Abstract
From 1984 to 1999, we collected 31 isolates of the rare serovar Salmonella bongori 48:z35:− in southern Italy. Twenty-four of the isolates were from cases of acute enteritis in humans. Pulsed-field gel electrophoresis analysis showed that all but one of our isolates were at least 80% similar. Our findings suggest that genetically related S. bongori 48:z35:− strains are endemically circulating in southern Italy.
Serovars of Salmonella other than subspecies enterica are associated mainly with cold-blooded animals and rarely colonize the intestines of warm-blooded animals. Human infections with serovars of Salmonella bongori or Salmonella enterica subspecies salamae, arizonae, diarizonae, houtenae, and indica are infrequent and are usually the result of contact with reptiles (2, 15). From 1985 to 1999, we collected 24 isolates from an epidemic cluster and from apparently sporadic cases of acute enteritis caused by S. bongori 48:z35:− in different cities in southern Italy (9, 10, 13). Seven further isolates of the same serovar were collected from a healthy human carrier; warm-blooded animals, i.e., two apparently healthy pigeons and a dog with diarrhea; and the environment (urban wastewater and food) (see Table 1).
TABLE 1.
Origin and characterization of the 31 human, animal, and environmental S. bongori 48:z35:- isolates from southern Italy (1984 to 1999) used in this study to determine PFGE XbaI-digested genomic DNA profiles
| Isolate no. | Source | Clinical manifestation | Year | Cityb | PFGE profile |
|---|---|---|---|---|---|
| CEIM 24450 | Pigeon | Apparently healthy | 1984 | Messina | 12 |
| CEIM 24686 | Child | Acute enteritis | 1985 | Messina | 12 |
| CEIM 24663 | Child | Acute enteritis | 1985 | Messina | 12 |
| CEIM 24682 | Child | Acute enteritis | 1985 | Messina | 12 |
| CEIM 24662 | Child | Acute enteritis | 1985 | Messina | 12 |
| CEIM 26851 | Pigeon | Apparently healthy | 1985 | Messina | 12 |
| CEIM 24665 | Child | Acute enteritis | 1985 | Messina | 12 |
| CEIM 26549 | Child | Acute enteritis | 1986 | Messina | 1 |
| C 285 | Child | Acute enteritis | 1985 | Catania | 14 |
| C 102 | Child | Acute enteritis | 1994 | Catania | 8 |
| C 250 | Child | Acute enteritis | 1995 | Catania | 13 |
| C 247 | Child | Acute enteritis | 1995 | Catania | 7 |
| C 261 | Child | Acute enteritis | 1995 | Catania | 6 |
| C 376 | Child | Acute enteritis | 1996 | Catania | 6 |
| C 368 | Child | Acute enteritis | 1996 | Catania | 9 |
| C 370 | Child | Acute enteritis | 1996 | Catania | 10 |
| C 485 | Child | Acute enteritis | 1997 | Catania | 1 |
| C 634 | Child | Acute enteritis | 1998 | Catania | 17 |
| CEIM 45048 | Child | Acute enteritis | 1998 | Carini (Palermo) | 3 |
| CEIM 44992 | Child | Acute enteritis | 1998 | Marineo (Palermo) | 2 |
| CEIM 44833 | Child | Acute enteritis | 1998 | Palermo | 2 |
| CEIM 44847 | Child | Acute enteritis | 1998 | Palma Montechiaro (Agrigento) | 2 |
| CEIM 44979 | Child | Acute enteritis | 1998 | Mezzojuso (Palermo) | 11 |
| CEIM 150 | Child | Acute enteritis | 1998 | Palermo | 18 |
| CEIM 44983 | Child | Acute enteritis | 1998 | Cinisi (Palermo) | 15 |
| CEIM 46295 | HIV+ adulta | Acute enteritis | 1999 | Palermo | 1 |
| CEIM 44712 | Waste water | 1998 | Ragusa | 1 | |
| CEIM 44721 | Waste water | 1998 | Ragusa | 4 | |
| CEIM 46162 | Adult | Healthy carrier | 1999 | Ragusa | 16 |
| CEIM 46049 | Cheese | 1999 | Agrigento | 21 | |
| CEIM 46082 | Dog | Diarrhea | 1999 | Cosenza | 5 |
HIV+, human immunodeficiency virus positive.
