Abstract
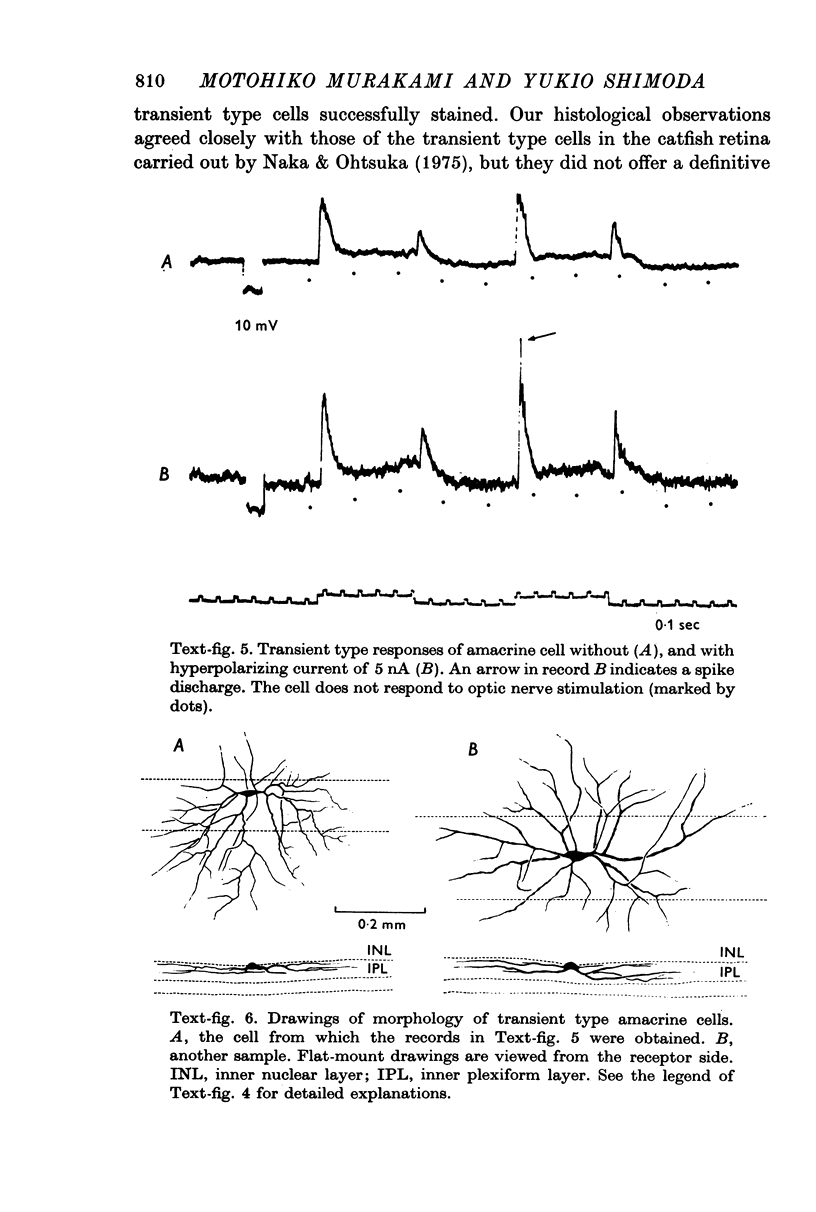
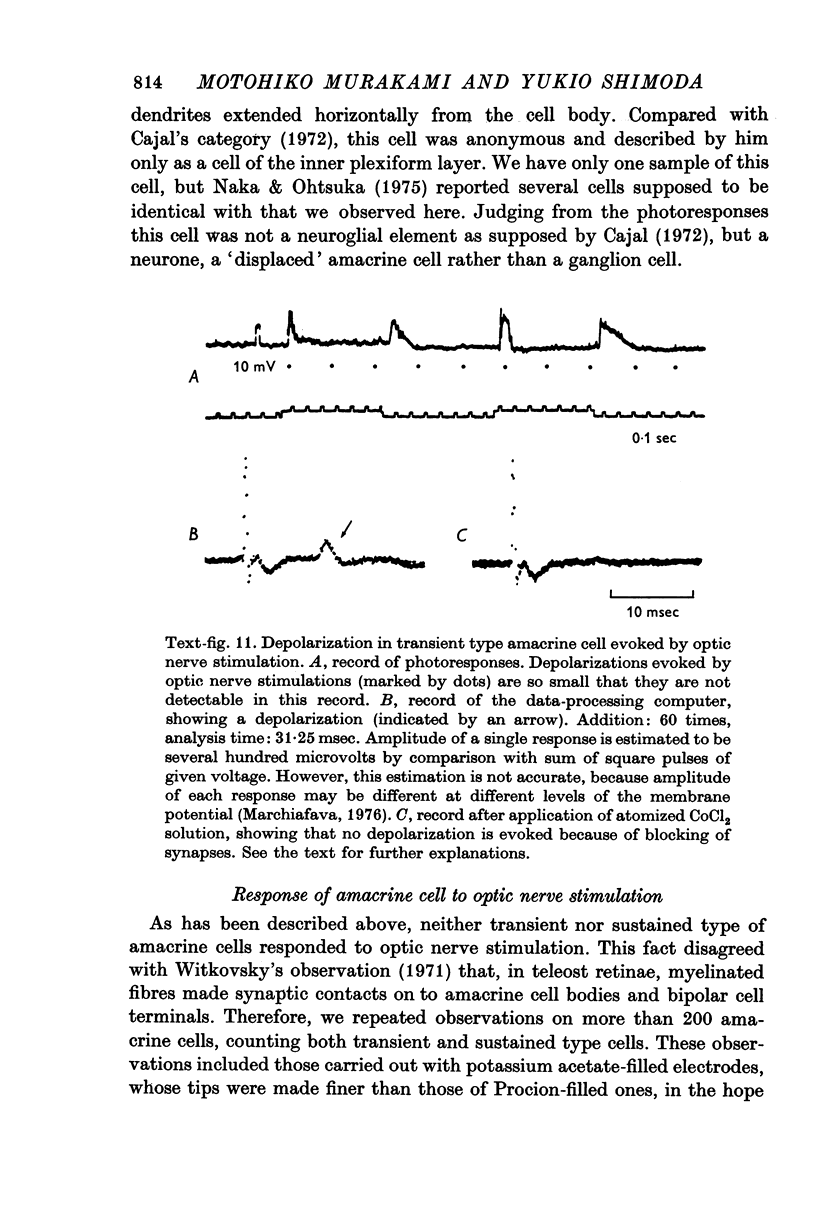
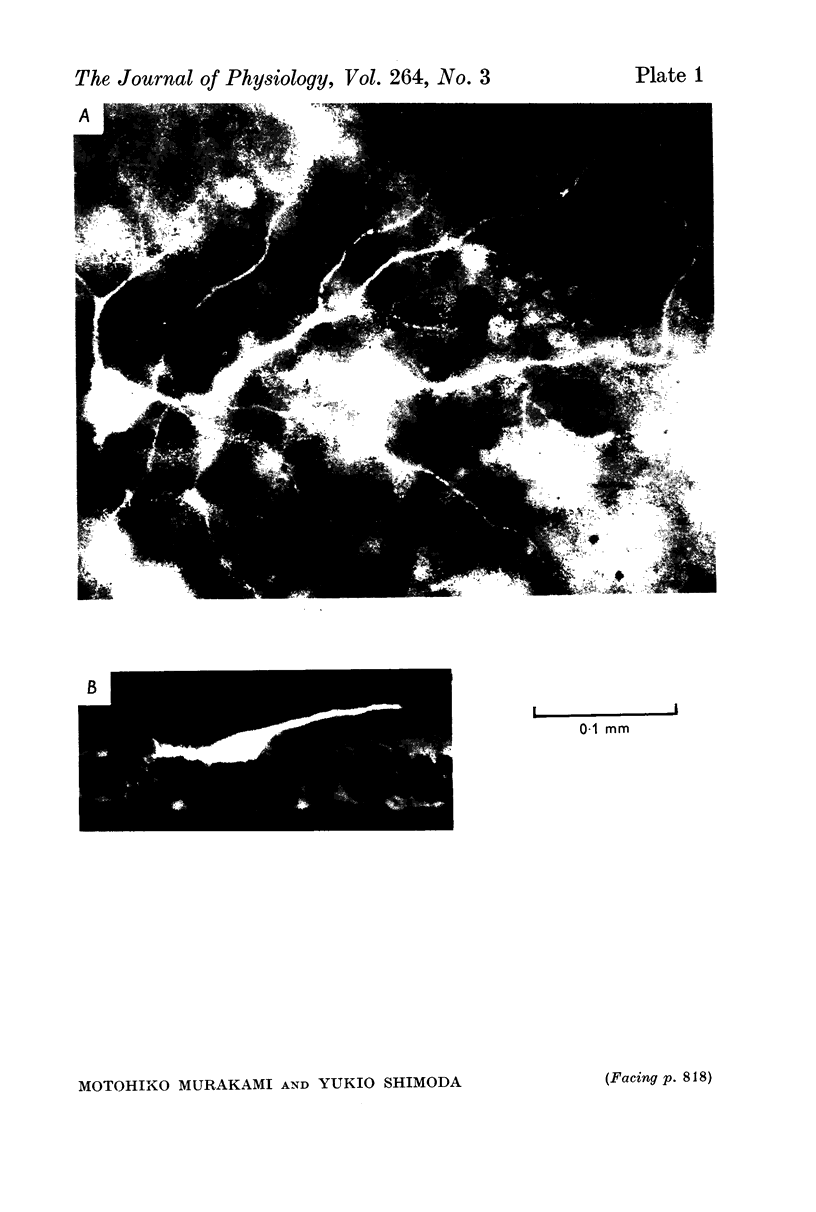
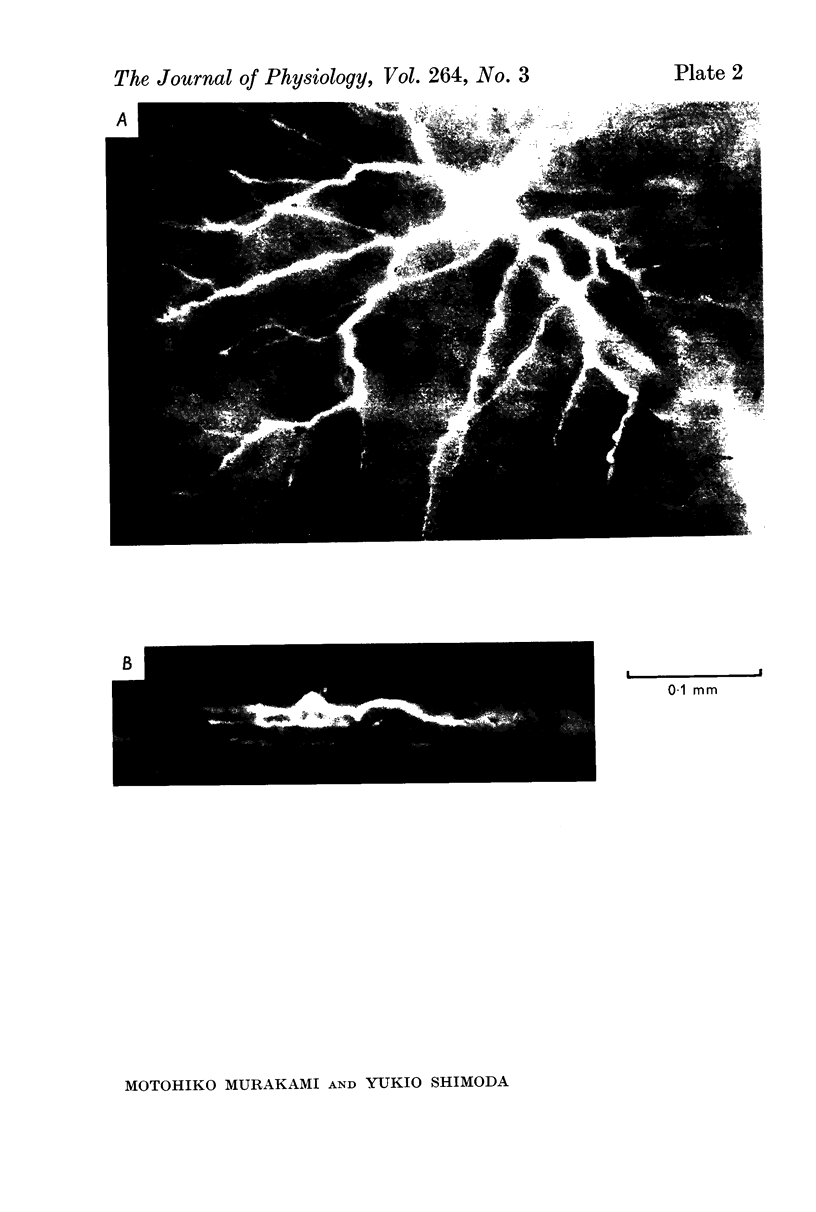
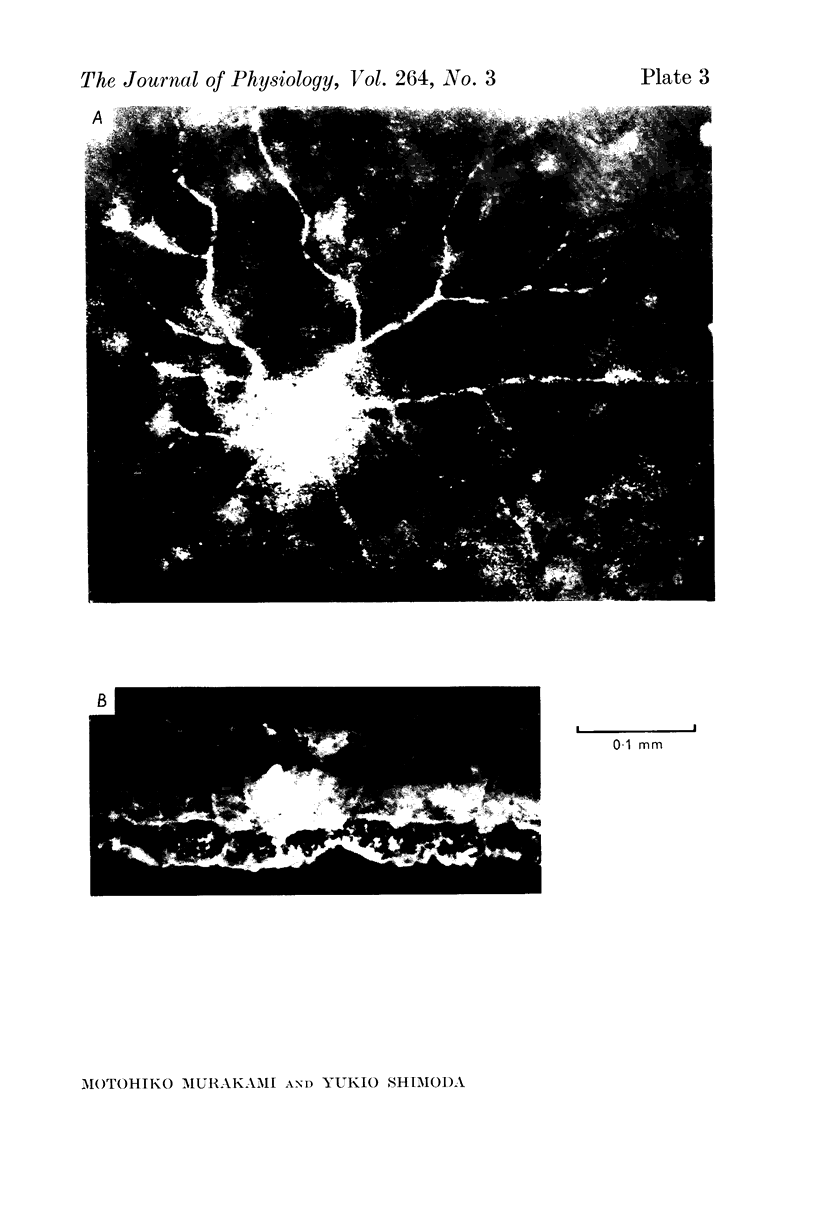
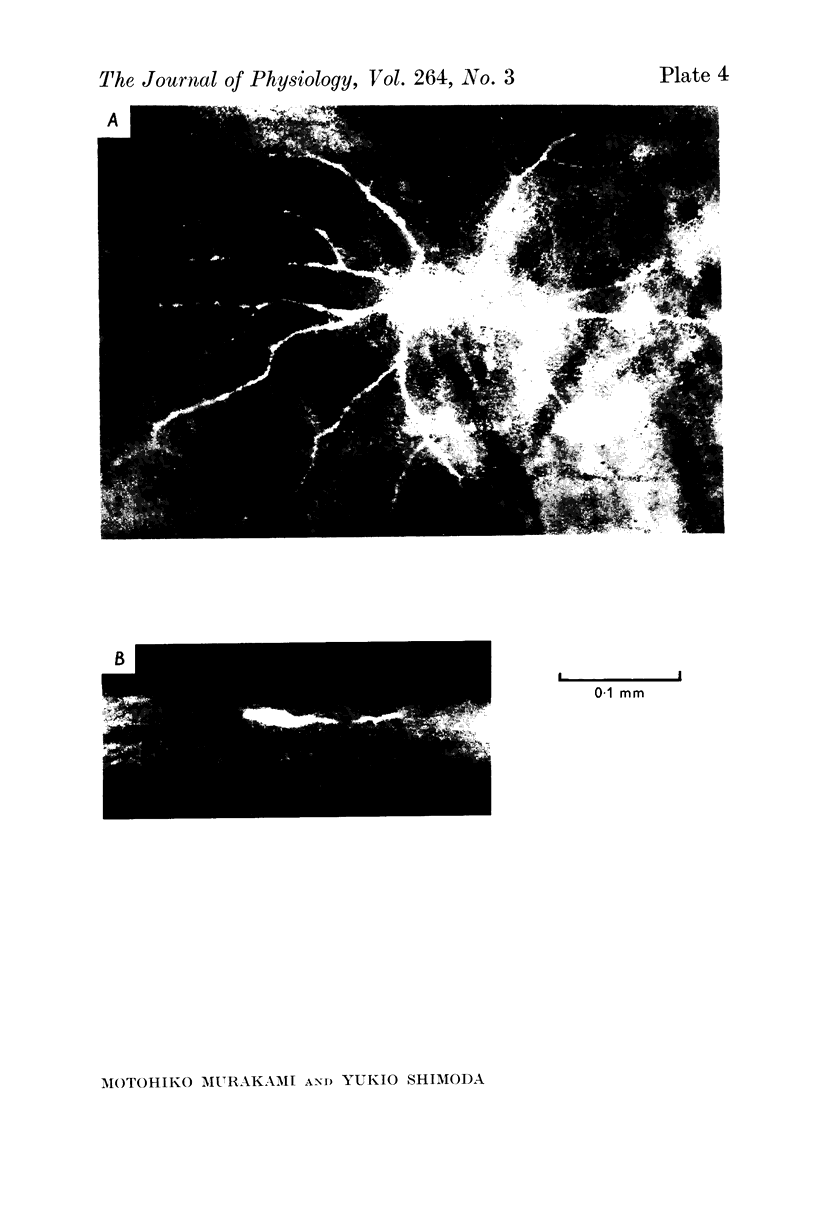
1. Amacrine and ganglion cells in the carp retina were identified from such criteria as photoresponses, intracellular dye staining, responses to optic nerve stimulation and behaviour to a synapse blocking agent. 2. Responses of ganglion cells were accompanied by spike discharges., either facilitated or suppressed by photic stimulation. The cells were also invaded by antidromic impulses, which survived after chemical synapses had been blocked by application of atomized CoCl2 solution. In subsequent histology of the Procion-stained neurones, the cell bodies were found in the ganglion cell layer and the axons were often traced. 