Abstract
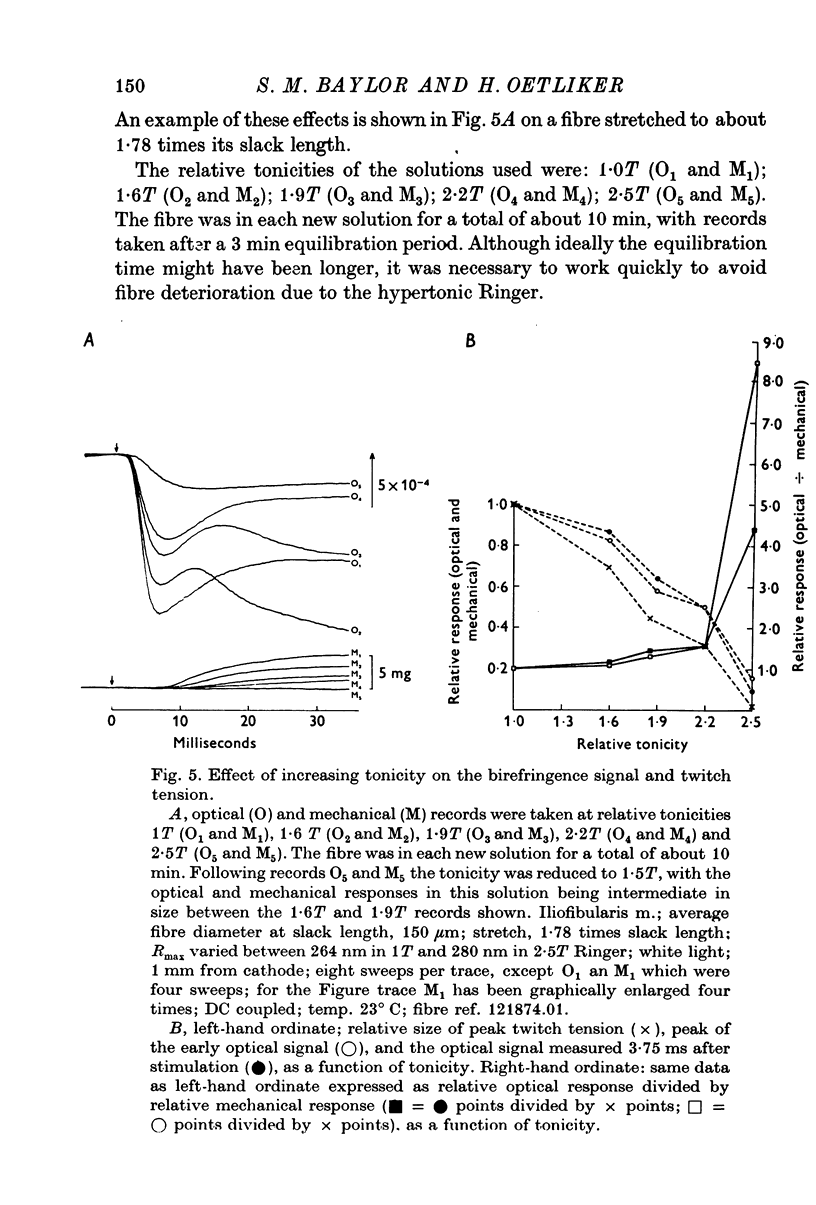
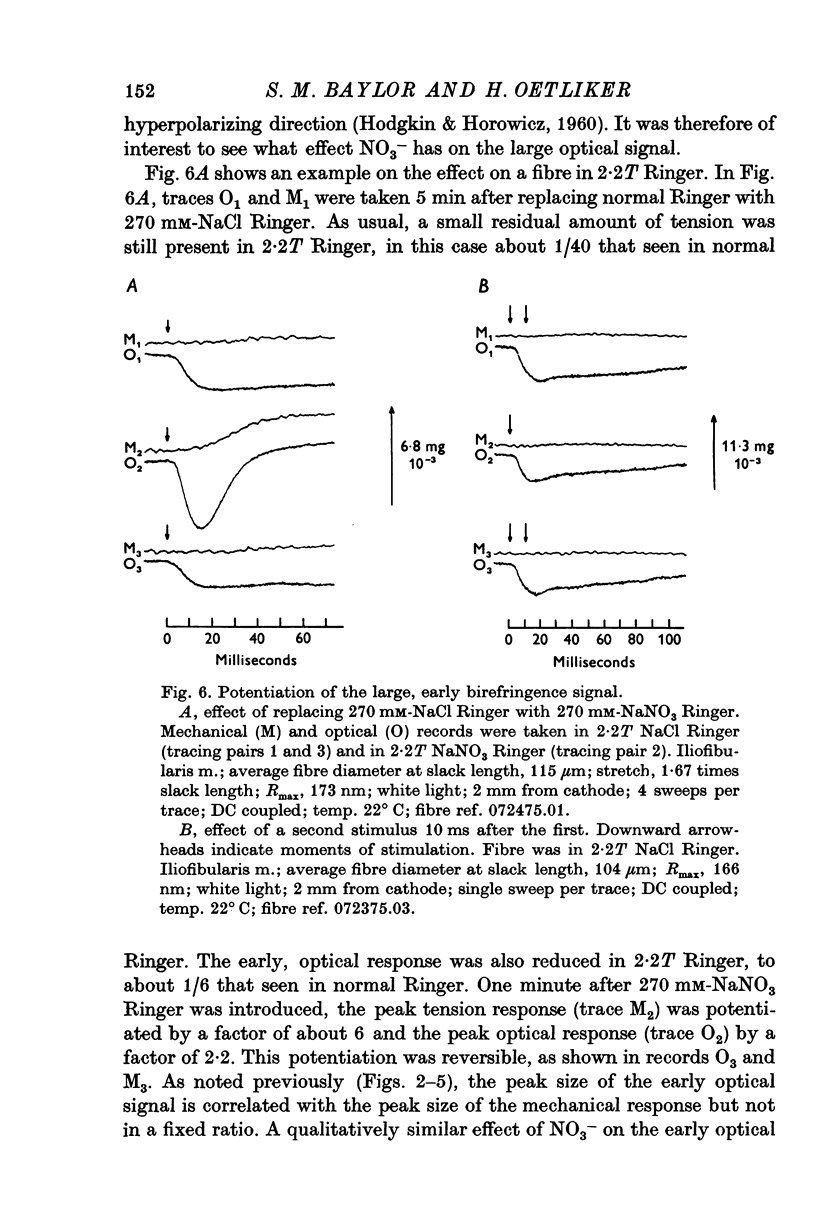
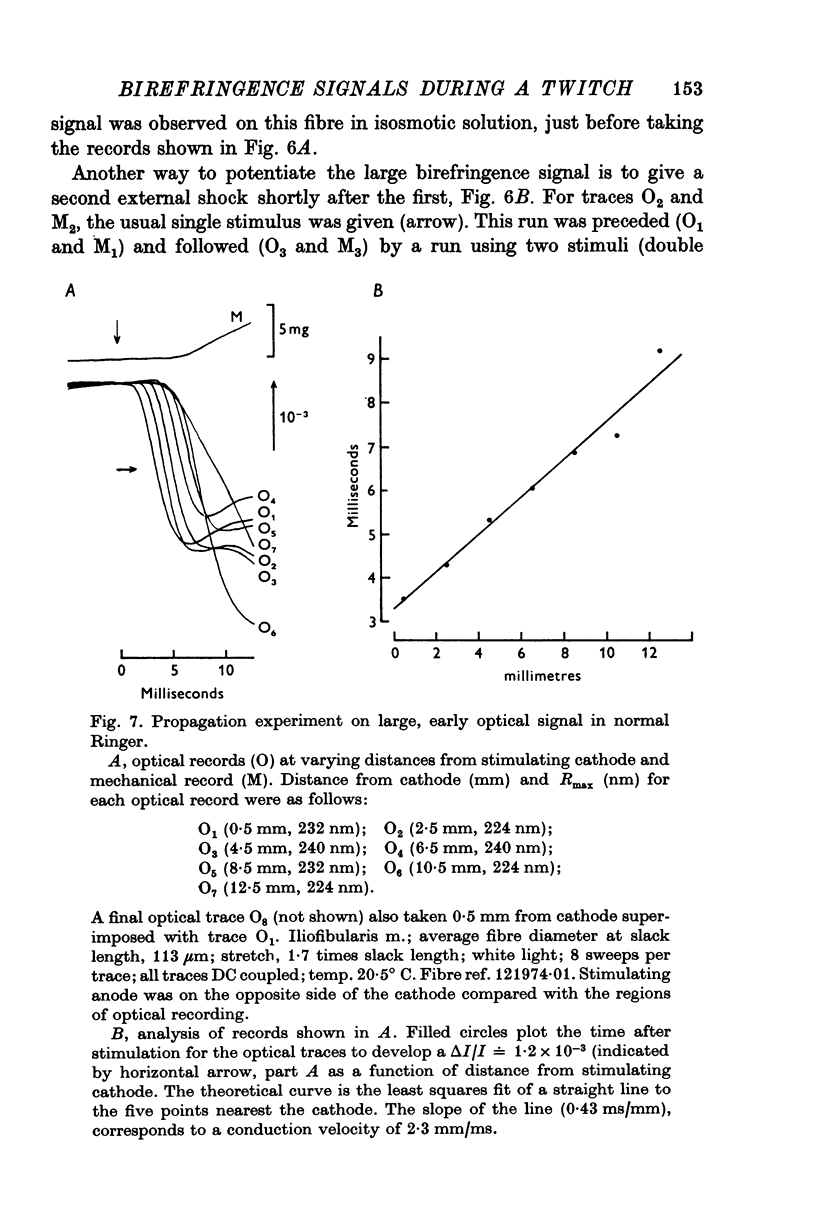
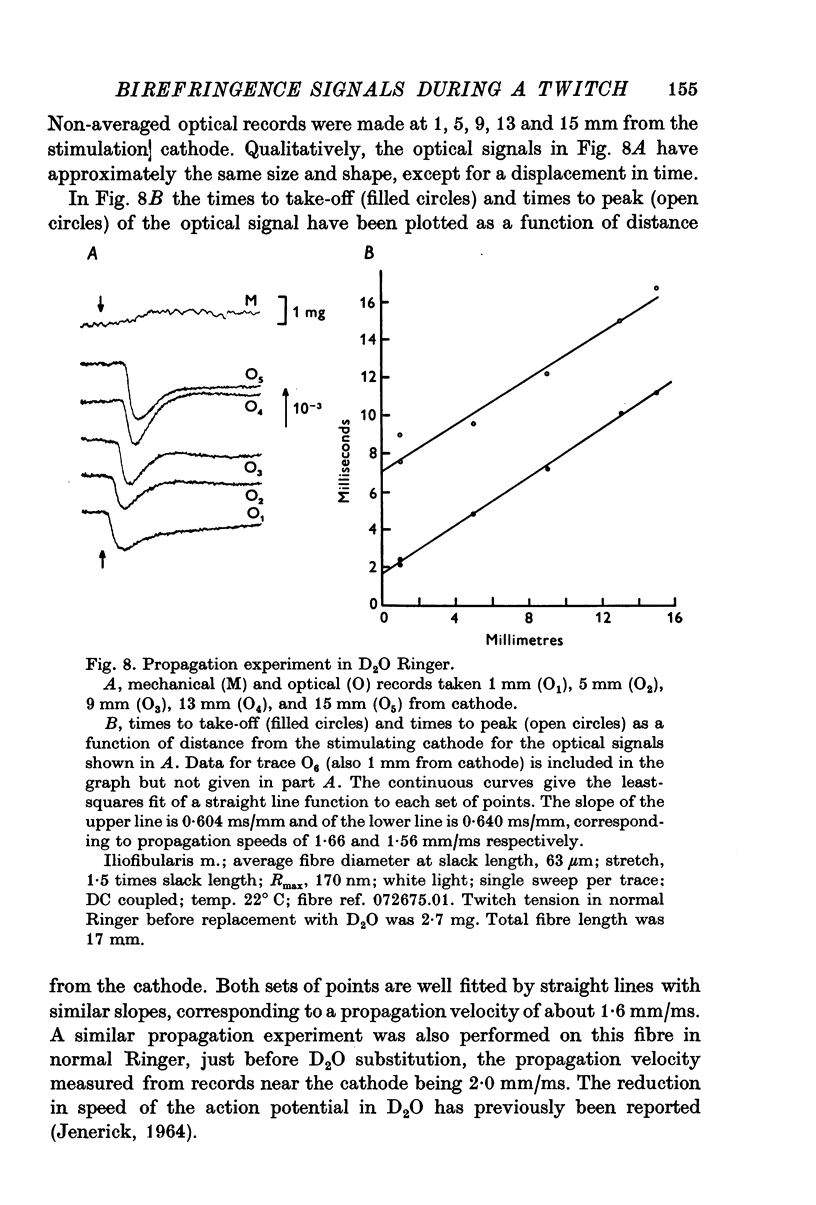
1. When a single muscle fibre was externally stimulated to give a propagated action potential, a large decrease in light intensity was measured with the fibre positioned between crossed-polarizers oriented at +/- 45 degrees with respect to the fibre axis. This large optical signal begins just after stimulation and its man phase precedes the development of positive tension. 2. The peak (or plateau) amplitude of the signal, expressed as the peak (plateau) change in light intensity, delta I, divided by the resting light intensity, I, was typically (minus) 1 to 3x10-3 and the time-to-peak (plateau) was 4 to 6 ms (20 degrees C). 3. When mechanical activity was minimized by stretch or Ringer replacement with D2O or hypertonic solution, the peak tension response was reduced in far greater proportion than the peak optical response, suggesting that the early optical signal is not due to changes in tension or gross fibre movement. 4. The magnitude of the optical response was increased by nitrate and double-shock stimulatin, procedures which potentiate the twitch response. 5. The optical signal was shown to propagate along the fibre length with a conduction velocity appropriate to the surface action potential. 6. The above results suggest that the large, early birefringence signal reflects a step or steps in the sequence of events leading to contractile activation.