City names are abbreviated as follows: Messina (ME), Catania (CT), Palermo (PA), Ragusa (RG), Agrigento (AG), and Cosenza (CS).
All but one of our isolates from human cases of diarrhea were from children aged 1 month to 3 years. The first five isolates were identified at the beginning of 1985 from a small epidemic cluster of cases of acute enteritis in children who presented with moderate to severe diarrhea with fever and who recovered 3 to 8 days after rehydration therapy. The only isolate from an adult case of diarrhea was from a human immunodeficiency virus-positive patient in 1999. This patient also recovered rapidly after antibiotic therapy.
To our knowledge, no cases of human infections with S. bongori 48:z35:− have ever been reported in other countries; the only recorded isolates of this serovar are the original S. bongori strain isolated in Chad from a lizard in 1966 (7) and four isolates from foodstuffs sent in 1985 from England to the World Health Organization Collaborating Centre for Reference and Research on Salmonella, Institut Pasteur, Paris, France (M. Y. Popoff, personal communication).
We subjected all the southern Italy isolates to molecular typing by pulsed-field gel electrophoresis (PFGE) analysis of digested DNA (Table 1). Two collection strains kindly supplied by the World Health Organization Collaborating Centre for Reference and Research on Salmonella (M. Y. Popoff), the original strain CIP 261-66, isolated in Chad, and the strain CIP 5040-85, isolated in England, were also analyzed. Analysis of digested DNA by PFGE was performed as described by Faith et al. (5). The XbaI restriction enzyme (Pharmacia LKB Biotechnology AB, Uppsala, Sweden) was used for digestion of genomic DNA. Similarities among restriction endonuclease digestion profiles were calculated by the Dice similarity index (3, 4) with the Taxotron software RestrictoTyper module (Taxolab, Institut Pasteur, Paris, France). A dendrogram tree was constructed by using the Adanson and Dendrograf module of Taxotron software and applying the unweighted pair group method of averages algorithm to the distance matrix resulting from the comparison of the profiles.
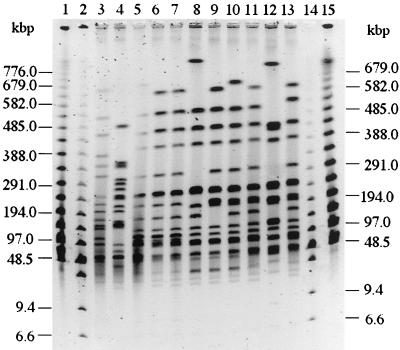
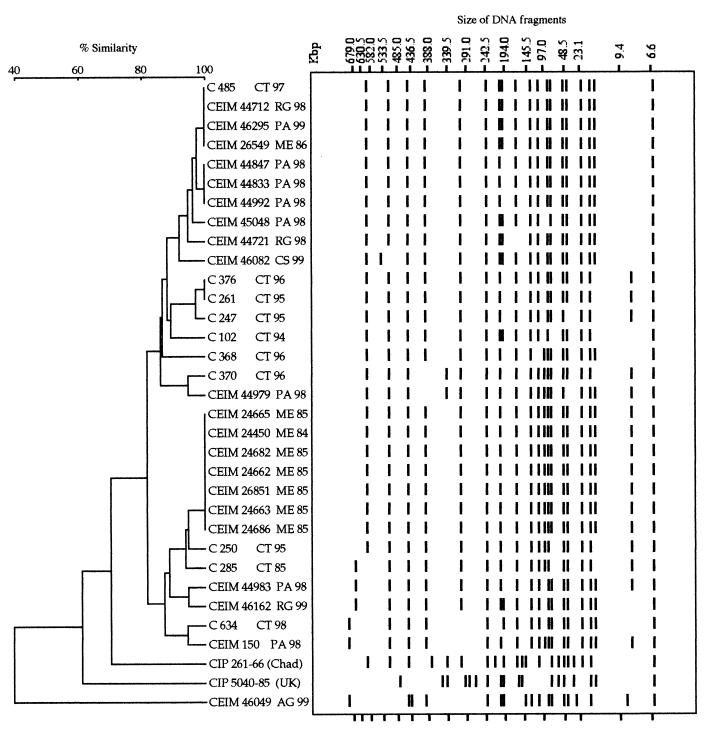
PFGE of XbaI-digested genomic DNA from the 31 S. bongori 48:z35:− isolates from southern Italy showed 19 profiles (profiles 1 to 18 and 21) differing by two or more fragments (Fig. 1 and 2). Profiles 19 and 20 were obtained, respectively, from the original strain isolated in Chad and from the strain isolated in England and differed from each other and from all the profiles of the isolates from southern Italy by several fragments. All but one of these showed identical or more than 79% similar profiles, differing from each other by two to seven bands. Only the profile of a soft cheese isolate apparently not linked to food-borne infection (profile 21) was clearly unrelated (less than 40% similarity) to those of the other isolates. The five isolates from the epidemic cluster collected from Messina in 1985 shared identical profiles with two other strains isolated in the same town from pigeons (profile 12). Three other small clusters of identical profiles were detected, but they all included isolates from different sources in terms of place and/or year of isolation. Considering profile 12 the original ancestor clone, which included the oldest isolates, all but one of the southern Italy isolates showed two- to five-band differences and should be considered either closely or possibly related to the ancestor's profile (14).
