Abstract
We developed a nested PCR assay that detects the recA gene of the Burkholderia cepacia complex in sputum. The product of the first PCR round is also used to identify the genomovar of the pathogen. The protocol achieves high sensitivity and specificity with simple interpretation of genomovar status.
Burkholderia cepacia is a multiresistant and transmissible opportunistic pathogen that frequently impairs the clinical state of patients with cystic fibrosis (CF) (1, 3, 9, 10). The group of B. cepacia complex (BCC) organisms consists of eight genomovars associated with different levels of virulence and patient-to-patient transmissibility (2, 15): B. cepacia genomovar I, B. multivorans (genomovar II), B. cepacia genomovar III, B. stabilis (genomovar IV), B. vietnamiensis (genomovar V), B. cepacia genomovar VI, B. ambifaria (genomovar VII), and B. pyrrocinia (5). To reduce the probability of BCC spreading among patients with CF, a reliable early test that detects small quantities of the bacteria in clinical samples is needed. Conventional microbiological diagnostics of the BCC based on the results of culture and subsequent biochemical identification is insufficient for this purpose because of the potential risk of misidentification or false negativity (7). Moreover, these methods are not able to reliably distinguish the genomovars of the BCC (21).
To increase the sensitivity and specificity of diagnostic routines and to identify the genomovars, use of molecular genetic methods is inevitable. Two target genes are commonly used for BCC analysis: the 16S rRNA gene (11, 19, 20) and the recA gene (13, 16). The recA gene polymorphisms enable both differentiation of the BCC from other closely related bacteria and its sorting into genomovars. Moreover, the differences in the recA sequences within genomovar III led to the establishment of two recA clusters designated III-A and III-B.
The present study sought (i) to develop a rapid diagnostic method for early detection of BCC organisms and determination of their genomovars directly from sputum and (ii) to assess the occurrence of the BCC genomovars in Czech patients with CF.
From May 2001 to April 2002, we collected 211 sputum samples from 134 consecutive CF patients attending the Prague CF center (55 males, 79 females; age range, 0 to 33 years). All samples were examined for the presence of the BCC by culture and our novel PCR protocol. For cultivation, specimens were liquefied with a homogenization solution (0.9% NaCl, 50 mM KH2PO4, 35 mM NaOH, 1% N-acetyl-l-cysteine), shaken for 20 min at 800 rpm, and cultured on blood, chocolate, Endo, Sabouraud, and MacConkey agars. The last 53 samples were also cultured on the selective B. cepacia agar (Oxoid, Basingstoke, United Kingdom) that came into use during the study. All of the BCC isolates were identified by the API 20 NE system (Biomerieux, Marcy l'Etoile, France) in accordance with the supplier's protocol. For PCR analysis, sputum was mixed with an equal volume of the homogenization solution and shaken for 1 h at 800 rpm. DNA was extracted from 100 μl of liquefied sputum with the AMPLICOR Respiratory Specimen Preparation Kit (Roche, Indianapolis, Ind.) in accordance with the manufacturer's instructions. To check the DNA content in extraction aliquots, real-time PCR quantitation of the human albumin gene as an equivalent of the human DNA content was done on an ABI 7700 system (Applied Biosystems, Foster City, Calif.). Samples with albumin gene quantities below the fifth percentile of the first 100 assays were re-extracted.
To detect the open reading frame of the BCC recA gene, DNA extracts were subjected to nested PCRs. For the first PCR round, the Taq PCR Core Kit (Qiagen, Hilden, Germany) was used. The PCRs were carried out in duplicate in a total volume of 20 μl with 1× PCR buffer, 1× solution Q (Qiagen, Hilden, Germany), 2 mM MgCl2, 200 μM (each) deoxynucleoside triphosphate, 14 pmol (each) of primers BCR1 and BCR2 (Table 1), 1 U of Taq polymerase, and 1 μl of the DNA extract. The PCR program was run on a GeneAmp 9700 thermocycler (Applied Biosystems, Foster City, Calif.): initial denaturation for 2 min at 94°C; 30 three-temperature cycles of 30 s at 94°C, 45 s at 62°C, and 90 s at 72°C; and a final extension of 5 min at 72°C. The second round of the nested PCR was performed with 1× PCR buffer (Promega), 2 mM MgCl2, 200 μM (each) deoxynucleoside triphosphate, 10 pmol (each) of inner recA primers REC-IN-5 and BCRBM2 (Table 1), 0.5 U of Taq polymerase (Promega, Madison, Wis.), and 0.5 μl of the first-round PCR product. The amplification profile consisted of 25 three-temperature cycles of 30 s (for denaturation) at 94°C; 45 s (for annealing) at 67°C (first 5 cycles), 65°C (next 5 cycles), and 63°C (remaining 15 cycles); and 60 s (for synthesis) at 72°C. A final extension step of 5 min at 72°C followed. The PCR products of both rounds were run on a 2% agarose gel in 0.5% Tris-borate-EDTA buffer for 20 min at 10 V/cm.