Full text
PDF


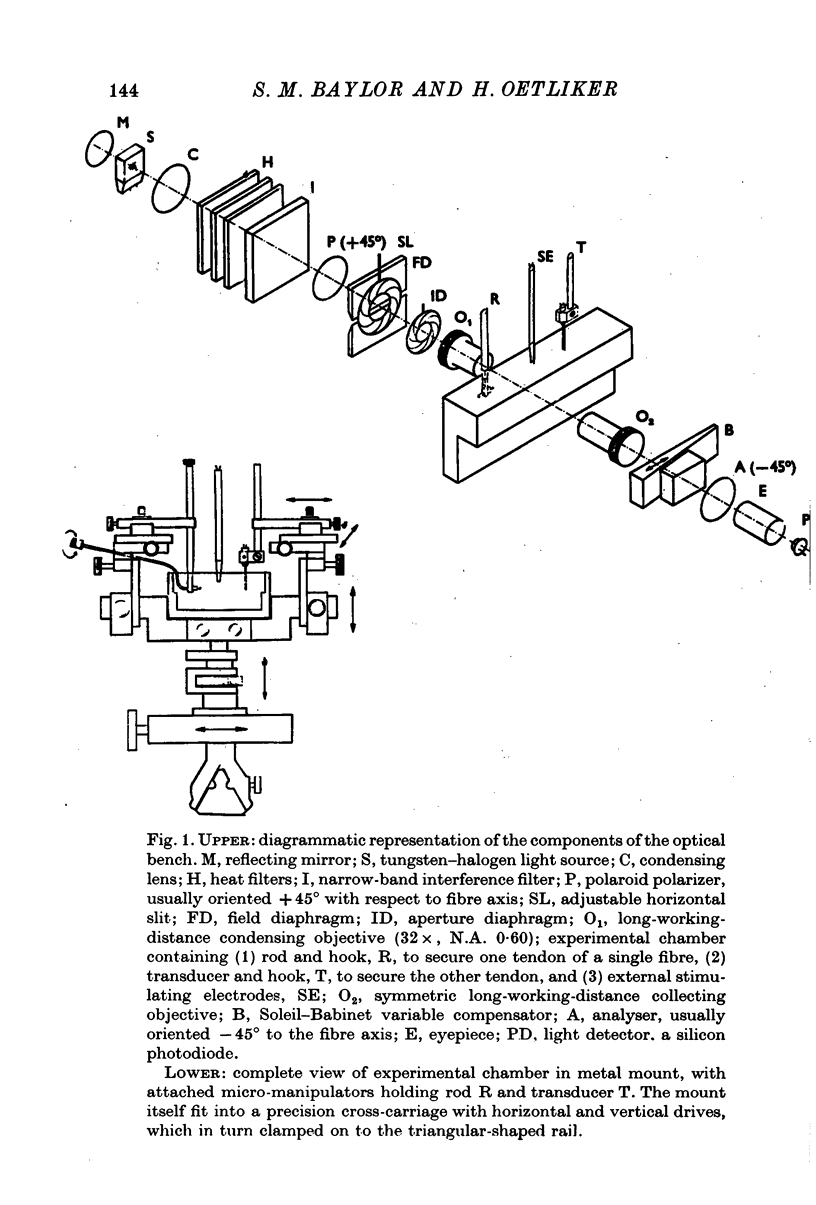


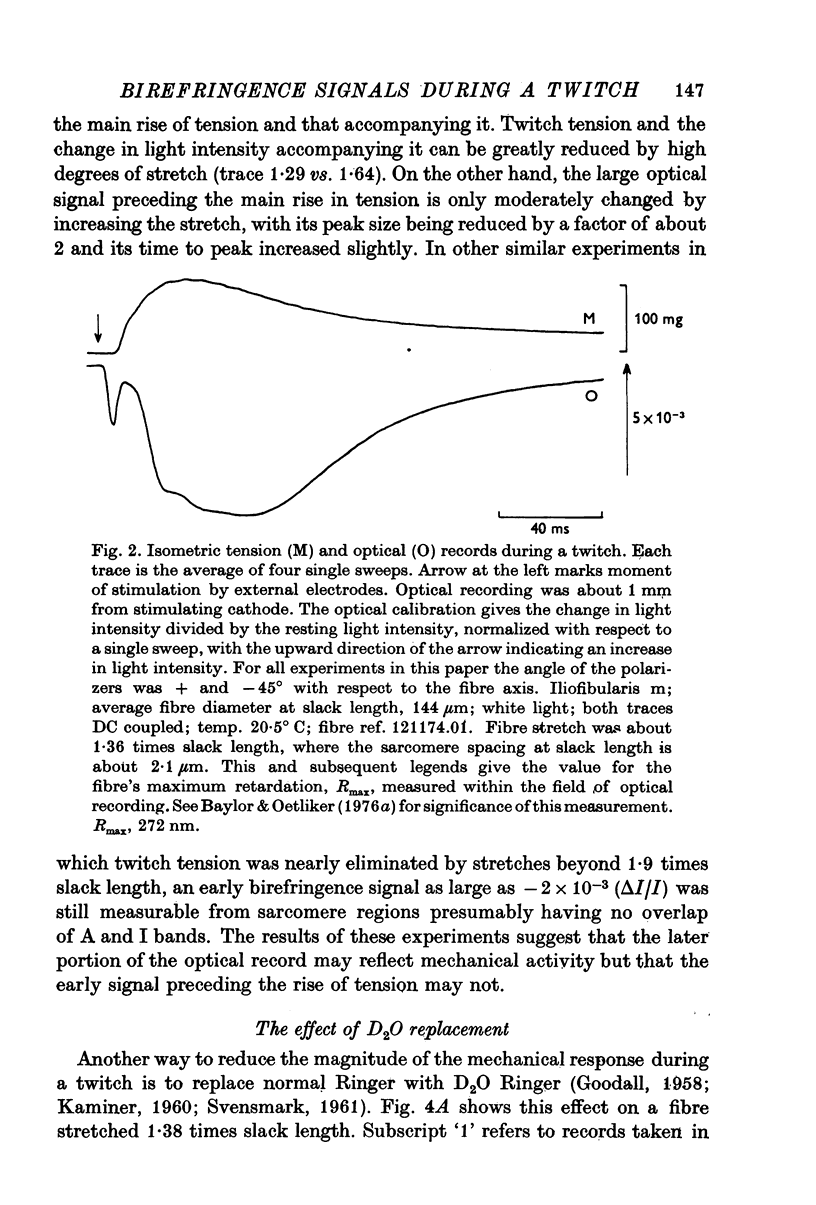
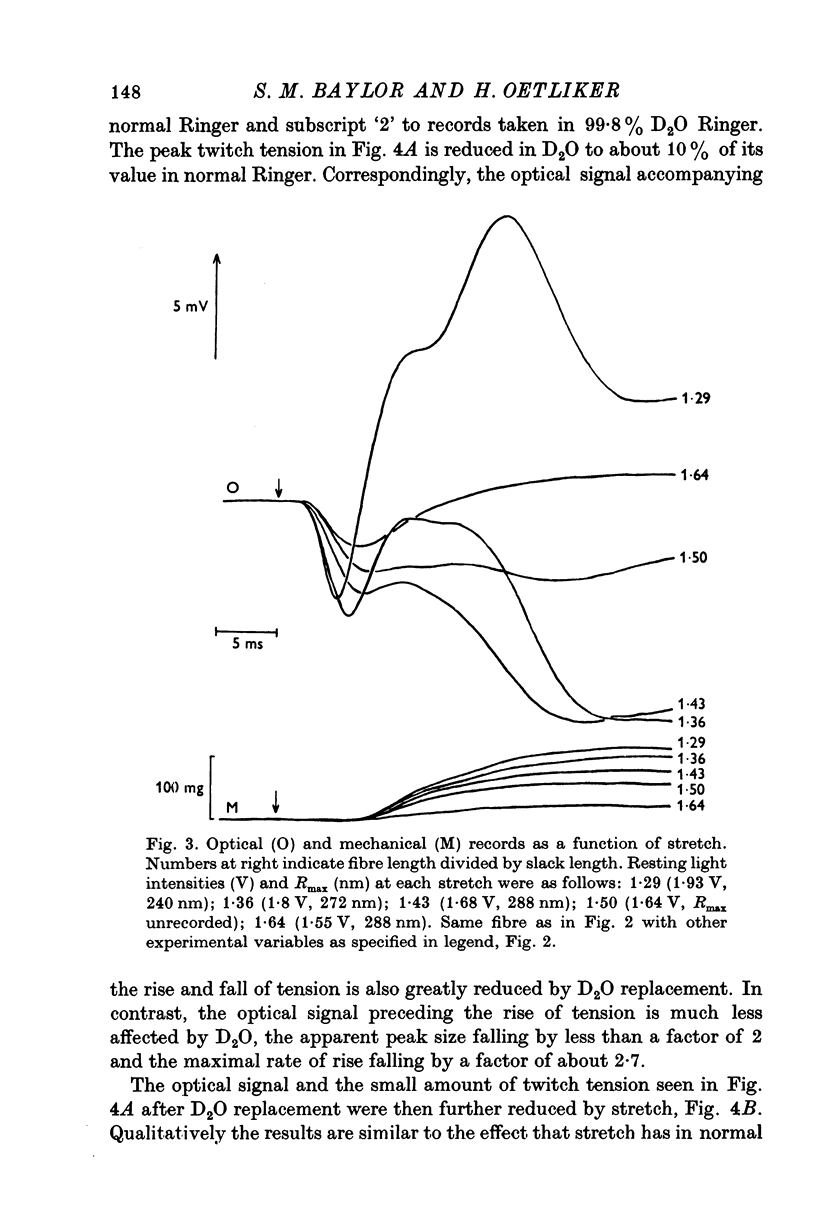
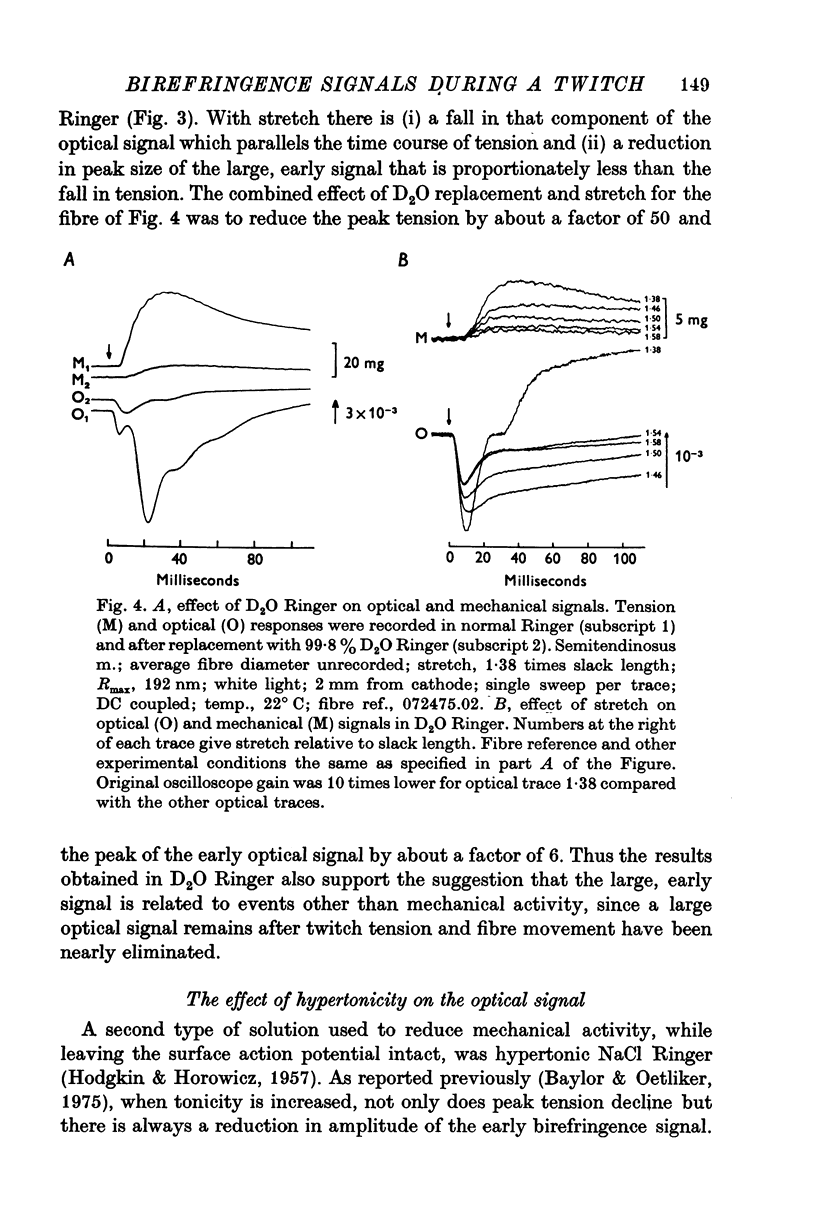








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott B. C., Ritchie J. M. Early tension relaxation during a muscle twitch. J Physiol. 1951 Apr 26;113(2-3):330–335. doi: 10.1113/jphysiol.1951.sp004576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry W. H., Carnay L. D. Changes in light scattered by striated muscle during excitation-contraction coupling. Am J Physiol. 1969 Nov;217(5):1425–1430. doi: 10.1152/ajplegacy.1969.217.5.1425. [DOI] [PubMed] [Google Scholar]
- Baylor S. M., Oetliker H. Birefringence experiments on isolated skeletal muscle fibres suggest a possible signal from the sarcoplasmic reticulum. Nature. 1975 Jan 10;253(5487):97–101. doi: 10.1038/253097a0. [DOI] [PubMed] [Google Scholar]
- Baylor S. M., Oetliker H. Birefringence signals from surface and t-system membranes of frog single muscle fibres. J Physiol. 1977 Jan;264(1):199–213. doi: 10.1113/jphysiol.1977.sp011663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Oetliker H. The optical properties of birefringence signals from single muscle fibres. J Physiol. 1977 Jan;264(1):163–198. doi: 10.1113/jphysiol.1977.sp011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Horowicz P. Fluorescence intensity changes associated with contractile activation in frog muscle stained with Nile Blue A. J Physiol. 1975 Apr;246(3):709–735. doi: 10.1113/jphysiol.1975.sp010912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnay L. D., Barry W. H. Turbidity, birefringence, and fluorescence changes in skeletal muscle coincident with the action potential. Science. 