3. Amacrine cells were subdivided into two types. The first type gave rise to transient depolarizations at both on- and offsets of spot and annulus illuminations, usually being associated with spike discharges of which the amplitudes varied in different cells. In histology, the cell bodies of this type were situated in the inner nuclear layer and dendrites ramified in two or more discrete sublayers of the inner plexiform layer (the stratified amacrine cell of Cajal). 4. The second type of amacrine cells produced sustained responses during illumination, being associated with no spike but with small oscillatory wavelets. The cell bodies were situated in the inner nuclear layer and the dendrites ramified in a single sublayer of the inner plexiform layer (the monolayered amacrine cell). 5. An attempt was made to see the effect of activation of centrifugal fibres on amacrine cells, but almost all of about 200 cells examined did not respond to optic nerve stimulation. Only two cells produced, with long latency, a small post-synaptic depolarization which disappeared after chemical synapses in the retina had been blocked. It is considered that the physiological role of the centrifugal system is insignificant in the carp retina.
Full text
PDF

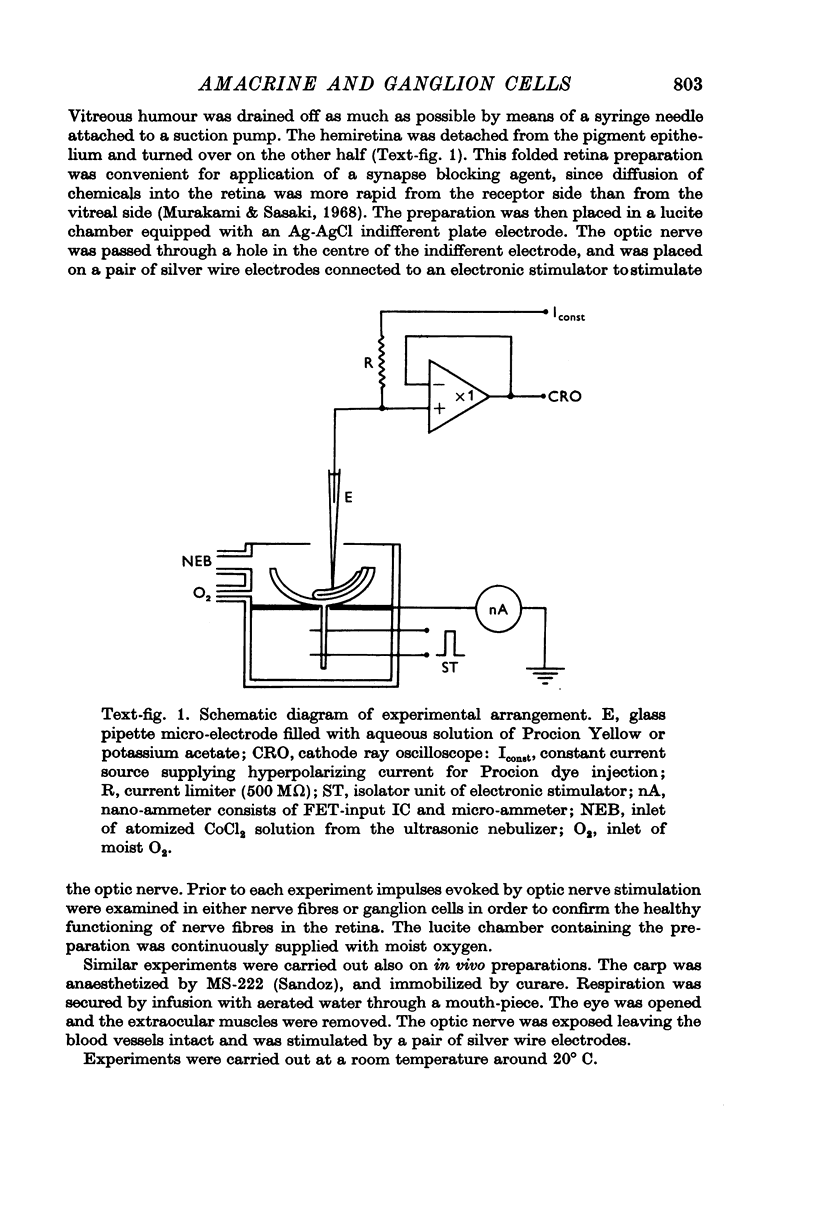


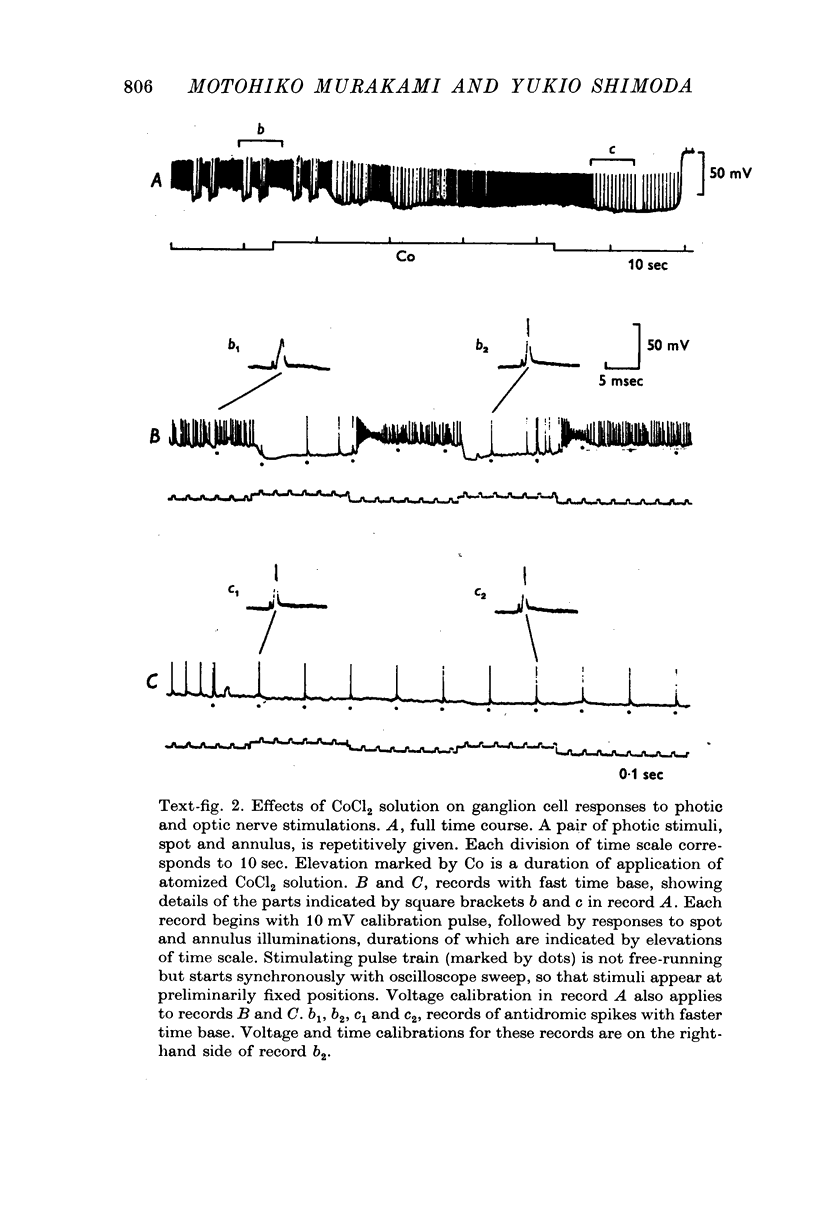
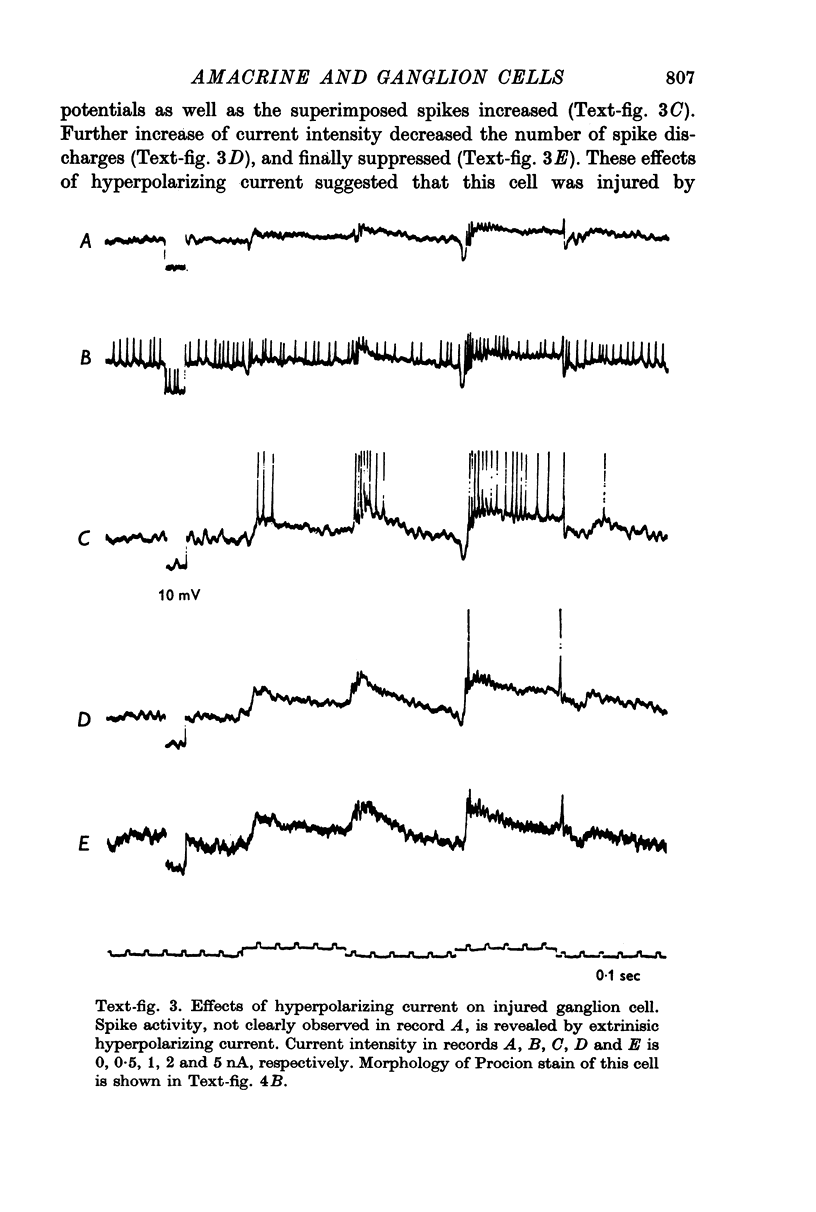
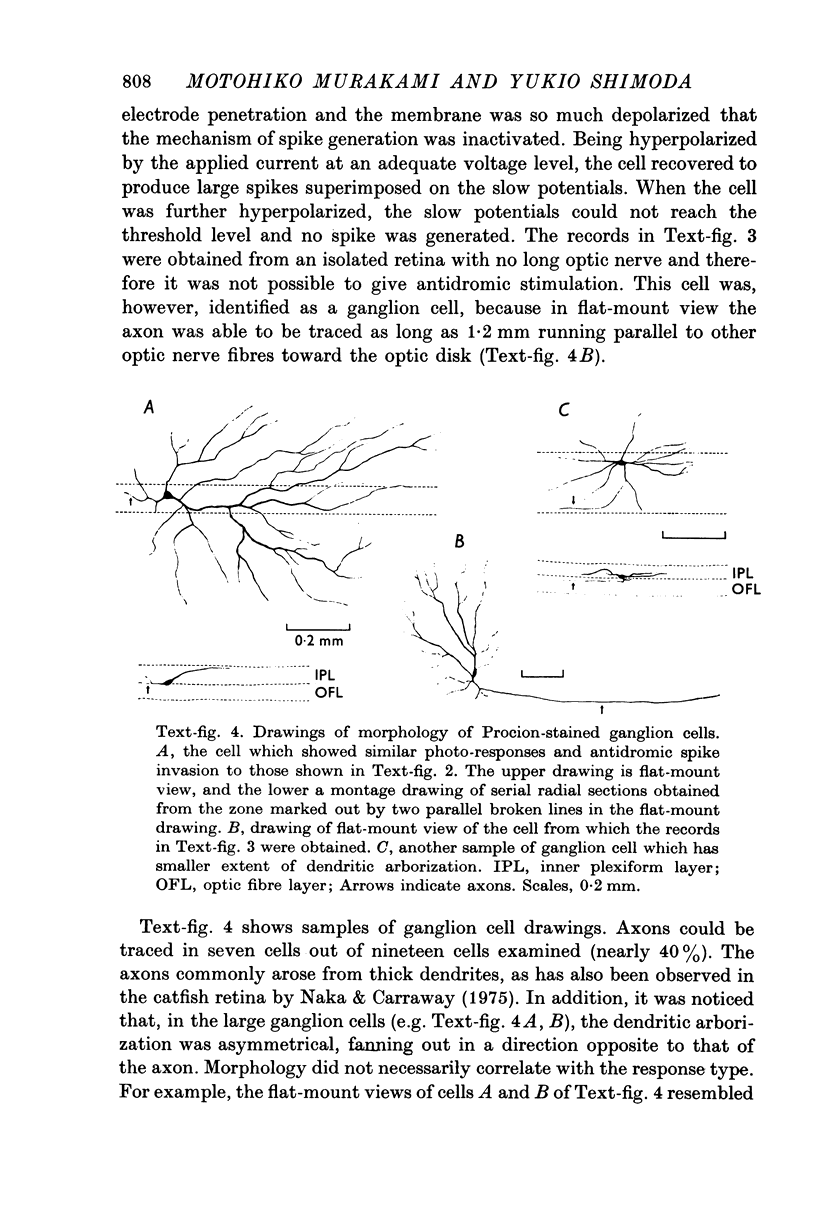

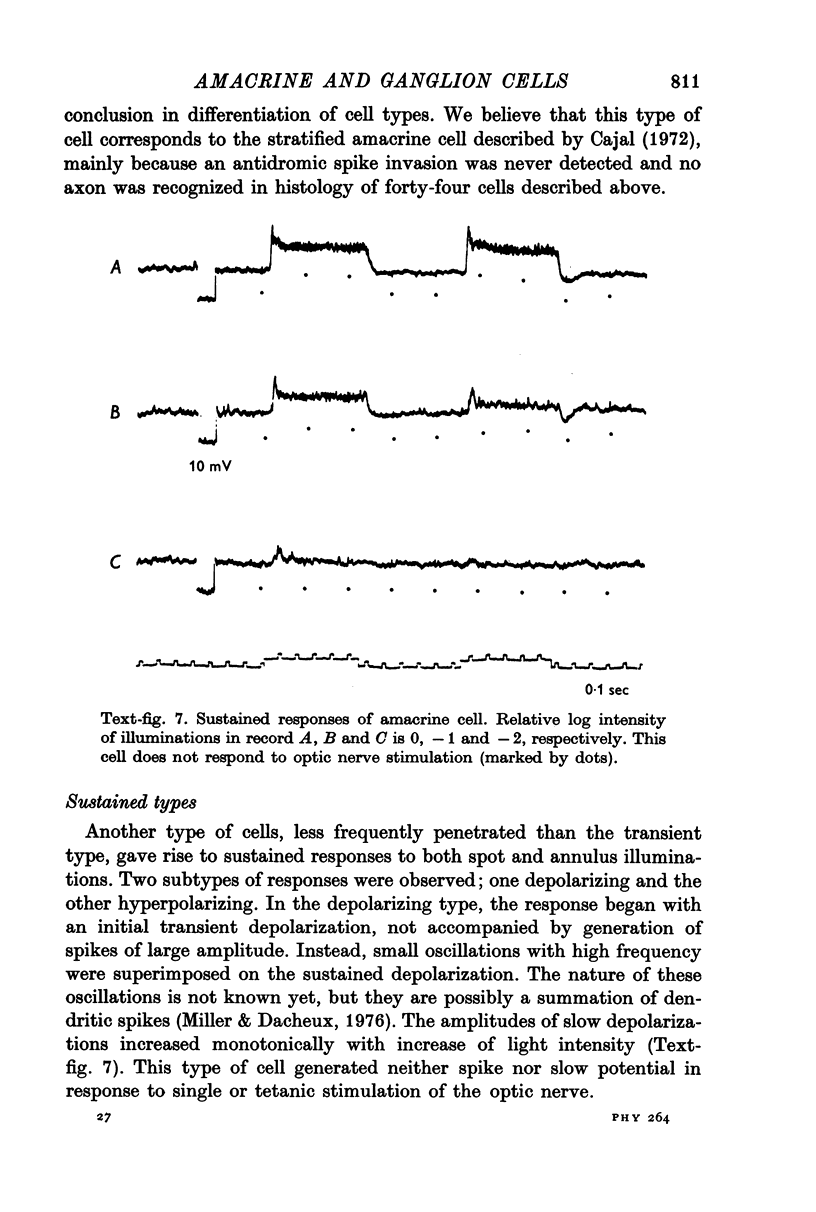
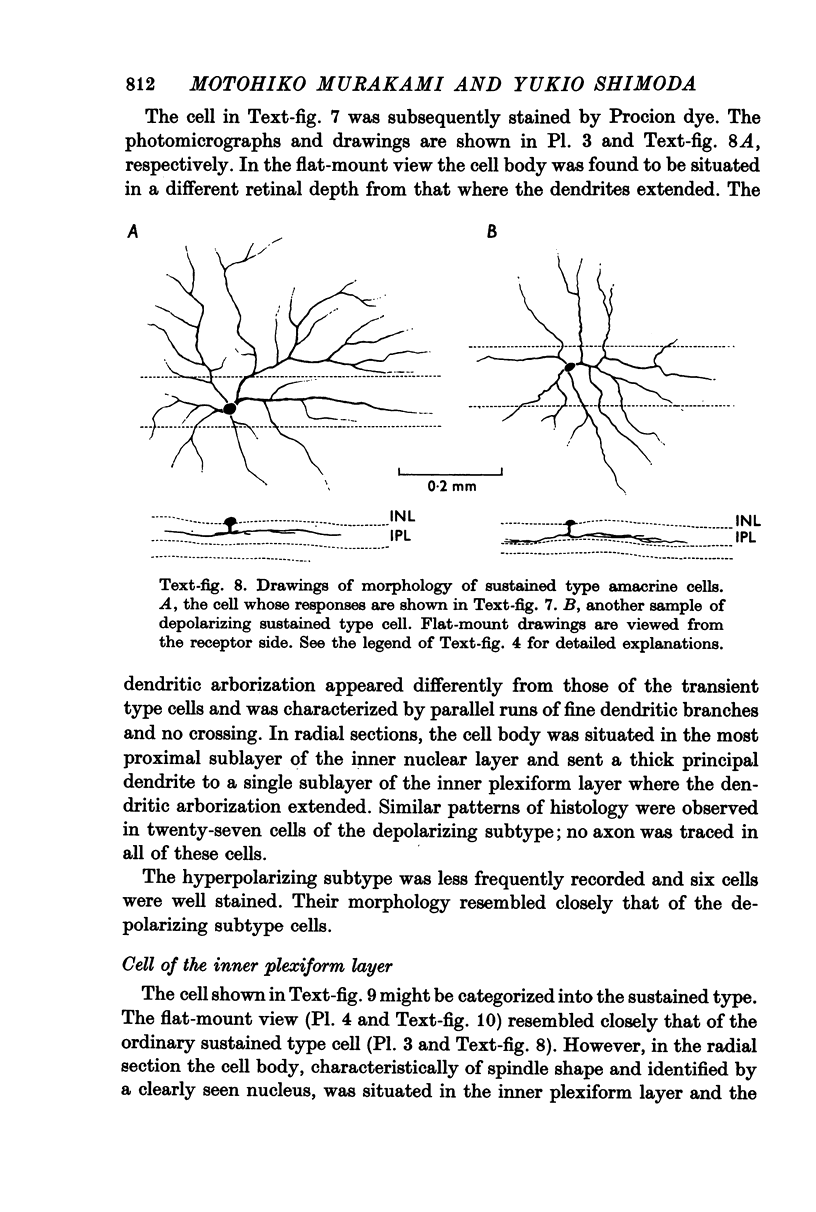
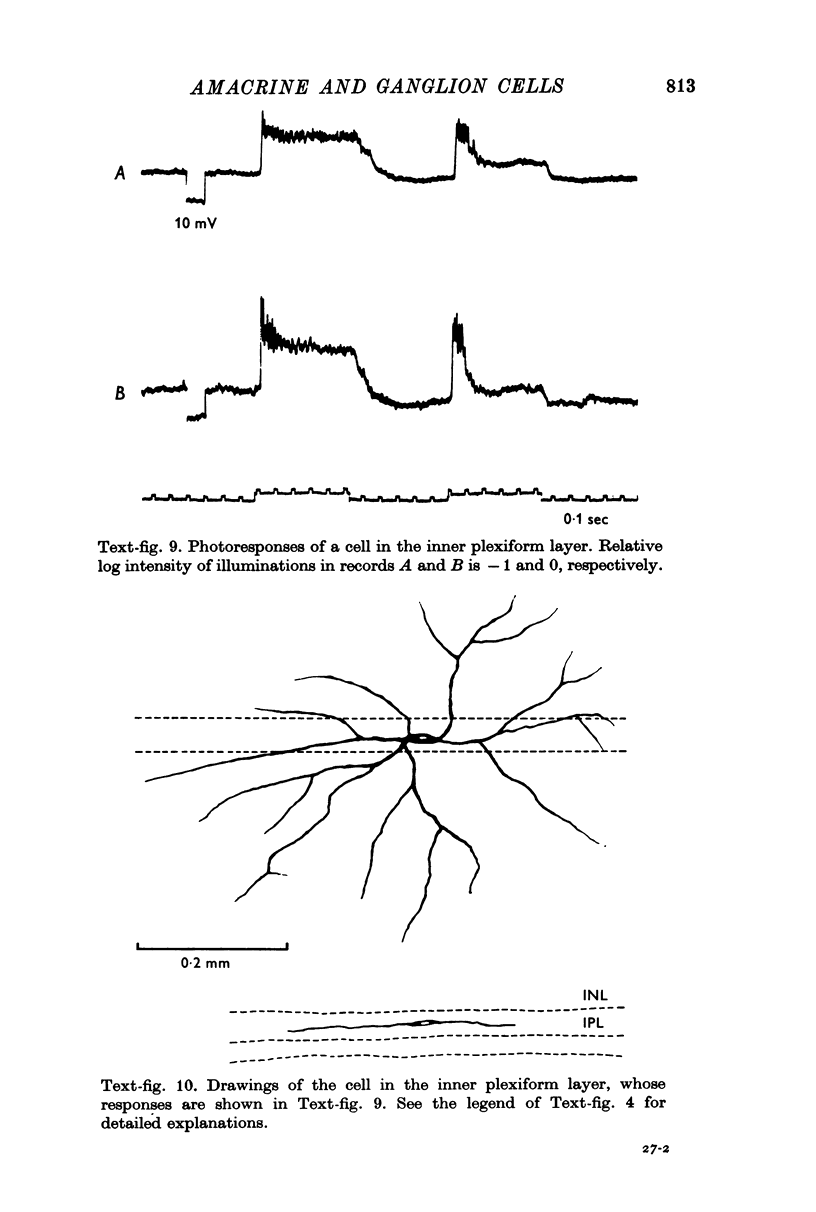