TABLE 1.
Primers used in this study
| Organism(s) detected | Primer mixture | 5′ primer name | Sequence (5′-3′) | 3′ primer name | Sequence (5′-3′) | Approximate product size (bp) |
|---|---|---|---|---|---|---|
| BCC (first run of nested PCR) | C-0 | BCR1a | TGACCGCCGAGAAGAGCAA | BCR2a | CTCTTCTTCGTCCATCGCCTC | 1040 |
| BCC (second run of nested PCR) | C-IN | REC-IN-5c | CATGATCGTCATCGACTCGGTC | BCRBM2b | TCCATCGCCTCGGCTTCGT | 620 |
| Genomovar I | C-1 | BCRG11a | CAGGTCGTCTCCACGGGT | BCRG12a | CACGCCGATCTTCATACGA | 490 |
| B. multivorans | C-2 | BCRBM1a | CGGCGTCAACGTGCCGGAT | C2-3c | CTCGGCTTCGTCGTGGC | 710 |
| Genomovar IIIA | C-3A | BCRG3A1a | GCTCGACGTTCAATATGCC | BCRG3A2a | TCGAGACGCACCGACGAG | 380 |
| Genomovar IIIB | C-3B | BCRG3B1a | GCTGCAAGTCATCGCTGAA | BCRG3B2a | TACGCCATCGGGCATGCT | 780 |
| B. stabilis | C-4 | BCRG41a | ACCGGCGAGCAGGCGCTT | BCRG42a | ACGCCATCGGGCATGGCA | 650 |
| B. vietnamiensis | C-5 | BCRBV1a | GGGCGACGGCGACGTGAA | BCRBV2a | TCGGCCTTCGGCACCAGT | 380 |
| Genomovar VI | C-6 | BCRBM1b | CGGCGTCAACGTGCCGGAT | C6-3c | TGATGAAGATCACGAGGCAA | 260 |
| B. ambifaria | C-7 | BCR1b | TGACCGCCGAGAAGAGCAA | CA-3c | CCTCGGCTTCGTCGTGGA | 1,030 |
In the BCC-positive samples, products of the first round were also used for genomovar status determination. The PCR product was diluted 1:10,000 (for samples positive in the first PCR round) or 1:100 (for samples that yielded a signal only in the second PCR round) with deionized, double-distilled water. The PCR setup was identical to that of the second round; the genomovar specificity of the eight reactions was maintained by the use of respective sequence-specific primers (Table 1).