1969 Aug 8;165(3893):608–609. doi: 10.1126/science.165.3893.608. [DOI] [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. A non-linear voltage dependent charge movement in frog skeletal muscle. J Physiol. 1976 Jan;254(2):245–283. doi: 10.1113/jphysiol.1976.sp011232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Hille B., Keynes R. D. Changes in axon birefringence during the action potential. J Physiol. 1970 Dec;211(2):495–515. doi: 10.1113/jphysiol.1970.sp009289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Hille B., Keynes R. D., Landowne D., Rojas E. Analysis of the potential-dependent changes in optical retardation in the squid giant axon. J Physiol. 1971 Oct;218(1):205–237. doi: 10.1113/jphysiol.1971.sp009611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Keynes R. D. Changes in light scattering associated with the action potential in crab nerves. J Physiol. 1971 Jan;212(1):259–275. doi: 10.1113/jphysiol.1971.sp009321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Keynes R. D., Hille B. Light scattering and birefringence changes during nerve activity. Nature. 1968 May 4;218(5140):438–441. doi: 10.1038/218438a0. [DOI] [PubMed] [Google Scholar]
- Cohen L. B., Salzberg B. M., Davila H. V., Ross W. N., Landowne D., Waggoner A. S., Wang C. H. Changes in axon fluorescence during activity: molecular probes of membrane potential. J Membr Biol. 1974;19(1):1–36. doi: 10.1007/BF01869968. [DOI] [PubMed] [Google Scholar]
