Abstract
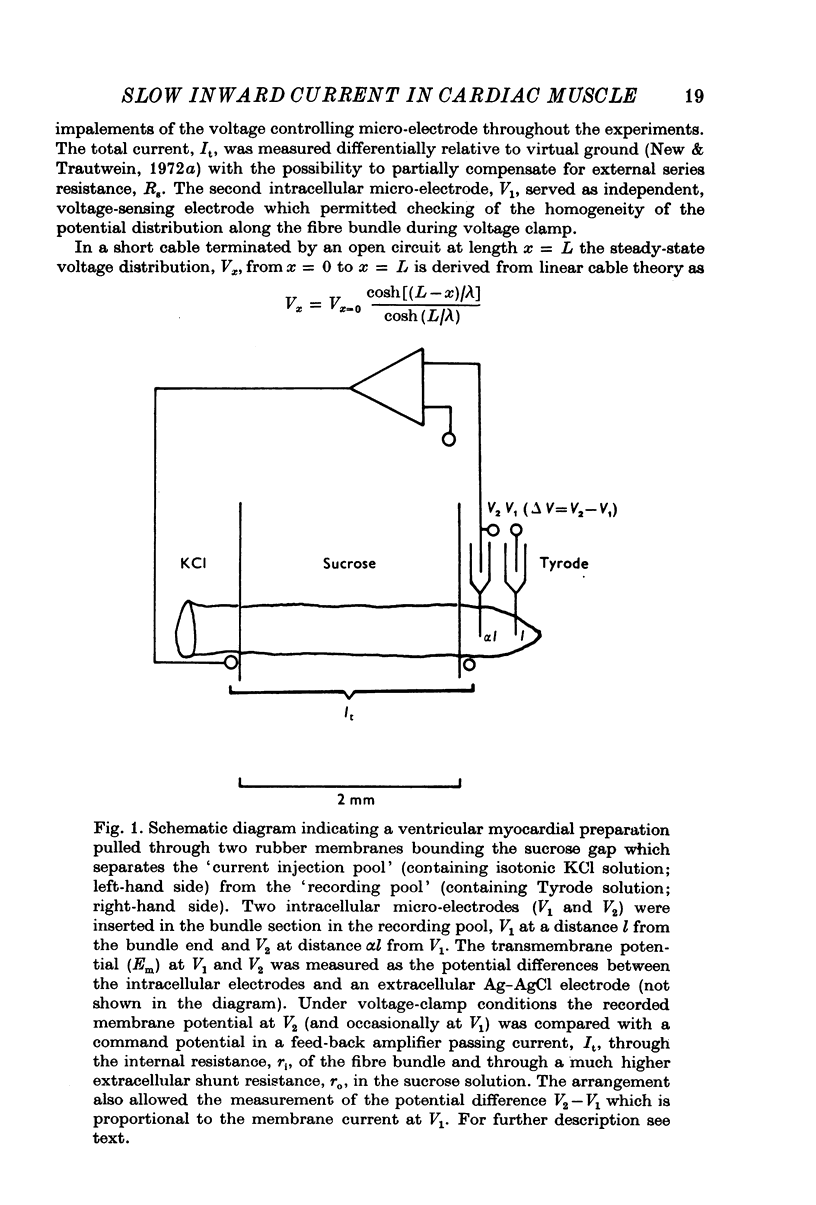
1. A voltage-clamp method combining a single surcose gap and two intracellular micro-electrodes was used to measure membrane currents in ventricullar myocardial fibres. 2. The adequacy of the voltage-clamp method is demonstrated by comparing the total current, It, across the gap with the voltage difference, delta V, between the two intracellular micro-electrodes, i.e. another independent way of measuring membrane currents. With both current measurements the slow inward current, Is, shows the same voltage- and time-dependences. 3. The sensitivity of the slow inward current to variation in external Ca and Na concentrations was investigated systematically. The reversal potential of the slow inward current was sensitive to variation of both ion species. 4. From the reversal potential measurements relative permeabilities of the conductance channels of the slow inward current were estimated as PCa/PNa approximately 1/0-01 and PCa/PK approximately 1/0-01 by means of the constant field equation. 5. The activation and inactivation kinetics of the slow inward current were explored in detail and related to the plateau of the action potential.