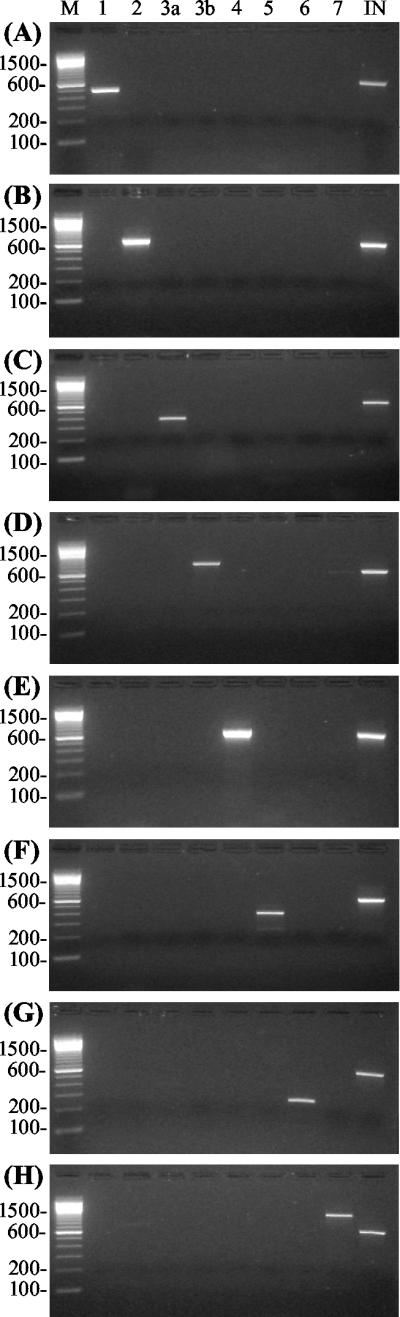
New primer pairs were designed to identify B. multivorans (C-2), genomovar VI (C-6), and B. ambifaria (C-7). To check the sensitivity and specificity of the assays, we used strains from the BCC strain panel (14) completed with genomovar VI and B. ambifaria strains from the Laboratorium voor Microbiologie, Ghent, Belgium. The recently described species B. pyrrocinia was not tested. Results are shown in Fig. 1. Each strain was correctly identified; moreover, our new 3′ primers eliminated the previously described cross-reactivity between B. multivorans and genomovar VI (6). We also tested for reactivity with other bacteria possibly present in CF sputum (i.e., Staphylococcus aureus, Pseudomonas aeruginosa, Haemophilus influenzae, Stenotrophomonas maltophilia, Alcaligenes xylosoxidans, Ralstonia pickettii, B. gladioli, Chryseobacterium meningosepticum, and Delftia acidovorans). No reactivity was observed for either the generic C-IN primer mixture (used in the second round of the nested PCR) or the genomovar-specific primer pairs. To determine the sensitivity of the nested PCR, the procedure described by McDowell et al. (16) was applied to strains LMG 16656 (genomovar III-A) and LMG 13010 (B. multivorans). The detection limit of the nested PCR was 103 CFU/ml of sputum.
FIG. 1.
Genomovar-specific reactions on BCC reference strains ATCC 25416 (A), LMG 16660 (B), LMG 16656 (C), LMG 14294 (E), LMG 10929 (F), LMG 18941 (G), and LMG 19182 (H) and a clinical sample of genomovar III-B (D). The lanes contained sequence-specific primer PCR products of the genomovars. Lanes: 1, genomovar I; 2, B. multivorans; 3a, genomovar III-A; 3b, genomovar III-B; 4, B. stabilis; 5, B. vietnamiensis; 6, genomovar VI; 7, B. ambifaria; IN, generic detection of the BCC species. Molecular size markers (100-bp ladder) were run in lane M.
Two hundred eleven sputum samples from 134 CF patients were tested. By culture techniques, the BCC organisms were recovered from 89 (42%) of 211 samples. Comparing results of growth on the nonselective agars and selective B. cepacia medium, we found no difference in the number of BCC organisms recovered. By the nested-PCR protocol, 110 samples (52%) were determined to be BCC positive, of which 28 yielded a signal only in the second PCR round. Of the 134 patients examined, the BCC was detected in 49 patients by culture whereas it was detected in 67 patients by PCR. Thus, 18 more CF patients were found to be BCC positive by PCR than by cultivation.
A nested or seminested design is known to increase PCR sensitivity. The improvement in sensitivity over the nonnested setup was evident in 28 positive samples that were found to be positive only after the second round of PCR. This represents 25% of the 110 positive samples. Similarly, the superiority of two PCR rounds was demonstrated by Moore et al. (18), who employed a seminested approach for detection of B. multivorans and genomovar III. Although the sensitivity of the reported seminested strategy was 1 order of magnitude greater than the sensitivity of the present method, our protocol offers the sorting of BCC organisms into seven genomovars. To further minimize the risk of false negativity, we checked the yields of DNA extraction by using real-time quantification of human genome equivalents per microliter of DNA. If human DNA was not present in a sufficient quantity in the extract, failure of DNA extraction was highly probable since sputum contains large quantities of leukocytes.