- Gordon A. M., Godt R. E. Some effects of hypertonic solutions on contraction and excitation-contraction coupling in frog skeletal muscles. J Gen Physiol. 1970 Feb;55(2):254–275. doi: 10.1085/jgp.55.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL A. V., MACPHERSON L. The effect of nitrate, iodide and bromide on the duration of the active state in skeletal muscle. Proc R Soc Lond B Biol Sci. 1954 Dec 15;143(910):81–102. doi: 10.1098/rspb.1954.0055. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The effect of nitrate and other anions on the mechanical response of single muscle fibres. J Physiol. 1960 Sep;153:404–412. doi: 10.1113/jphysiol.1960.sp006542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen P., Sten-Knudsen O. Sarcomere lengthening and tension drop in the latent period of isolated frog skeletal muscle fibers. J Gen Physiol. 1976 Sep;68(3):247–265. doi: 10.1085/jgp.68.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. K. Tension due to interaction between the sliding filaments in resting striated muscle. The effect of stimulation. J Physiol. 1968 Dec;199(3):637–684. doi: 10.1113/jphysiol.1968.sp008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Nakajima S. Analysis of the membrane capacity in frog muscle. J Physiol. 1972 Feb;221(1):121–136. doi: 10.1113/jphysiol.1972.sp009743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENERICK H. ACTION CURRENT OF STRIATED MUSCLE IN HEAVY WATER. Am J Physiol. 1964 Oct;207:944–946. doi: 10.1152/ajplegacy.1964.207.4.944. [DOI] [PubMed] [Google Scholar]
- KAHN A. J., SANDOW A. The potentiation of muscular contraction by the nitrate-ion. Science. 1950 Dec 1;112(2918):647–649. doi: 10.1126/science.112.2918.647. [DOI] [PubMed] [Google Scholar]
