Abstract
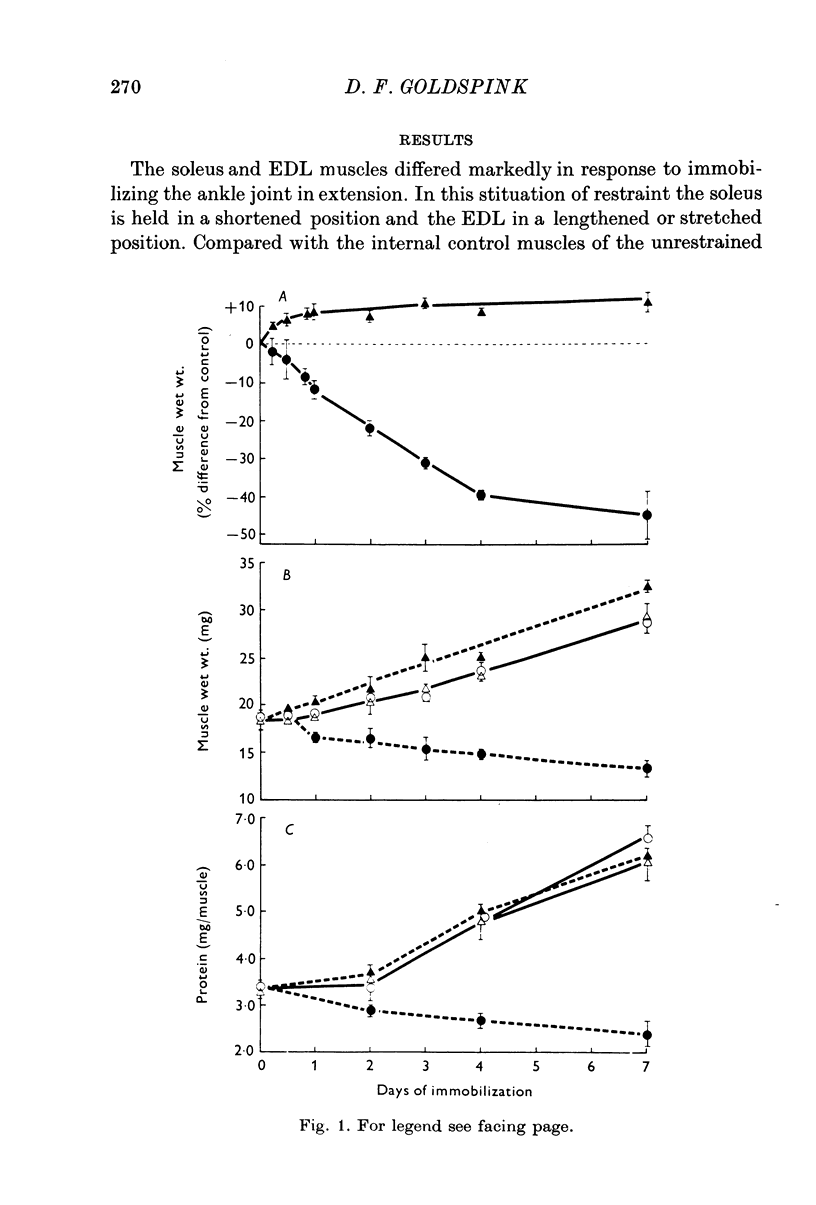
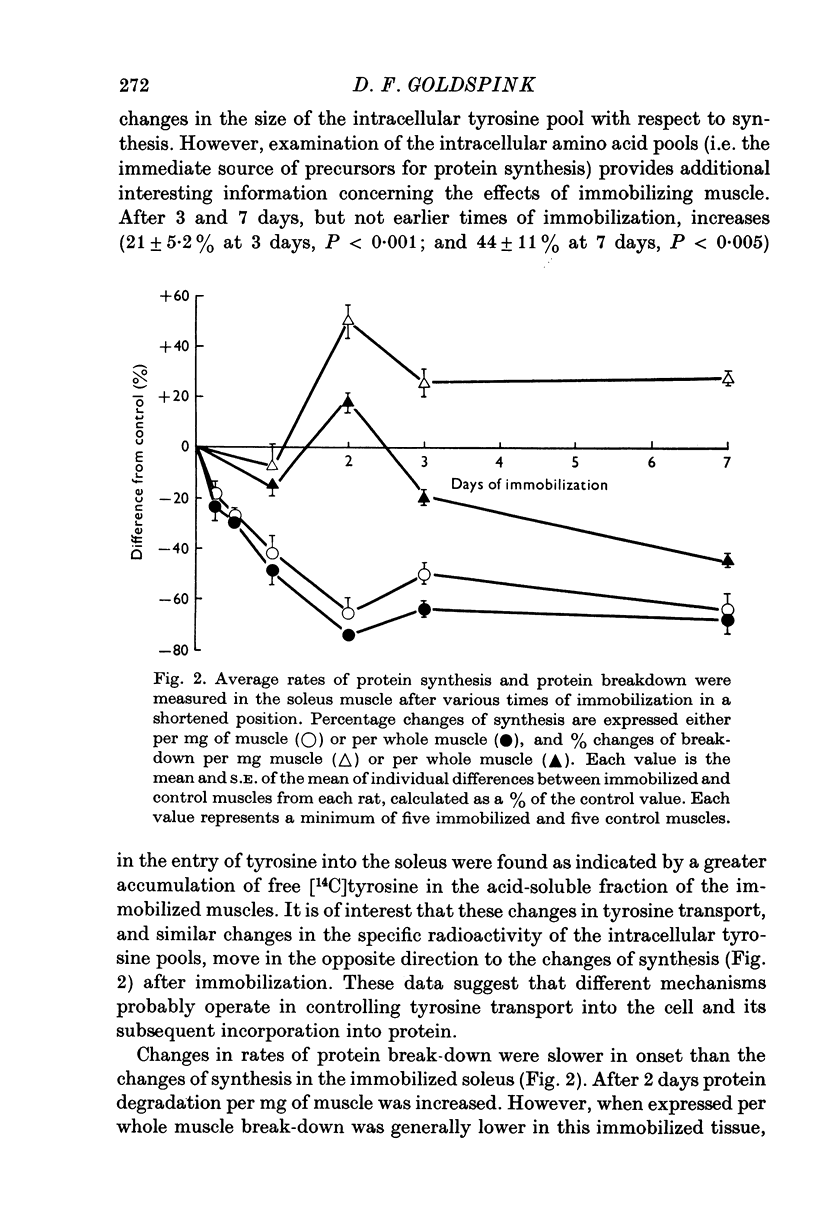
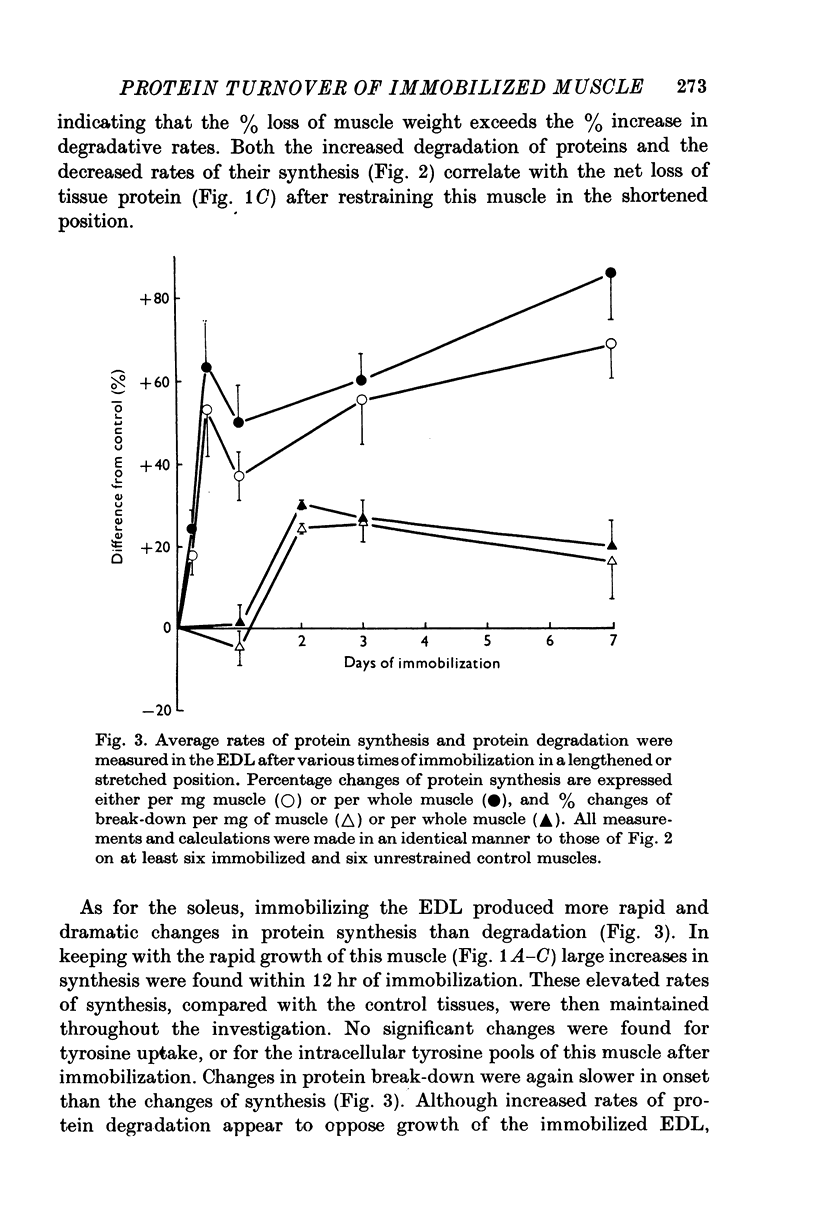
1. Changes in muscle size have been studied as a function of time after immobilization and in relation to the functional length of the muscle. 2. When immobilized in a shortened position the soleus and extensor digitorum longus muscles underwent atrophy, showing net losses of tissue protein. This atrophy appears to be caused by decreases in protein synthesis and increases in protein break-down. Average rates of protein synthesis and degradation were measured in immobilized and unrestrained control muscles by sensitive in vitro methods. 3. When the two muscles were held in a lengthened position they grew compared with internal controls. This rapid growth was primarily due to increased rats of protein synthesis, which possibly arise from a more active involvement of ribosomes in translation. In keeping with these situations of growth, DNA synthesis, DNA and RNA concentrations were found to be higher in the immobilized tissues. 4. Passive stretch and the development of isometric tension via the stretch reflux are suggested as factors stimulating protein synthesis, and hence inducing the growth of muscles held in the lengthened state. Probably neither of these factors will be experienced appreciably in the shortened position of restraint, and the lower level of activity in these immobilized muscles leads to their atrophy.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buresová M., Gutmann E., Klicpera M. Effect of tension upon rate of incorporation of amino acids into proteins of cross-striated muscle. Experientia. 1969 Feb 15;25(2):144–145. doi: 10.1007/BF01899088. [DOI] [PubMed] [Google Scholar]
- Cheek D. B., Hill D. E. Muscle and liver cell growth: role of hormones and nutritional factors. Fed Proc. 1970 Jul-Aug;29(4):1503–1509. [PubMed] [Google Scholar]
