Abstract
1. The electrical properties and the active transport processes of the isolated urinary bladder of the urodele, Amphiuma means, were studied by mounting this tissue as a flat sheet between two halves of a lucite chamber. The mean transepithelial potential difference was 70-2 +/- 2-3 mV (serosa positive), the mean short-circuit current was 10-9 +/- 0-5 micrionA/mg of dry weight and the mean transepithelial d.c. resistance was 6540 +/- 374 omega mg of dry weight. 2. The short-circuit current (Isc) accounted for 92% of the net 22Na+ flux from the mucosa to the serosa. The difference resulted from a transport of 36Cl- in the same direction as sodium. 3. The active sodium transport exhibited typical saturation kinetics, having a Km of 15-4 m-equiv/l. and approaching zero order at 60-70 m-equiv/l. The transepithelial potential difference increased linearly with the log of the mucosal sodium concentration at a rate of 50-3 mV per tenfold concentration change. 4. In the absence of the major anions (HCO3- and Cl-) from the bathing solutions, the electrical properties and the sodium influx decreased to less than 40% of their control values. The presence of only one of these two anions in the serosal bathing solution was sufficient to maintain these parameters. 5. Amiloride (10(-5)M) and ouabain (10(-6)M) inhibited the sodium transport 97% and 85% respectively. Amphotericin B (10(-6)M) stimulated the sodium transport 47%. Furosemide (10(-3)M) inhibited the chloride transport 43%. The sodium transport was insensitive to the action of two enurohypophyseal peptides tested, lysine-vasotocin and pitocin.
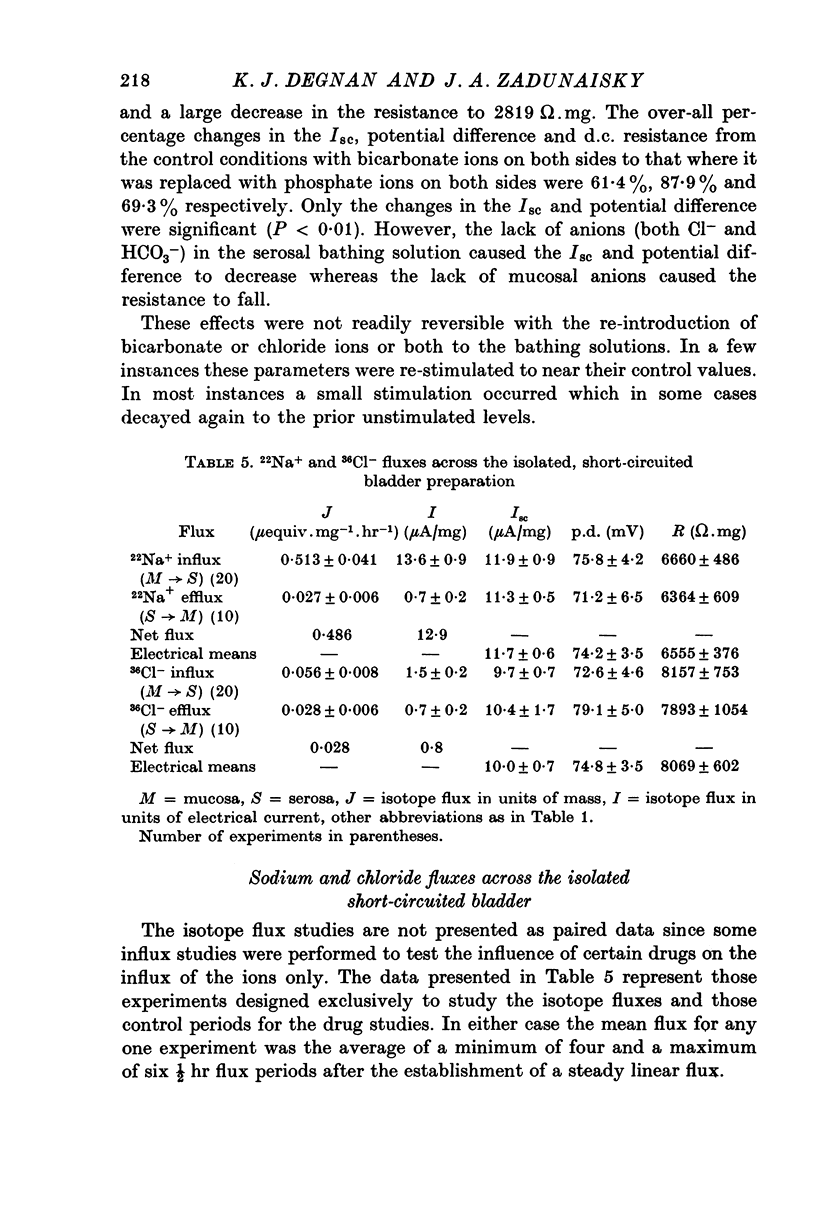
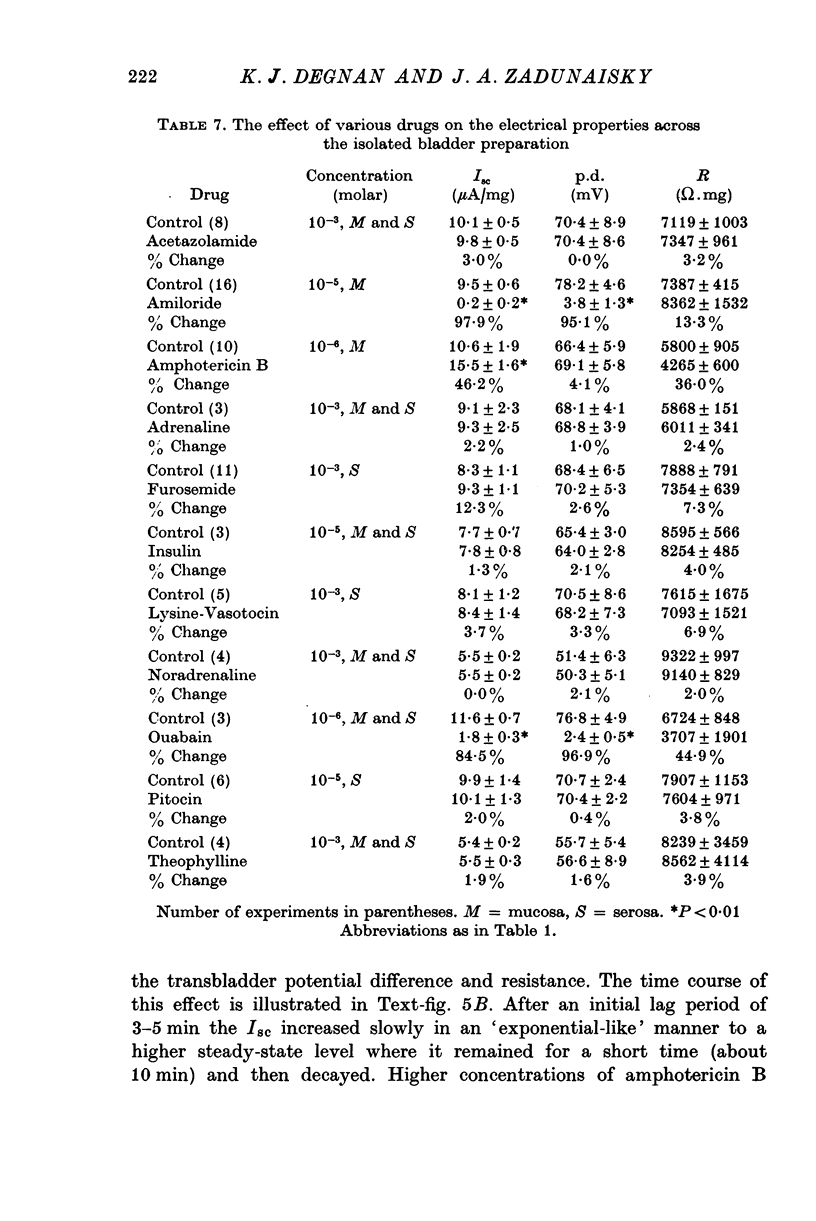
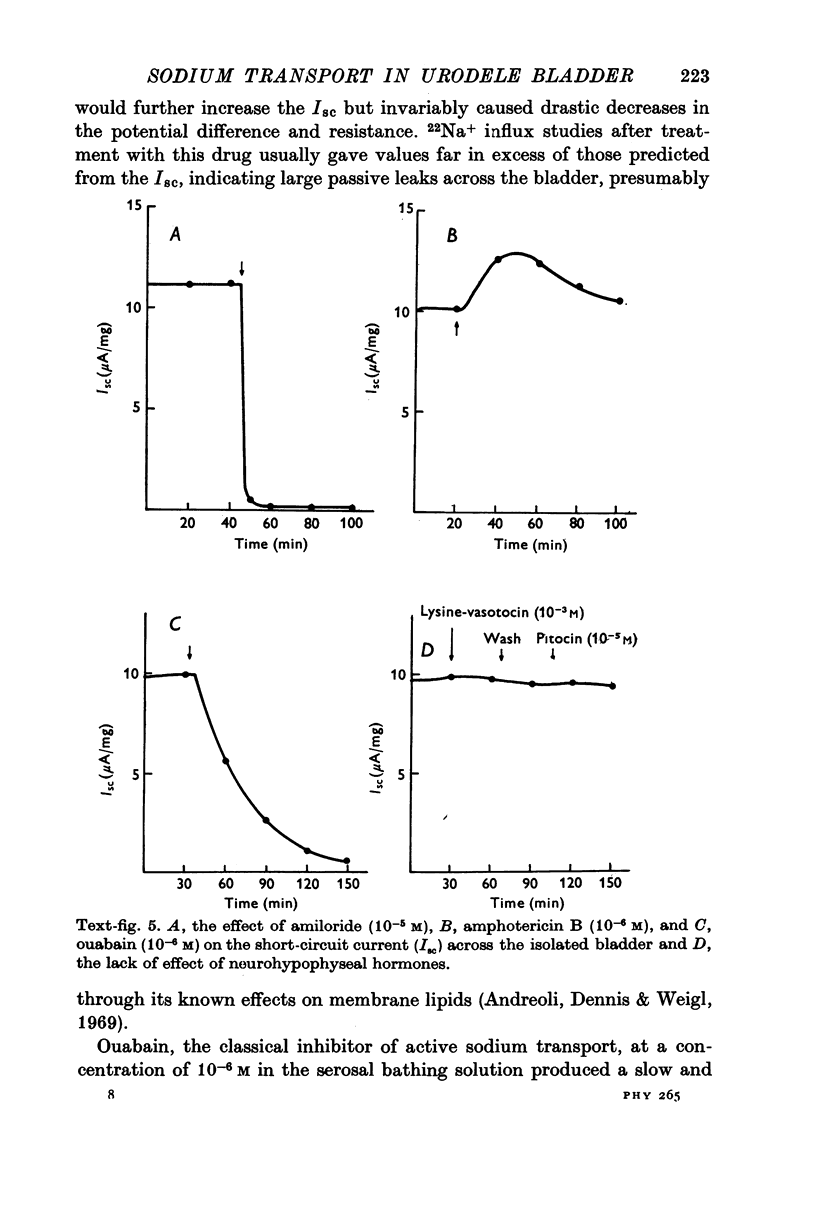
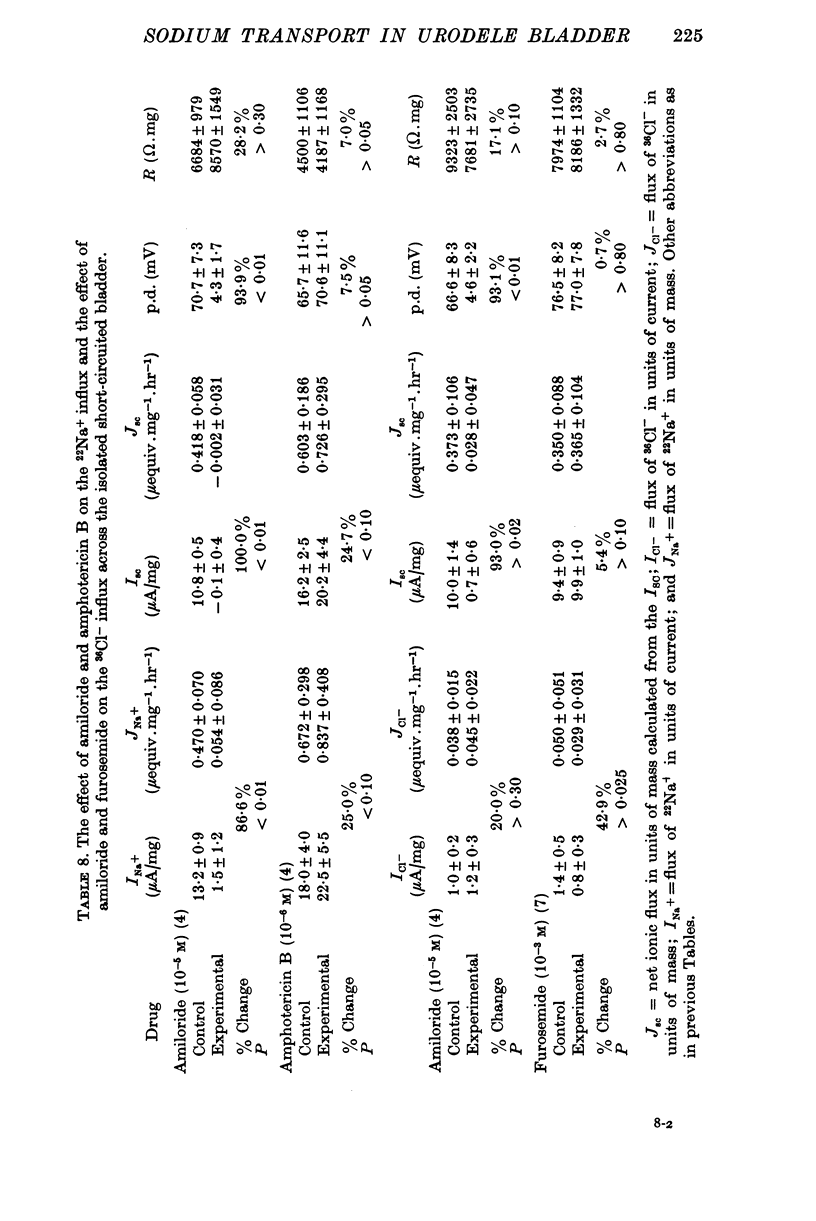
Full text
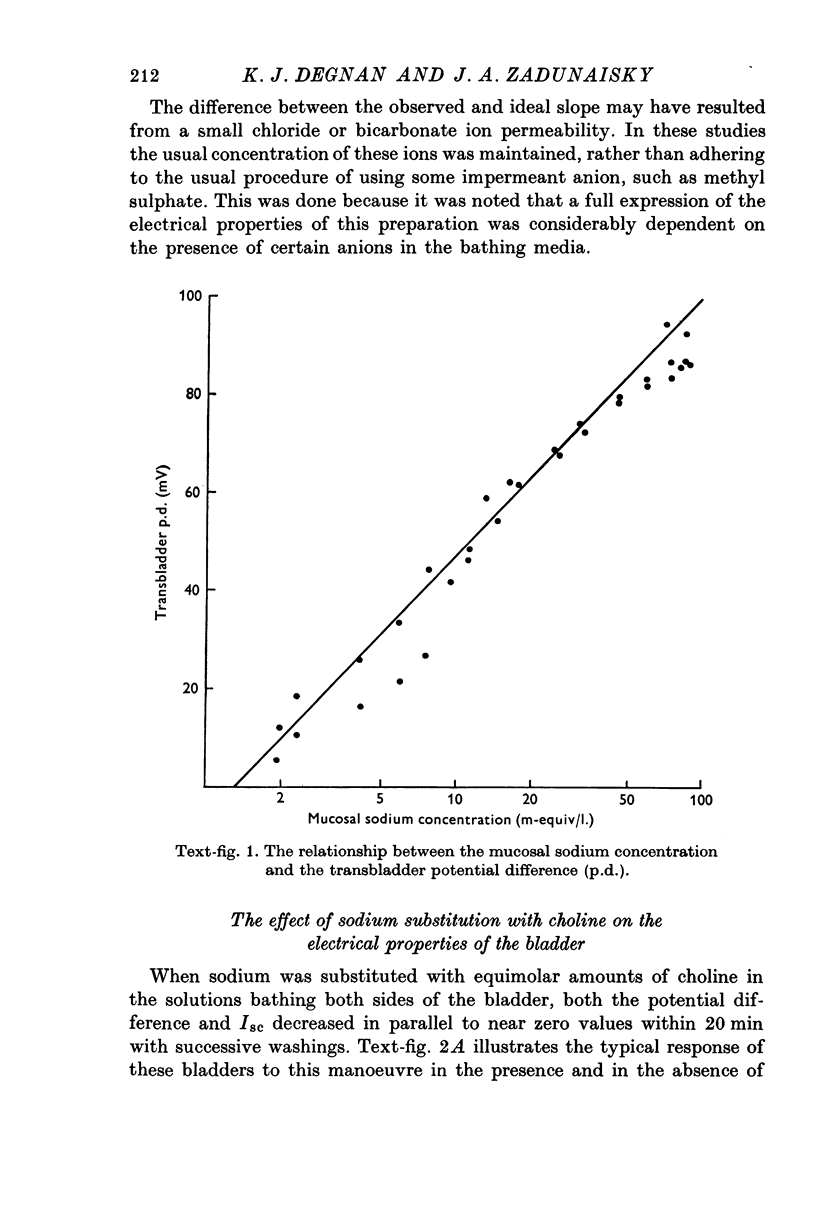
PDF




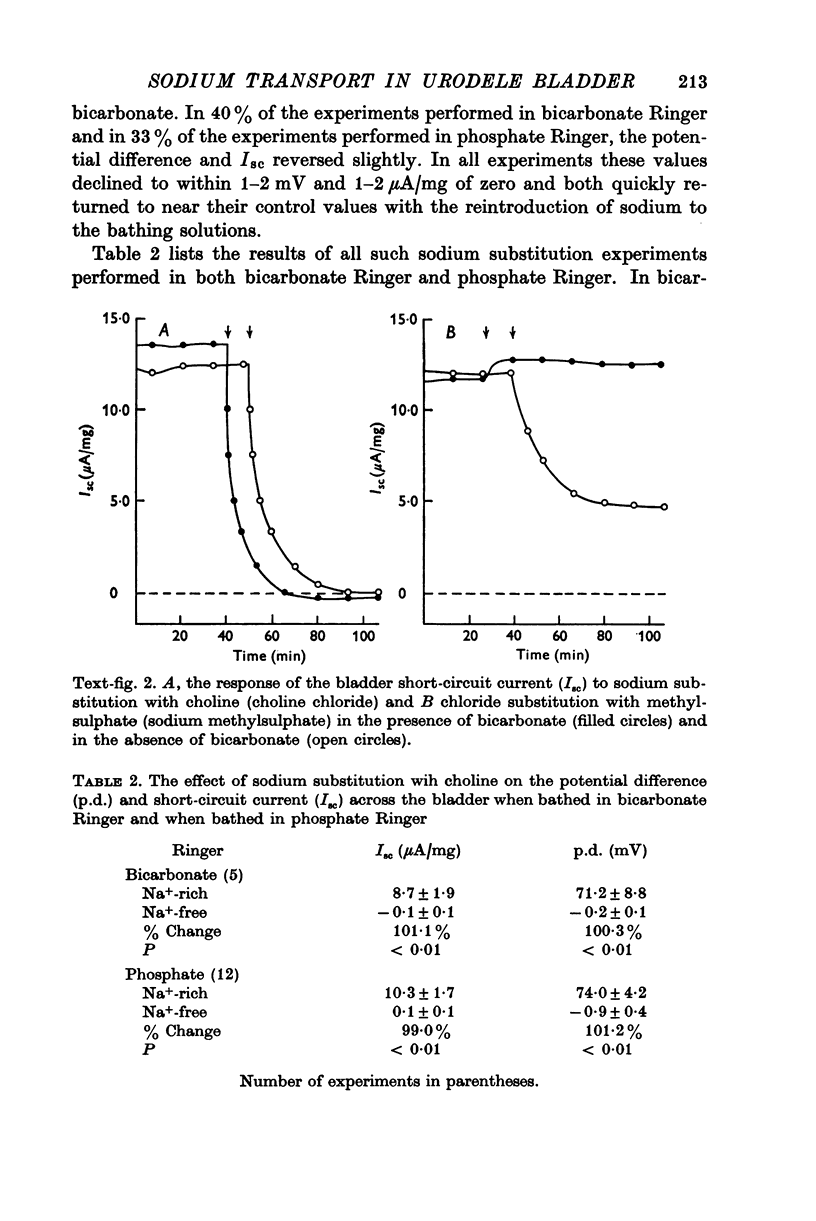
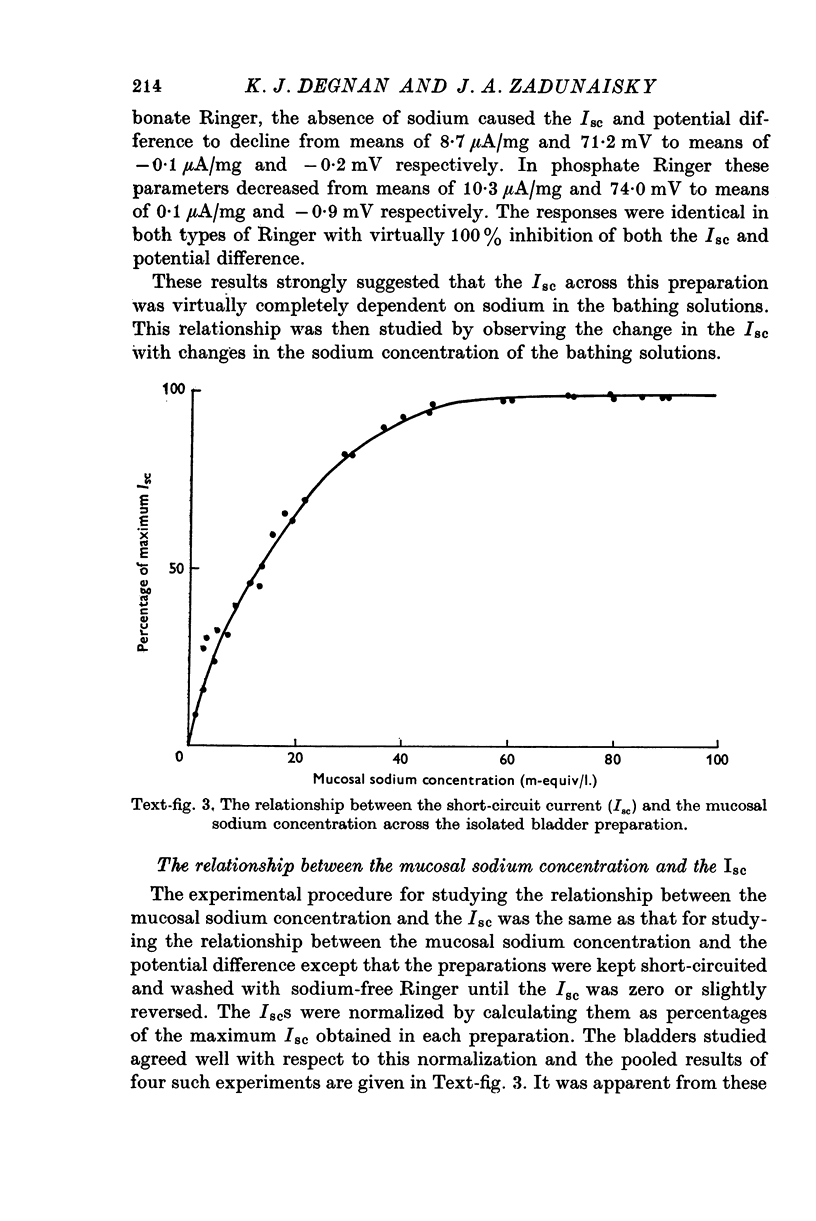