As regards the genomovar identification of BCC organisms, 62 patients (92.5%) were colonized by genomovar III, recA group III-A; 3 were colonized by genomovar III, recA group III-B; and 2 were colonized by B. multivorans. None of the patients was infected by two or more genomovars, and replacement of one genomovar by another was not observed during the study period. In addition to analysis of clinical samples, we examined 44 BCC isolates recovered from sputum samples of 36 Czech CF patients collected from 1997 to 1999 and archived at the National Institute of Public Health, Prague, Czech Republic. All of them were identified as genomovar III, recA group III-A, which has been previously associated with the most severe course of the infection caused by the BCC (7, 12). The almost absolute uniformity of the BCC genomovar spectrum in the Czech CF community is alarming, and the identical finding among the archived samples suggests a possible epidemic origin of the infections. However, to fully explain this unfavorable phenomenon, further studies based on molecular typing have to be performed.
In conclusion, the protocol described herein offers rapid PCR detection of BCC in sputum and identification of its genomovars. Previous assays for direct PCR detection of the BCC in sputum were based on amplification of the 16S rRNA gene (4, 8) or of the 16S-23S spacer region of the rRNA operon (22). Recently, McDowell et al. described PCR of the recA gene followed by restriction fragment length polymorphism (RFLP) analysis (16), concluding that the recA gene is a more suitable target than the 16S region. However, the PCR-RFLP assay can yield many different RFLP patterns, demanding attentive comparison (17). On the contrary, the algorithm presented here with nested PCR and genomovar-specific recA primers achieves high sensitivity and specificity with simple interpretation of genomovar results.
Acknowledgments
We are grateful to E. Mahenthiralingam for helpful advice and to P. Vandamme for providing the reference strains.
This work was supported by the Ministry of Health (projects NM/6568-3 and 64203) and the Ministry of Education (grant 111300003), Czech Republic.
REFERENCES
- 1.Aaron, S. D., W. Ferris, D. A. Henry, D. P. Speert, and N. E. Macdonald. 2000. Multiple combination bactericidal antibiotic testing for patients with cystic fibrosis infected with Burkholderia cepacia. Am. J. Respir. Crit. Care Med. 161:1206-1212. [DOI] [PubMed] [Google Scholar]
- 2.Agodi, A., E. Mahenthiralingam, M. Barchitta, V. Giannino, A. Sciacca, and S. Stefani. 2001. Burkholderia cepacia complex infection in Italian patients with cystic fibrosis: prevalence, epidemiology, and genomovar status. J. Clin. Microbiol. 39:2891-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beringer, P. M., and M. D. Appleman. 2000. Unusual respiratory bacterial flora in cystic fibrosis: microbiologic and clinical features. Curr. Opin. Pulm. Med. 6:545-550. [DOI] [PubMed] [Google Scholar]
- 4.Campbell, P. W., J. A. Phillips, G. J. Heidecker, M. R. Krishnamani, R. Zahorchak, and T. L. Stull. 1995. Detection of Pseudomonas (Burkholderia) cepacia using PCR. Pediatr. Pulmonol. 20:44-49. [DOI] [PubMed] [Google Scholar]
- 5.Coenye, T., P. Vandamme, J. R. Govan, and J. J. LiPuma. 2001. Taxonomy and identification of the Burkholderia cepacia complex. J. Clin. Microbiol. 39:3427-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henry, D. A., E. Mahenthiralingam, P. Vandamme, T. Coenye, and D. P. Speert. 2001. Phenotypic methods for determining genomovar status of the Burkholderia cepacia complex. J. Clin. Microbiol. 39:1073-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones, A. M., M. E. Dodd, and A. K. Webb. 2001. Burkholderia cepacia: current clinical issues, environmental controversies and ethical dilemmas. Eur. Respir. J. 17:295-301. [DOI] [PubMed] [Google Scholar]
- 8.Karpati, F., and J. Jonasson. 1996. Polymerase chain reaction for the detection of Pseudomonas aeruginosa, Stenotrophomonas maltophilia and Burkholderia cepacia in sputum of patients with cystic fibrosis. Mol. Cell. Probes 10:397-403. [DOI] [PubMed] [Google Scholar]