- Csapo A., Erdos T., De Mattos C. R., Gramss E., Moscowitz C. Stretch-induced uterine growth, protein synthesis and function. Nature. 1965 Sep 25;207(5004):1378–1379. doi: 10.1038/2071378a0. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D., Robbins N. Changes in contractile properties of disused soleus muscles. J Physiol. 1969 Apr;201(2):305–320. doi: 10.1113/jphysiol.1969.sp008757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- Goldberg A. L., Goldspink D. F. Influence of food deprivation and adrenal steroids on DNA synthesis in various mammalian tissues. Am J Physiol. 1975 Jan;228(1):310–317. doi: 10.1152/ajplegacy.1975.228.1.310. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Jablecki C., Li J. B. Trophic functions of the neuron. 3. Mechanisms of neurotrophic interactions. Effects of use and disuse on amino acid transport and protein turnover in muscle. Ann N Y Acad Sci. 1974 Mar 22;228(0):190–201. doi: 10.1111/j.1749-6632.1974.tb20510.x. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Work-induced growth of skeletal muscle in normal and hypophysectomized rats. Am J Physiol. 1967 Nov;213(5):1193–1198. doi: 10.1152/ajplegacy.1967.213.5.1193. [DOI] [PubMed] [Google Scholar]
- Goldspink D. F., Goldberg A. L. Influence of pituitary growth hormone on DNA synthesis in rat tissues. Am J Physiol. 1975 Jan;228(1):302–309. doi: 10.1152/ajplegacy.1975.228.1.302. [DOI] [PubMed] [Google Scholar]
- Goldspink D. F., Goldberg A. L. Problems in the use of (Me- 3H) thymidine for the measurement of DNA synthesis. Biochim Biophys Acta. 1973 Apr 11;299(4):521–532. doi: 10.1016/0005-2787(73)90224-4. [DOI] [PubMed] [Google Scholar]