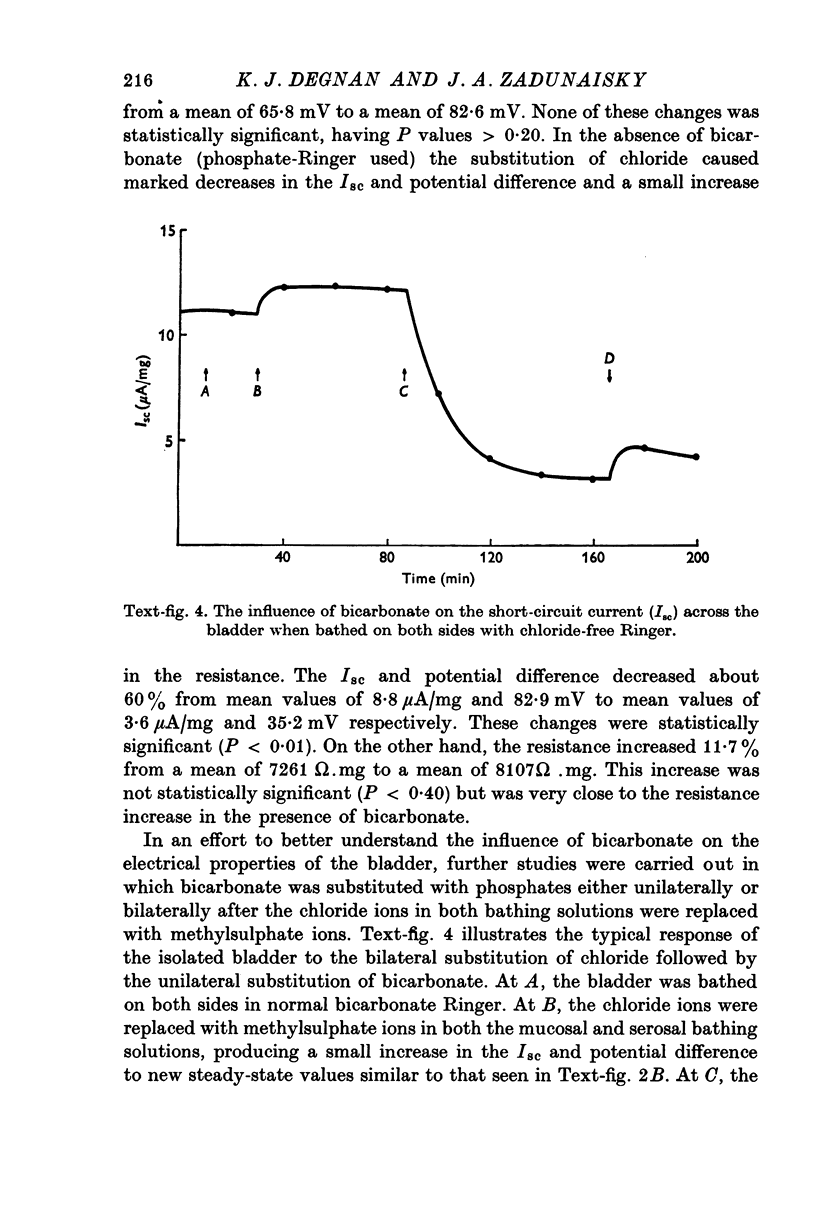











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aceves J., Erlij D., Edwards C. Na+ transport acrosss the isolated skin of Ambystoma mexicanus. Biochim Biophys Acta. 1968 Jun 11;150(4):744–746. doi: 10.1016/0005-2736(68)90068-0. [DOI] [PubMed] [Google Scholar]
- Andreoli T. E., Dennis V. W., Weigl A. M. The effect of amphotericin B on the water and nonelectrolyte permeability of thin lipid membranes. J Gen Physiol. 1969 Feb;53(2):133–156. doi: 10.1085/jgp.53.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENTLEY P. J. The effects of neurohypophysial extracts on the water transfer across the wall of the isolated urinary bladder of the toad Bufo marinus. J Endocrinol. 1958 Sep;17(3):201–209. doi: 10.1677/joe.0.0170201. [DOI] [PubMed] [Google Scholar]
- Bentley P. J. Neurohypophyseal hormones in amphibia: a comparison of their actions and storage. Gen Comp Endocrinol. 1969 Aug;13(1):39–44. doi: 10.1016/0016-6480(69)90219-6. [DOI] [PubMed] [Google Scholar]
