian rhythm, at certain periods of the day, VMN and LHA neurones showed short duration oscillations in rate of firing, roughly every 7-15 sec and every 3-5 min. 4. Activities in neurones of the VMN and LHA were reciprocally related; a decrease in firing rate of one was associated with an increase in the other. This phenomenon was shown clearly by analysis of auto- and cross-correlation functions of firing patterns of VMN and LHA neurones. 5. The effects of stimulations of the prefrontal cortex and splanchnic afferents on VMN and LHA neurones depended on the basic firing frequency, thus they varied with the time of day. Definite relationships exist between basic firing frequency of a cell and the magnitude of changes evoked by these stimuli. Reactions of VMN and LHA neurones were the opposite in most instances. Septal stimulations (at more than 10/sec) always produced inhibition of LHA neurone activity. 6. Intravenous injection of glucose inhibited LHA neurones and accelerated firing of VMN cells. This was true during the day period as well as at night when background activities of VMN and LHA neurones were different from that of the day. 7. Stimulation of the septal area with subthreshold pulses at a low rate (1-0.3/sec) suppressed or altered oscillations in firing frequency of LHA neurones. Severance of connection between LHA and structures caudal thereto had no effect on LHA neurone firing rates or rhythms. Sections between the septal area and LHA, however, abolished or greatly altered the oscillatory rhythms of LHA cell activity, although spontaneous discharges continued at a somewhat lower rate for periods of hours. 8. Stimulation of suprachiasmatic nuclei with weak intensity and low frequency also changed oscillatory fluctuations in firing of LHA neurones. 9. Possible origins of circadian rhythm and oscillations of short duration in firing pattern of VMN and LHA neurones were discussed.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANAND B. K., CHHINA G. S., SHARMA K. N., DUA S., SINGH B. ACTIVITY OF SINGLE NEURONS IN THE HYPOTHALAMIC FEEDING CENTERS: EFFECT OF GLUCOSE. Am J Physiol. 1964 Nov;207:1146–1154. doi: 10.1152/ajplegacy.1964.207.5.1146. [DOI] [PubMed] [Google Scholar]