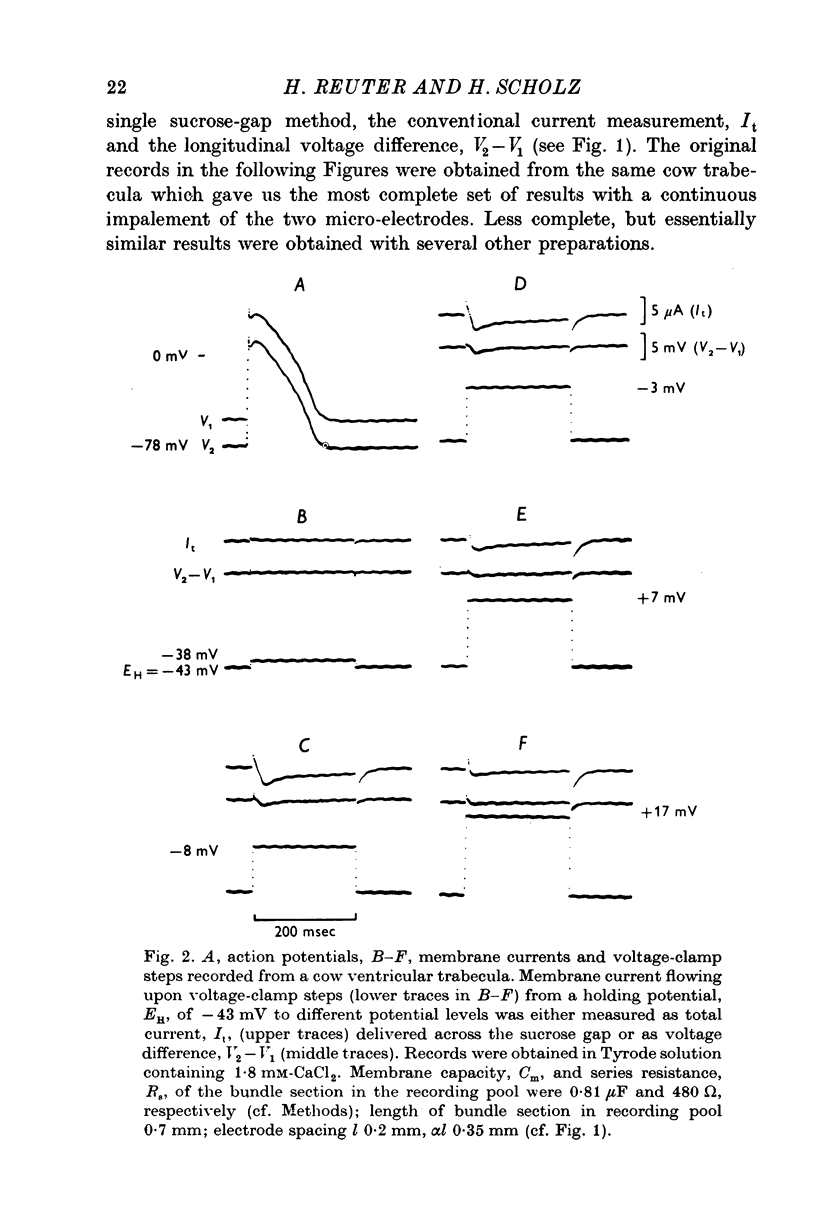
Full text
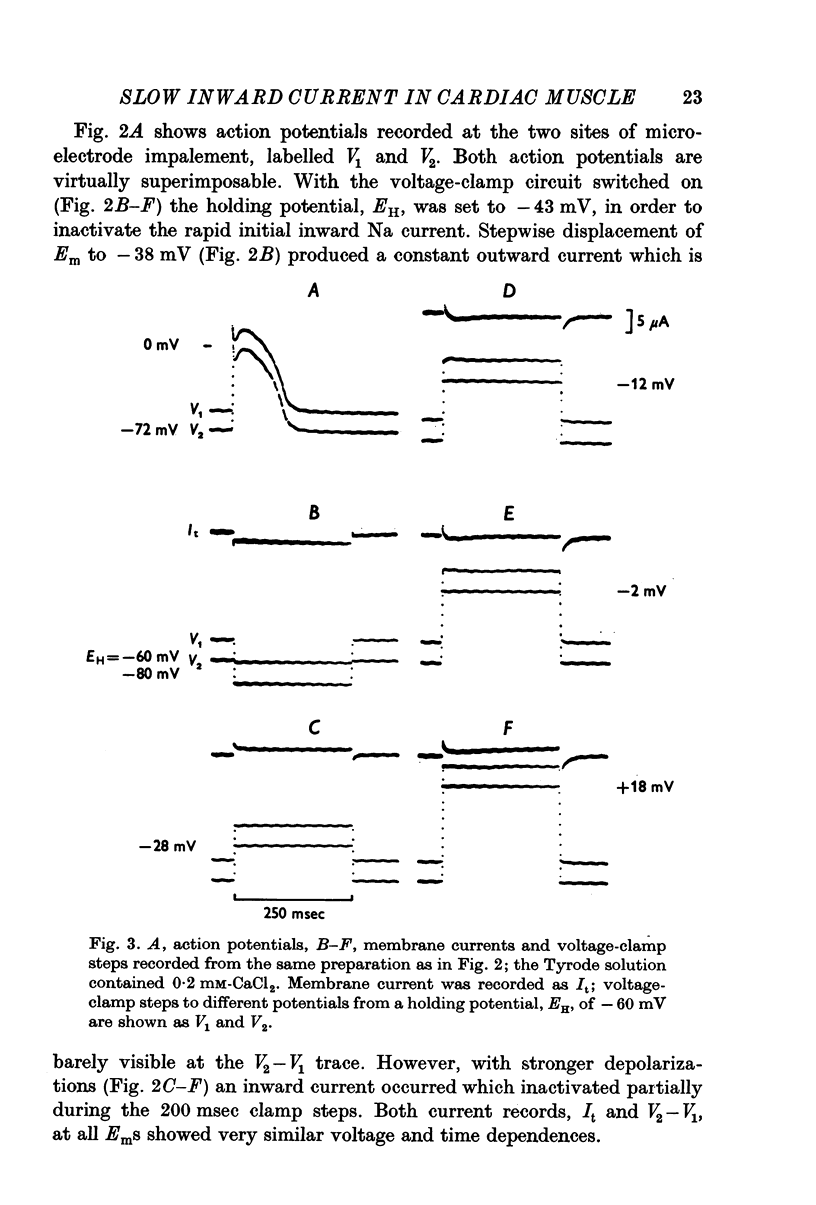
PDF



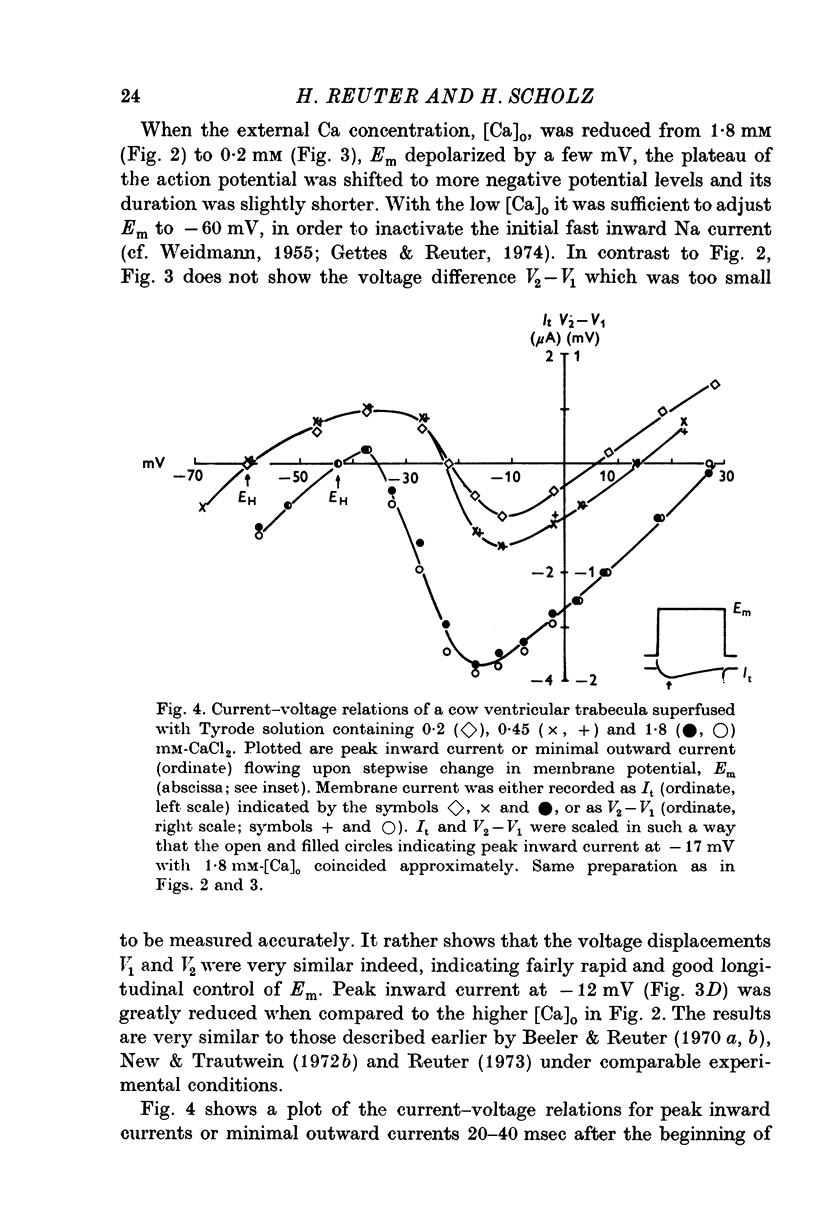












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. Membrane calcium current in ventricular myocardial fibres. J Physiol. 1970 Mar;207(1):191–209. doi: 10.1113/jphysiol.1970.sp009056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. Voltage clamp experiments on ventricular myocarial fibres. J Physiol. 1970 Mar;207(1):165–190. doi: 10.1113/jphysiol.1970.sp009055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begenisich T. Magnitude and location of surface charges on Myxicola giant axons. J Gen Physiol. 1975 Jul;66(1):47–65. doi: 10.1085/jgp.66.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P. The interrelationship between sodium and calcium fluxes across cell membranes. Rev Physiol Biochem Pharmacol. 1974;70:33–82. doi: 10.1007/BFb0034293. [DOI] [PubMed] [Google Scholar]
- Brown R. H., Jr Membrane surface charge: discrete and uniform modelling. Prog Biophys Mol Biol. 1974;28:341–370. doi: 10.1016/0079-6107(74)90021-2. [DOI] [PubMed] [Google Scholar]
- Eckert R., Lux H. D. A voltage-sensitive persistent calcium conductance in neuronal somata of Helix. J Physiol. 1976 Jan;254(1):129–151. doi: 10.1113/jphysiol.1976.sp011225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., GINSBORG B. L. The ionic requirements for the production of action potentials in crustacean muscle fibres. J Physiol. 1958 Aug 6;142(3):516–543. doi: 10.1113/jphysiol.1958.sp006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozzard H. A., Beeler G. W., Jr The voltage clamp and cardiac electrophysiology. Circ Res. 1975 Oct;37(4):403–413. doi: 10.1161/01.res.37.4.403. [DOI] [PubMed] [Google Scholar]