- Bentley P. J. Osmoregulation in the aquatic urodeles Amphiuma means (the Congo eel) and Siren lacertina (the mud eel). Effects of vasotocin. Gen Comp Endocrinol. 1973 Apr;20(2):386–391. doi: 10.1016/0016-6480(73)90192-5. [DOI] [PubMed] [Google Scholar]
- Brodsky W. A., Schilb T. P. Ionic mechanisms for sodium and chloride transport across turtle bladders. Am J Physiol. 1966 May;210(5):987–996. doi: 10.1152/ajplegacy.1966.210.5.987. [DOI] [PubMed] [Google Scholar]
- Burg M., Stoner L., Cardinal J., Green N. Furosemide effect on isolated perfused tubules. Am J Physiol. 1973 Jul;225(1):119–124. doi: 10.1152/ajplegacy.1973.225.1.119. [DOI] [PubMed] [Google Scholar]
- CHOI J. K. The fine structure of the urinary bladder of the toad, Bufo marinus. J Cell Biol. 1963 Jan;16:53–72. doi: 10.1083/jcb.16.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRABBE J. Stimulation of active sodium transport across the isolated toad bladder after injection of aldosterone to the animal. Endocrinology. 1961 Oct;69:673–682. doi: 10.1210/endo-69-4-673. [DOI] [PubMed] [Google Scholar]
- Cuthbert A. W., Painter E., Prince W. T. The effects of anions on sodium transport. Br J Pharmacol. 1969 May;36(1):97–106. doi: 10.1111/j.1476-5381.1969.tb08307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira K. T. Anionic dependence of sodium transport in the frog skin. Biochim Biophys Acta. 1968 Jun 11;150(4):587–598. doi: 10.1016/0005-2736(68)90048-5. [DOI] [PubMed] [Google Scholar]
- Finn A. L., Handler J. S., Orloff J. Active chloride transport in the isolated toad bladder. Am J Physiol. 1967 Jul;213(1):179–184. doi: 10.1152/ajplegacy.1967.213.1.179. [DOI] [PubMed] [Google Scholar]
- Frazier L. W., Vanatta J. C. Mechanism of acidification of the mucosal fluid by the toad urinary bladder. Biochim Biophys Acta. 1972 Dec 1;290(1):168–177. doi: 10.1016/0005-2736(72)90061-2. [DOI] [PubMed] [Google Scholar]