- KAMINER B. Effect of heavy water on different types of muscle and on glycerol-extracted psoas fibres. Nature. 1960 Jan 16;185:172–173. doi: 10.1038/185172a0. [DOI] [PubMed] [Google Scholar]
- LUETTGAU H. C. THE ACTION OF CALCIUM IONS ON POTASSIUM CONTRACTURES OF SINGLE MUSCLE FIBRES. J Physiol. 1963 Oct;168:679–697. doi: 10.1113/jphysiol.1963.sp007215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUETTGAU H. C. THE EFFECT OF METABOLIC INHIBITORS ON THE FATIGUE OF THE ACTION POTENTIAL IN SINGLE MUSCLE FIBRES. J Physiol. 1965 May;178:45–67. doi: 10.1113/jphysiol.1965.sp007613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley B. A., Eisenberg B. R. Sizes of components in frog skeletal muscle measured by methods of stereology. J Gen Physiol. 1975 Jul;66(1):31–45. doi: 10.1085/jgp.66.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulieri L. A. The dependence of the latency relaxation on sarcomere length and other characteristics of isolated muscle fibres. J Physiol. 1972 Jun;223(2):333–354. doi: 10.1113/jphysiol.1972.sp009850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oetliker H., Baylor S. M., Chandler W. K. Simultaneous changes in fluorescence and optical retardation in single muscle fibres during activity. Nature. 1975 Oct 23;257(5528):693–696. doi: 10.1038/257693a0. [DOI] [PubMed] [Google Scholar]