- Fozzard H. A., Lee C. O. Influence of changes in external potassium and chloride ions on membrane potential and intracellular potassium ion activity in rabbit ventricular muscle. J Physiol. 1976 Apr;256(3):663–689. doi: 10.1113/jphysiol.1976.sp011345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettes L. S., Reuter H. Slow recovery from inactivation of inward currents in mammalian myocardial fibres. J Physiol. 1974 Aug;240(3):703–724. doi: 10.1113/jphysiol.1974.sp010630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebisch G., Weidmann S. Membrane currents in mammalian ventricular heart muscle fibers using a voltage-clamp technique. J Gen Physiol. 1971 Mar;57(3):290–296. doi: 10.1085/jgp.57.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas H. G., Kern R., Einwächter H. M., Tarr M. Kinetics of Na inactivation in frog atria. Pflugers Arch. 1971;323(2):141–157. doi: 10.1007/BF00586445. [DOI] [PubMed] [Google Scholar]
- Hagiwara S. Ca spike. Adv Biophys. 1973;4:71–102. [PubMed] [Google Scholar]
- Hille B., Woodhull A. M., Shapiro B. I. Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions, and pH. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):301–318. doi: 10.1098/rstb.1975.0011. [DOI] [PubMed] [Google Scholar]
- Isnberg G. Is potassium conductance of cardiac Purkinje fibres controlled by (Ca2+)? Nature. 1975 Jan 24;253(5489):273–274. doi: 10.1038/253273a0. [DOI] [PubMed] [Google Scholar]
- Jakobsson E., Barr L., Connor J. A. An equivalent circuit for small atrial trabeculae of frog. Biophys J. 1975 Oct;15(10):1069–1085. doi: 10.1016/S0006-3495(75)85883-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. A., Lieberman M. Heart: excitation and contraction. Annu Rev Physiol. 1971;33:479–532. doi: 10.1146/annurev.ph.33.030171.002403. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Tsien R. W. Control of action potential duration by calcium ions in cardiac Purkinje fibers. J Gen Physiol. 1976 May;67(5):599–617. doi: 10.1085/jgp.67.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kléber A. G. Effects of sucrose solution on the longitudinal tissue resistivity of trabecular muscle from mammalian heart. Pflugers Arch. 1973 Dec 18;345(3):195–205. doi: 10.1007/BF00586334. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Krause H., Kübler M., Herdey A. Kinetics of inactivation and recovery of the slow inward current in the mammalian ventricular myocardium. Pflugers Arch. 1975 Mar 22;355(1):1–17. doi: 10.1007/BF00584795. [DOI] [PubMed] [Google Scholar]
- Kootsey J. M., Johnson E. A. Voltage clamp of cardiac muscle. A theoretical analysis of early currents in the single sucrose gap. Biophys J. 1972 Nov;12(11):1496–1508. doi: 10.1016/S0006-3495(72)86177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. O., Fozzard H. A. Activities of potassium and sodium ions in rabbit heart muscle. J Gen Physiol. 1975 Jun;65(6):695–708. doi: 10.1085/jgp.65.6.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister R. E., Noble D., Tsien R. W. Reconstruction of the electrical activity of cardiac Purkinje fibres. J Physiol. 1975 Sep;251(1):1–59. doi: 10.1113/jphysiol.1975.sp011080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H., Vogel W. Calcium inward currents in internally perfused giant axons. J Physiol. 1973 Nov;235(1):225–265. doi: 10.1113/jphysiol.1973.sp010386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New W., Trautwein W. Inward membrane currents in mammalian myocardium. Pflugers Arch. 1972;334(1):1–23. doi: 10.1007/BF00585997. [DOI] [PubMed] [Google Scholar]
- New W., Trautwein W. The ionic nature of slow inward current and its relation to contraction. Pflugers Arch. 1972;334(1):24–38. doi: 10.1007/BF00585998. [DOI] [PubMed] [Google Scholar]
- Ochi R. The slow inward current and the action of manganese ions in guinea-pig's myocardium. Pflugers Arch. 1970;316(1):81–94. doi: 10.1007/BF00587898. [DOI] [PubMed] [Google Scholar]