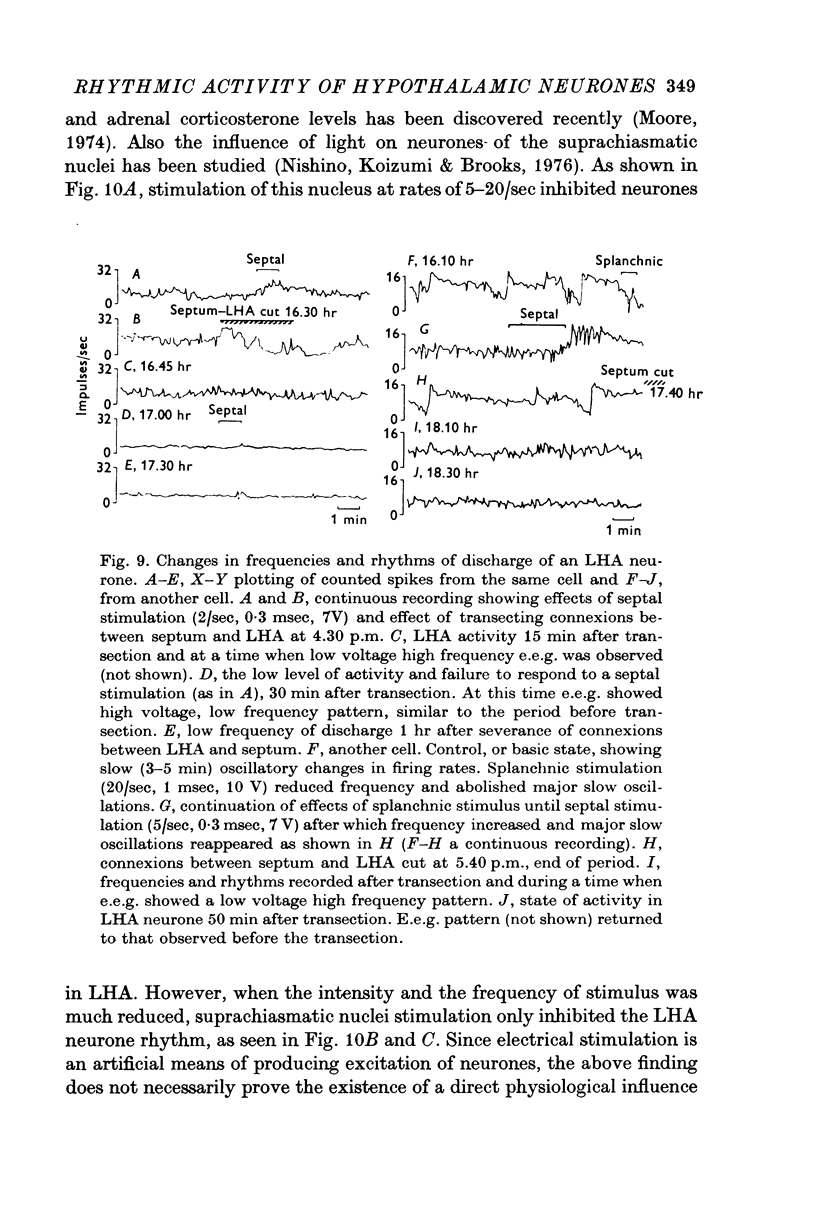
- Brooks C. M., Koizumi K., Zeballos G. A. A study of factors controlling activity of neurons within the paraventricular, supraoptic and ventromedian nuclei of the hypothalamus. Acta Physiol Lat Am. 1966;16:83–96. [PubMed] [Google Scholar]
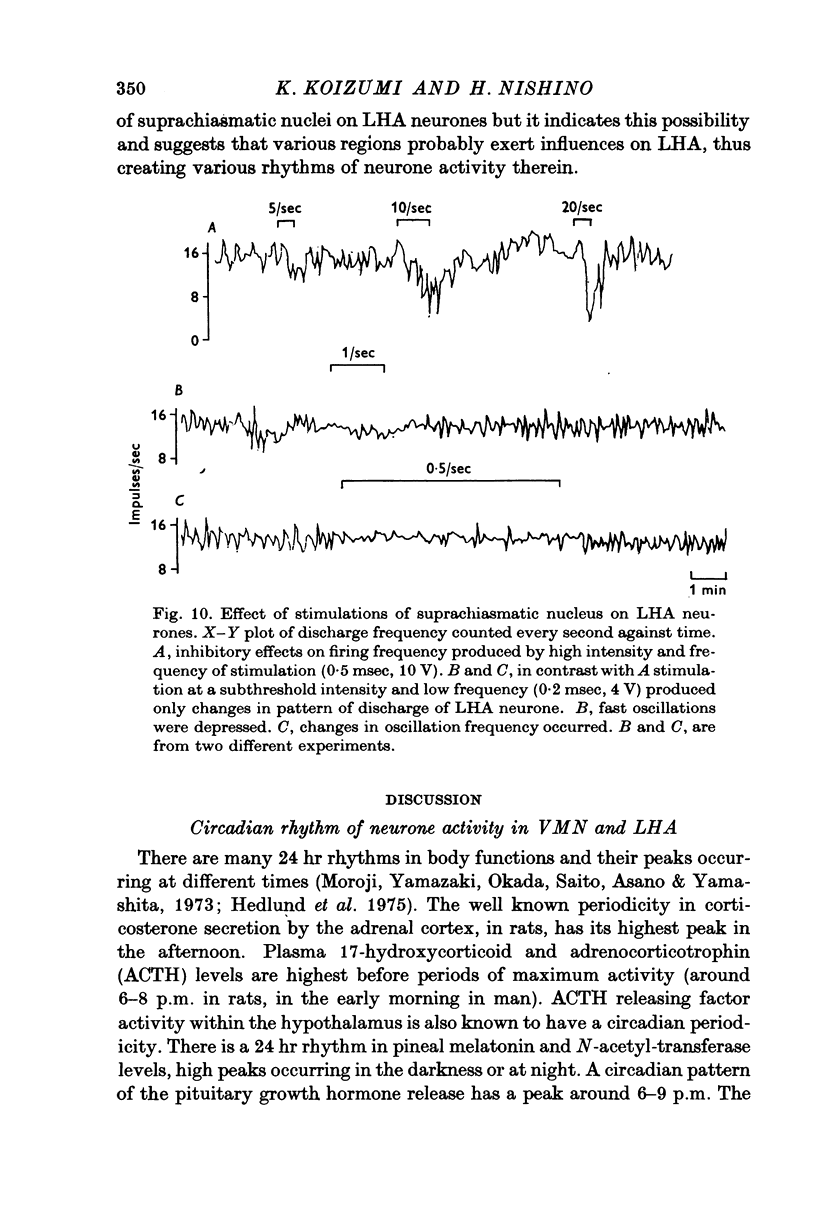
- Macadar O., Roig J. A., Monti J. M., Budelli R. The functional relationship between septal and hippocampal unit activity and hippocampal theta rhythm. Physiol Behav. 1970 Dec;5(12):1443–1449. doi: 10.1016/0031-9384(70)90134-4. [DOI] [PubMed] [Google Scholar]
- Millhouse O. E. Certain ventromedial hypothalamic afferents. Brain Res. 1973 May 30;55(1):89–105. doi: 10.1016/0006-8993(73)90490-3. [DOI] [PubMed] [Google Scholar]
- Millhouse O. E. The organization of the ventromedial hypothalamic nucleus. Brain Res. 1973 May 30;55(1):71–87. [PubMed] [Google Scholar]
- Moberg G. P., Scapagnini U., de Groot J., Ganong W. F. Effect of sectioning the fornix on diurnal fluctuation in plasma corticosterone levels in the rats. Neuroendocrinology. 1971;7(1):11–15. doi: 10.1159/000121950. [DOI] [PubMed] [Google Scholar]
- Morales F. R., Roig J. A., Monti J. M., Macadar O., Budelli R. Septal unit activity and hippocampal EEG during the sleep-wakefulness cycle of the rat. Physiol Behav. 1971 May;6(5):563–567. doi: 10.1016/0031-9384(71)90206-x. [DOI] [PubMed] [Google Scholar]
- Murphy J. T., Renaud L. P. Mechanisms of inhibition in the ventromedial nucleus of the hypothalamus. J Neurophysiol. 1969 Jan;32(1):85–102. doi: 10.1152/jn.1969.32.1.85. [DOI] [PubMed] [Google Scholar]
- Nishino H., Kiyomi K., Brooks C. M. The role of suprachiasmatic nuclei of the hypothalamus in the production of circadian rhythm. Brain Res. 1976 Aug 6;112(1):45–59. doi: 10.1016/0006-8993(76)90333-4. [DOI] [PubMed] [Google Scholar]
- OOMURA Y., KIMURA K., OOYAMA H., MAENO T., IKI M., KUNIYOSHI M. RECIPROCAL ACTIVITIES OF THE VENTROMEDIAL AND LATERAL HYPOTHALAMIC AREAS OF CATS. Science. 1964 Jan 31;143(3605):484–485. doi: 10.1126/science.143.3605.484. [DOI] [PubMed] [Google Scholar]