- 9.LiPuma, J. J. 1998. Burkholderia cepacia. Management issues and new insights. Clin. Chest Med. 19:473-486. [DOI] [PubMed] [Google Scholar]
- 10.LiPuma, J. J., S. E. Dasen, D. W. Nielson, R. C. Stern, and T. L. Stull. 1990. Person-to-person transmission of Pseudomonas cepacia between patients with cystic fibrosis. Lancet 336:1094-1096. [DOI] [PubMed] [Google Scholar]
- 11.LiPuma, J. J., B. J. Dulaney, J. D. McMenamin, P. W. Whitby, T. L. Stull, T. Coenye, and P. Vandamme. 1999. Development of rRNA-based PCR assays for identification of Burkholderia cepacia complex isolates recovered from cystic fibrosis patients. J. Clin. Microbiol. 37:3167-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LiPuma, J. J., T. Spilker, L. H. Gill, P. W. Campbell III, L. Liu, and E. Mahenthiralingam. 2001. Disproportionate distribution of Burkholderia cepacia complex species and transmissibility markers in cystic fibrosis. Am. J. Respir. Crit. Care Med. 164:92-96. [DOI] [PubMed] [Google Scholar]
- 13.Mahenthiralingam, E., J. Bischof, S. K. Byrne, C. Radomski, J. E. Davies, Y. Av-Gay, and P. Vandamme. 2000. DNA-based diagnostic approaches for identification of Burkholderia cepacia complex, Burkholderia vietnamiensis, Burkholderia multivorans, Burkholderia stabilis, and Burkholderia cepacia genomovars I and III. J. Clin. Microbiol. 38:3165-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahenthiralingam, E., T. Coenye, J. W. Chung, D. P. Speert, J. R. Govan, P. Taylor, and P. Vandamme. 2000. Diagnostically and experimentally useful panel of strains from the Burkholderia cepacia complex. J. Clin. Microbiol. 38:910-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahenthiralingam, E., P. Vandamme, M. E. Campbell, D. A. Henry, A. M. Gravelle, L. T. Wong, A. G. Davidson, P. G. Wilcox, B. Nakielna, and D. P. Speert. 2001. Infection with Burkholderia cepacia complex genomovars in patients with cystic fibrosis: virulent transmissible strains of genomovar III can replace Burkholderia multivorans. Clin. Infect Dis. 33:1469-1475. [DOI] [PubMed] [Google Scholar]
- 16.McDowell, A., E. Mahenthiralingam, J. E. Moore, K. E. Dunbar, A. K. Webb, M. E. Dodd, S. L. Martin, B. C. Millar, C. J. Scott, M. Crowe, and J. S. Elborn. 2001. PCR-based detection and identification of Burkholderia cepacia complex pathogens in sputum from cystic fibrosis patients. J. Clin. Microbiol. 39:4247-4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore, J. E., B. C. Millar, J. Xu, M. Crowe, A. O. Redmond, and J. S. Elborn. 2002. Misidentification of a genomovar of Burkholderia cepacia by recA restriction fragment length polymorphism. J. Clin. Pathol. 55:309-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore, J. E., J. Xu, B. C. Millar, M. Crowe, and J. S. Elborn. 2002. Improved molecular detection of Burkholderia cepacia genomovar III and Burkholderia multivorans directly from sputum of patients with cystic fibrosis. J. Microbiol. Methods 49:183-191. [DOI] [PubMed] [Google Scholar]
- 19.O'Callaghan, E. M., M. S. Tanner, and G. J. Boulnois. 1994. Development of a PCR probe test for identifying Pseudomonas aeruginosa and Pseudomonas (Burkholderia) cepacia. J. Clin. Pathol. 47:222-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Segonds, C., T. Heulin, N. Marty, and G. Chabanon. 1999. Differentiation of Burkholderia species by PCR-restriction fragment length polymorphism analysis of the 16S rRNA gene and application to cystic fibrosis isolates. J. Clin. Microbiol. 37:2201-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Pelt, C., C. M. Verduin, W. H. Goessens, M. C. Vos, B. Tummler, C. Segonds, F. Reubsaet, H. Verbrugh, and A. van Belkum. 1999. Identification of Burkholderia spp. in the clinical microbiology laboratory: comparison of conventional and molecular methods. J. Clin. Microbiol. 37:2158-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitby, P. W., H. L. Dick, P. W. Campbell, 3rd, D. E. Tullis, A. Matlow, and T. L. Stull. 1998. Comparison of culture and PCR for detection of Burkholderia cepacia in sputum samples of patients with cystic fibrosis. J. Clin. Microbiol. 36:1642-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]