- Ramón F., Anderson N., Joyner R. W., Moore J. W. Axon voltage-clamp simulations. A multicellular preparation. Biophys J. 1975 Jan;15(1):55–69. doi: 10.1016/S0006-3495(75)85791-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. Divalent cations as charge carriers in excitable membranes. Prog Biophys Mol Biol. 1973;26:1–43. doi: 10.1016/0079-6107(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Reuter H. Exchange of calcium ions in the mammalian myocardium. Mechanisms and physiological significance. Circ Res. 1974 May;34(5):599–605. doi: 10.1161/01.res.34.5.599. [DOI] [PubMed] [Google Scholar]
- Reuter H. Localization of beta adrenergic receptors, and effects of noradrenaline and cyclic nucleotides on action potentials, ionic currents and tension in mammalian cardiac muscle. J Physiol. 1974 Oct;242(2):429–451. doi: 10.1113/jphysiol.1974.sp010716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougier O., Vassort G., Garnier D., Gargouil Y. M., Coraboeuf E. Existence and role of a slow inward current during the frog atrial action potential. Pflugers Arch. 1969;308(2):91–110. doi: 10.1007/BF00587018. [DOI] [PubMed] [Google Scholar]
- Trautwein W., McDonald T. F., Tripathi O. Calcium conductance and tension in mammalian ventricular muscle. Pflugers Arch. 1975;354(1):55–74. doi: 10.1007/BF00584503. [DOI] [PubMed] [Google Scholar]
- Vereecke J., Carmeliet E. Sr action potentials in cardiac Purkyne fibres. II. Dependence of the Sr conductance on the external Sr concentration and Sr-Ca antagonism. Pflugers Arch. 1971;322(1):73–82. doi: 10.1007/BF00586666. [DOI] [PubMed] [Google Scholar]
- Vitek M., Trautwein W. Slow inward current and action potential in cardiac Purkinje fibres. The effect of Mn plus,plus-ions. Pflugers Arch. 1971;323(3):204–218. doi: 10.1007/BF00586384. [DOI] [PubMed] [Google Scholar]
- WEIDMANN S. Effects of calcium ions and local anesthetics on electrical properties of Purkinje fibres. J Physiol. 1955 Sep 28;129(3):568–582. doi: 10.1113/jphysiol.1955.sp005379. [DOI] [PMC free article] [PubMed] [Google Scholar]