- Oomura Y. Central mechanism of feeding. Adv Biophys. 1973;5(0):65–142. [PubMed] [Google Scholar]
- Oomura Y., Ono T., Ooyama H., Wayner M. J. Glucose and osmosensitive neurones of the rat hypothalamus. Nature. 1969 Apr 19;222(5190):282–284. doi: 10.1038/222282a0. [DOI] [PubMed] [Google Scholar]
- Oomura Y., Ooyama H., Naka F., Yamamoto T., Ono T., Kobayashi N. Some stochastical patterns of single unit discharges in the cat hypothalamus under chronic conditions. Ann N Y Acad Sci. 1969 May 15;157(2):666–689. doi: 10.1111/j.1749-6632.1969.tb12913.x. [DOI] [PubMed] [Google Scholar]
- Renaud L. P., Martin J. B. Electrophysiological studies of connections of hypothalamic ventromedial nucleus neurons in the rat: evidence for a role in neuroendocrine regulation. Brain Res. 1975 Jul 25;93(1):145–151. doi: 10.1016/0006-8993(75)90293-0. [DOI] [PubMed] [Google Scholar]
- Rusak B., Zucker I. Biological rhythms and animal behavior. Annu Rev Psychol. 1975;26:137–171. doi: 10.1146/annurev.ps.26.020175.001033. [DOI] [PubMed] [Google Scholar]
- Schmitt M. Circadian rhythmicity in responses of cells in the lateral hypothalamus. Am J Physiol. 1973 Nov;225(5):1096–1101. doi: 10.1152/ajplegacy.1973.225.5.1096. [DOI] [PubMed] [Google Scholar]
- Schmitt M. Influences of hepatic portal receptors on hypothalamic feeding and satiety centers. Am J Physiol. 1973 Nov;225(5):1089–1095. doi: 10.1152/ajplegacy.1973.225.5.1089. [DOI] [PubMed] [Google Scholar]
- Strumwasser F. Seventeenth Bowditch lecture. Neural and humoral factors in the temporal organization of behavior. Physiologist. 1973 Feb;16(1):9–42. [PubMed] [Google Scholar]
- Yamaoka S., Hagino N. Spontaneous septal neuron activity in the rat. Brain Res. 1974 Feb 15;67(1):147–152. doi: 10.1016/0006-8993(74)90305-9. [DOI] [PubMed] [Google Scholar]
- Yokota T., Reeves A. G., MacLean P. D. Differential effects of septal and olfactory volleys on intracellular responses of hippocampal neurons in awake, sitting monkeys. J Neurophysiol. 1970 Jan;33(1):96–107. doi: 10.1152/jn.1970.33.1.96. [DOI] [PubMed] [Google Scholar]