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cervetto L., Marchiafava P. L., Pasino E. Influence of efferent retinal fibres on responsiveness of ganglion cells to light. Nature. 1976 Mar 4;260(5546):56–57. doi: 10.1038/260056a0. [DOI] [PubMed] [Google Scholar]
- Kaneko A., Hashimoto H. Electrophysiological study of single neurons in the inner nuclear layer of the carp retina. Vision Res. 1969 Jan;9(1):37–55. doi: 10.1016/0042-6989(69)90030-3. [DOI] [PubMed] [Google Scholar]
- Kaneko A., Hashimoto H. Localization of spike-producing cells in the frog retina. Vision Res. 1968 Mar;8(3):259–262. doi: 10.1016/0042-6989(68)90013-8. [DOI] [PubMed] [Google Scholar]
- Kaneko A., Hashimoto H. Recording site of the single cone response determined by an electrode marking technique. Vision Res. 1967 Nov;7(11):847–851. doi: 10.1016/0042-6989(67)90005-3. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970 May;207(3):623–633. doi: 10.1113/jphysiol.1970.sp009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiafava P. L. Centrifugal actions on amacrine and ganglion cells in the retina of the turtle. J Physiol. 1976 Feb;255(1):137–155. doi: 10.1113/jphysiol.1976.sp011273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto N., Naka K. I. Identification of intracellular responses in the frog retina. Brain Res. 1972 Jul 13;42(1):59–71. doi: 10.1016/0006-8993(72)90042-x. [DOI] [PubMed] [Google Scholar]
- Miller R. F., Dacheux R. Dendritic and somatic spikes in mudpuppy amacrine cells: indentification and TTX sensitivity. Brain Res. 1976 Mar 5;104(1):157–162. doi: 10.1016/0006-8993(76)90657-0. [DOI] [PubMed] [Google Scholar]