- Goldspink D. F. The effects of denervation on protein turnover of rat skeletal muscle. Biochem J. 1976 Apr 15;156(1):71–80. doi: 10.1042/bj1560071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink D. F. The influence of activity on muscle size and protein turnover. J Physiol. 1977 Jan;264(1):283–296. doi: 10.1113/jphysiol.1977.sp011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink G., Tabary C., Tabary J. C., Tardieu C., Tardieu G. Effect of denervation on the adaptation of sarcomere number and muscle extensibility to the functional length of the muscle. J Physiol. 1974 Feb;236(3):733–742. doi: 10.1113/jphysiol.1974.sp010463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann E., Schiaffino S., Hanzliková V. Mechanism of compensatory hypertrophy in skeletal muscle of the rat. Exp Neurol. 1971 Jun;31(3):451–464. doi: 10.1016/0014-4886(71)90248-2. [DOI] [PubMed] [Google Scholar]
- Kimberg D. V., Loeb J. N. Differential sensitivity of nuclear and mitochondrial DNA synthesis to suppression by cortisone treatment. Biochim Biophys Acta. 1971 Sep 24;246(3):412–420. doi: 10.1016/0005-2787(71)90777-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lesch M., Gorlin R., Sonnenblick E. H. Myocardial amino acid transport in the isolated rabbit right ventricular papillary muscle. General characteristics and effects of passive stretch. Circ Res. 1970 Sep;27(3):445–460. doi: 10.1161/01.res.27.3.445. [DOI] [PubMed] [Google Scholar]
- Mallet L. E., Exton J. H., Park C. R. Control of gluconeogenesis from amino acids in the perfused rat liver. J Biol Chem. 1969 Oct 25;244(20):5713–5723. [PubMed] [Google Scholar]
- Millward D. J., Garlick P. J., James W. P., Nnanyelugo D. O., Ryatt J. S. Relationship between protein synthesis and RNA content in skeletal muscle. Nature. 1973 Jan 19;241(5386):204–205. doi: 10.1038/241204a0. [DOI] [PubMed] [Google Scholar]