FIG. 1.
PFGE restriction patterns of nine representative S. bongori 48:z35:− isolates from Sicily and Calabria and two collection strains from Chad and England. Lanes 1 and 15, lambda ladder pulsed-field gel (PFG) marker 340 (New England Biolabs, Inc., Beverly, Mass.); lanes 2 and 14, low-range PFG marker 350 (New England Biolabs); lane 3, CIP 261-66 (Chad); lane 4, CIP 5040-85 (United Kingdom); lane 5, C 285 CT 85; lane 6, CEIM 24662 ME 85; lane 7, CEIM 26851 ME 85; lane 8, C 634 CT 98; lane 9, CEIM 44721 RG 98; lane 10, CEIM 46162 RG 99; lane 11, CEIM 46295 PA 99; lane 12, CEIM 46049 AG 99; lane 13, CEIM 46082 CS 99. The two-digit numbers after the city abbreviation (see Table 1, footnote b) indicate the year of isolation.
FIG. 2.
Dendrogram showing percent similarity calculated by the Dice similarity index of PFGE restriction endonuclease digestion profiles among the 31 S. bongori 48:z35:− isolates from southern Italy and two collection strains from Chad (CIP 261-66) and England (CIP 5040-85).
Genetic homogeneity of southern Italy S. bongori 48:z35:− strains had already been shown by ribotyping (9), which is known to be less discriminating than PFGE. In profiles produced by PFGE, differences of two to three fragments in the banding patterns suggest that a single genetic mutation has occurred while differences of four to six bands are observed when two independent genetic events occur (14). None except one of the southern Italy S. bongori 48:z35:− isolates differed in their PFGE profiles from the supposed original clone (profile 12) by more than five fragments. This result is consistent with a large circulation due to a prolonged endemic presence in our population. The presence of S. bongori 48:z35:− in southern Italy before 1984 is not documented (8) but nevertheless cannot be excluded. In past years, many rare and new serovars of Salmonella have been identified in Sicily from wild reptiles but S. bongori 48:z35:− has never been isolated from these animals (11). Transmission of salmonellae from reptiles to children has been repeatedly observed in other countries (1, 6). Although reptiles are not present in households of southern Italy either as pets or as food, lizards are widely present in rural Italy as well as in urban areas (12); thus, children aged less than 3 years might be exposed to lizard droppings while crawling on the floor or being in a playground. The diffuse endemic presence of S. bongori 48:z35:− in our population is confirmed by the finding of isolates from urban wastewaters whose PFGE patterns were identical or closely related to those of isolates from cases of acute enteritis. The significance of the isolation of this serovar from domestic animals as occurred in the city of Messina from pigeons and in the city of Cosenza from a dog is questionable. Although pet animals can be sources of infections, mainly for children, they can also be infected by humans. Finally, considering that the majority of our cases of enteritis occurred in infants in the first months of life, the infection may have been transmitted by person-to-person contact and originated in adult healthy carriers within the family.