- Peachey L. D., Schild R. F. The distribution of the T-system along the sarcomeres of frog and toad sartorius muscles. J Physiol. 1968 Jan;194(1):249–258. doi: 10.1113/jphysiol.1968.sp008405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- Ross W. N., Salzberg B. M., Cohen L. B., Davila H. V. A large change in dye absorption during the action potential. Biophys J. 1974 Dec;14(12):983–986. doi: 10.1016/S0006-3495(74)85963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVENSMARK O. The effect of deuterium oxide on the mechanical properties of muscle. Acta Physiol Scand. 1961 Sep;53:75–84. doi: 10.1111/j.1748-1716.1961.tb02265.x. [DOI] [PubMed] [Google Scholar]
- Salzberg B. M., Davila H. V., Cohen L. B. Optical recording of impulses in individual neurones of an invertebrate central nervous system. Nature. 1973 Dec 21;246(5434):508–509. doi: 10.1038/246508a0. [DOI] [PubMed] [Google Scholar]
- Schoenberg M., Wells J. B., Podolsky R. J. Muscle compliance and the longitudinal transmission of mechanical impulses. J Gen Physiol. 1974 Dec;64(6):623–642. doi: 10.1085/jgp.64.6.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki I., Watanabe A., Hallett M. Fluorescence of squid axon membrane labelled with hydrophobic probes. J Membr Biol. 1972;8(2):109–132. doi: 10.1007/BF01868097. [DOI] [PubMed] [Google Scholar]
- Tasaki I., Watanabe A., Hallett M. Properties of squid axon membrane as revealed by a hydrophobic probe, 2-p-toluidinylnaphthalene-6-sulfonate. Proc Natl Acad Sci U S A. 1971 May;68(5):938–941. doi: 10.1073/pnas.68.5.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki I., Watanabe A., Sandlin R., Carnay L. Changes in fluorescence, turbidity, and birefringence associated with nerve excitation. Proc Natl Acad Sci U S A. 1968 Nov;61(3):883–888. doi: 10.1073/pnas.61.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]