- Gonzalez C. F., Shamoo Y. E., Brodsky W. A. Electrical nature of active chloride transport across short-circuited turtle bladders. Am J Physiol. 1967 Mar;212(3):641–650. doi: 10.1152/ajplegacy.1967.212.3.641. [DOI] [PubMed] [Google Scholar]
- Gonzalez C. F., Shamoo Y. E., Brodsky W. A. The accelerating effect of serosal HCO3- on Na+ transport in short-circuited turtle bladders. Biochim Biophys Acta. 1969;193(2):403–418. doi: 10.1016/0005-2736(69)90200-4. [DOI] [PubMed] [Google Scholar]
- Helman S. I., Miller D. A. In vitro techniques for avoiding edge damage in studies of frog skin. Science. 1971 Jul 9;173(3992):146–148. doi: 10.1126/science.173.3992.146. [DOI] [PubMed] [Google Scholar]
- Kristensen P. Chloride transport across isolated frog skin. Acta Physiol Scand. 1972 Mar;84(3):338–346. doi: 10.1111/j.1748-1716.1972.tb05185.x. [DOI] [PubMed] [Google Scholar]
- LEAF A., ANDERSON J., PAGE L. B. Active sodium transport by the isolated toad bladder. J Gen Physiol. 1958 Mar 20;41(4):657–668. doi: 10.1085/jgp.41.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAFFLY R. H., HAYS R. M., LAMDIN E., LEAF A. The effect of neurohypophyseal hormones on the permeability of the toad bladder to urea. J Clin Invest. 1960 Apr;39:630–641. doi: 10.1172/JCI104078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. W., Curran P. F. Reversed potentials in isolated frog skin. II. Active transport of chloride. J Cell Physiol. 1966 Jun;67(3):367–373. doi: 10.1002/jcp.1040670302. [DOI] [PubMed] [Google Scholar]
- PEACHEY L. D., RASMUSSEN H. Structure of the toad's urinary bladder as related to its physiology. J Biophys Biochem Cytol. 1961 Aug;10:529–553. doi: 10.1083/jcb.10.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss L., Finn A. L. Passive electrical properties of toad urinary bladder epithelium. Intercellular electrical coupling and transepithelial cellular and shunt conductances. J Gen Physiol. 1974 Jul;64(1):1–25. doi: 10.1085/jgp.64.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USSING H. H., ZERAHN K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951 Aug 25;23(2-3):110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]
- WHITTEMBURY G. ELECTRICAL POTENTIAL PROFILE OF THE TOAD SKIN EPITHELIUM. J Gen Physiol. 1964 Mar;47:795–808. doi: 10.1085/jgp.47.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZADUNAISKY J. A., CANDIA O. A., CHIARANDINI D. J. THE ORIGIN OF THE SHORT-CIRCUIT CURRENT IN THE ISOLATED SKIN OF THE SOUTH AMERICAN FROG LEPTODACTYLUS OCELLATUS. J Gen Physiol. 1963 Nov;47:393–402. doi: 10.1085/jgp.47.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadunaisky J. A. Active transport of chloride in frog cornea. Am J Physiol. 1966 Aug;211(2):506–512. doi: 10.1152/ajplegacy.1966.211.2.506. [DOI] [PubMed] [Google Scholar]