In conclusion, S. bongori 48:z35:−, a rare serovar that has never been recorded as being responsible for human or animal infections in other countries, proved to be able to cause acute enteritis in children and, occasionally, in immunodeficient adults and in animals in southern Italy. PFGE analysis of the XbaI-digested genomic DNA of isolates showed them to be genetically distant from the only two previously isolated S. bongori 48:z35:− strains from Chad and England. On the contrary, PFGE profiles of all but one of the isolates from southern Italy did not differ substantially from each other. Since these genetically related isolates have been detected over a 15-year period and are mostly epidemiologically unrelated, we can affirm that S. bongori 48:z35:− is endemically circulating in our population.
REFERENCES
- 1.Ackman, D. M., P. Drabkin, G. Birkhead, and P. Cieslak. 1995. Reptile-associated salmonellosis in New York state. Pediatr. Infect. Dis. J. 14:955-959. [DOI] [PubMed] [Google Scholar]
- 2.Aleksic, S., F. Heinzerling, and J. Bockemuhl. 1996. Human infection caused by Salmonellae of subspecies II to VI in Germany, 1977-1992. Zentbl. Bakteriol. 283:391-398. [DOI] [PubMed] [Google Scholar]
- 3.Brosch, R., J. Chen, and J. B. Luchansky. 1994. Pulsed-field fingerprinting of listeriae: identification of genomic divisions for Listeria monocytogenes and their correlation with serovar. Appl. Environ. Microbiol. 60:2584-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dice, L. R. 1945. Measures of the amount of ecological association between species. Ecology 26:297-302. [Google Scholar]
- 5.Faith, N. G., J. A. Shere, R. Brosch, K. W. Arnold, S. E. Ansay, M. S. Lee, J. B. Luchansky, and C. W. Kaspar. 1996. Prevalence and clonal nature of Escherichia coli O157:H7 on dairy farms in Wisconsin. Appl. Environ. Microbiol. 62:1519-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly, J., R. Hopkin, and M. E. Rimsza. 1995. Rattlesnake meat ingestion and Salmonella arizona infection in children: case report and review of the literature. Pediatr. Infect. Dis. J. 14:320-322. [DOI] [PubMed] [Google Scholar]
- 7.Le Minor, L., G. Chamoiseau, E. Barbe, C. Charie-Marsaines, and L. Egron. 1969. Dix nouveaux sérotypes de Salmonella isolés au Tchad. Ann. Inst. Pasteur (Paris) 116:775-780. [PubMed] [Google Scholar]
- 8.Nastasi, A., C. Mammina, M. R. Villafrate, M. F. Massenti, G. Scarlata, and M. Diquattro. 1988. Multiple typing of strains of Salmonella enterica subsp. bongori ser. 48:z35:− isolated in southern Italy. Ann. Inst. Pasteur Microbiol. 139:605-612. [DOI] [PubMed] [Google Scholar]
- 9.Nastasi, A., C. Mammina, and C. Sacco. 2000. Epidemiology of Salmonella bongori 48:z35:− in southern Italy, 1984-2000. J. Prev. Med. Hyg. 41:31-33. [Google Scholar]
- 10.Nastasi, A., C. Mammina, and L. Salsa. 1999. Outbreak of Salmonella bongori 48:z35:− enteritis in Sicily. Eurosurveillance 4:97-98. [DOI] [PubMed] [Google Scholar]
- 11.Orlandella, B. M. 1997. Sulle nuove salmonelle isolate in Italia e considerazioni sulla infezione salmonellare. Ig. Mod. 107:283-293. [Google Scholar]
- 12.Orlandella, V., C. Alosi, A. Campagna, and L. Coppola. 1969. The epidemiological-epizootic role of lizards in the distribution of Salmonella. G. Batteriol. Virol. Immunol. 62:840-865. [PubMed] [Google Scholar]
- 13.Pignato, S., G. Giammanco, C. Santangelo, and G. M. Giammanco. 1998. Endemic presence of Salmonella bongori 48:z35:− causing enteritis in children in Sicily. Res. Microbiol. 149:429-431. [DOI] [PubMed] [Google Scholar]
- 14.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodward, D. L., R. Khakhria, and W. M. Johnson. 1997. Human salmonellosis associated with exotic pets. J. Clin. Microbiol. 35:2786-2790. [DOI] [PMC free article] [PubMed] [Google Scholar]