- Moss F. P., Leblond C. P. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec. 1971 Aug;170(4):421–435. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- Rowe R. W., Goldspink G. Muscle fibre growth in five different muscles in both sexes of mice. II. Dystrophic mice. J Anat. 1969 May;104(Pt 3):531–538. [PMC free article] [PubMed] [Google Scholar]
- SOLA O. M., MARTIN A. W. Denervation hypertrophy and atrophy of the hemidiaphragm of the rat. Am J Physiol. 1953 Feb;172(2):324–332. doi: 10.1152/ajplegacy.1953.172.2.324. [DOI] [PubMed] [Google Scholar]
- Schreiber S. S., Rothschild M. A., Evans C., Reff F., Oratz M. The effect of pressure or flow stress on right ventricular protein synthesis in the face of constant and restricted coronary perfusion. J Clin Invest. 1975 Jan;55(1):1–11. doi: 10.1172/JCI107899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola O. M., Christensen D. L., Martin A. W. Hypertrophy and hyperplasia of adult chicken anterior latissimus dorsi muscles following stretch with and without denervation. Exp Neurol. 1973 Oct;41(1):76–100. doi: 10.1016/0014-4886(73)90182-9. [DOI] [PubMed] [Google Scholar]
- Tabary J. C., Tabary C., Tardieu C., Tardieu G., Goldspink G. Physiological and structural changes in the cat's soleus muscle due to immobilization at different lengths by plaster casts. J Physiol. 1972 Jul;224(1):231–244. doi: 10.1113/jphysiol.1972.sp009891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAALKES T. P., UDENFRIEND S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med. 1957 Nov;50(5):733–736. [PubMed] [Google Scholar]
- Williams P. E., Goldspink G. The effect of denervation and dystrophy on the adaptation of sarcomere number to the functional length of the muscle in young and adult mice. J Anat. 1976 Nov;122(Pt 2):455–465. [PMC free article] [PubMed] [Google Scholar]
- Williams P. E., Goldspink G. The effect of immobilization on the longitudinal growth of striated muscle fibres. J Anat. 1973 Oct;116(Pt 1):45–55. [PMC free article] [PubMed] [Google Scholar]