- Miller W. H., Hashimoto Y., Saito T., Tomita T. Physiological and morphological identification of L- and C-type S-potentials in the turtle retina. Vision Res. 1973 Feb;13(2):443–447. doi: 10.1016/0042-6989(73)90121-1. [DOI] [PubMed] [Google Scholar]
- Murakami M., Otsu K., Otsuka T. Effects of chemicals on receptors and horizontal cells in the retina. J Physiol. 1972 Dec;227(3):899–913. doi: 10.1113/jphysiol.1972.sp010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Sasaki Y. Analysis of spatial distribution of the ERG components in the carp retina. Jpn J Physiol. 1968 Jun 15;18(3):326–336. doi: 10.2170/jjphysiol.18.326. [DOI] [PubMed] [Google Scholar]
- Naka K., Garraway N. R. Morphological and functional identifications of catfish retinal neurons. I. Classical morphology. J Neurophysiol. 1975 Jan;38(1):53–71. doi: 10.1152/jn.1975.38.1.53. [DOI] [PubMed] [Google Scholar]
- Naka K., Marmarelis P. Z., Chan R. Y. Morphological and functional identifications of catfish retinal neurons. III. Functional identification. J Neurophysiol. 1975 Jan;38(1):92–131. doi: 10.1152/jn.1975.38.1.92. [DOI] [PubMed] [Google Scholar]
- Naka K., Otsuka T. Morphological and functional identifications of catfish retinal neurons. II. Morphological identification. J Neurophysiol. 1975 Jan;38(1):72–91. doi: 10.1152/jn.1975.38.1.72. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Organization of on-off cells in the retina of the turtle. J Physiol. 1973 Apr;230(1):1–14. doi: 10.1113/jphysiol.1973.sp010171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg R. H., Schmidt R. Identification of horizontal cells as S-potential generators in the cat retina by intracellular dye injection. Vision Res. 1970 Sep;10(9):817–820. doi: 10.1016/0042-6989(70)90160-4. [DOI] [PubMed] [Google Scholar]
- Tomita T., Kaneko A., Murakami M., Pautler E. L. Spectral response curves of single cones in the carp. Vision Res. 1967 Jul;7(7):519–531. doi: 10.1016/0042-6989(67)90061-2. [DOI] [PubMed] [Google Scholar]
- Toyoda J., Hashimoto H., Anno H., Tomita T. The rod response in the frog and studies by intracellular recording. Vision Res. 1970 Nov;10(11):1093–1100. doi: 10.1016/0042-6989(70)90026-x. [DOI] [PubMed] [Google Scholar]
- Weakly J. N. The action of cobalt ions on neuromuscular transmission in the frog. J Physiol. 1973 Nov;234(3):597–612. doi: 10.1113/jphysiol.1973.sp010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- de Moraes S., Carvalho F. V. On the mechanism of the neuromuscular blocking action of cobalt ion. Pharmacology. 1969;2(4):237–242. doi: 10.1159/000136024. [DOI] [PubMed] [Google Scholar]






