Abstract
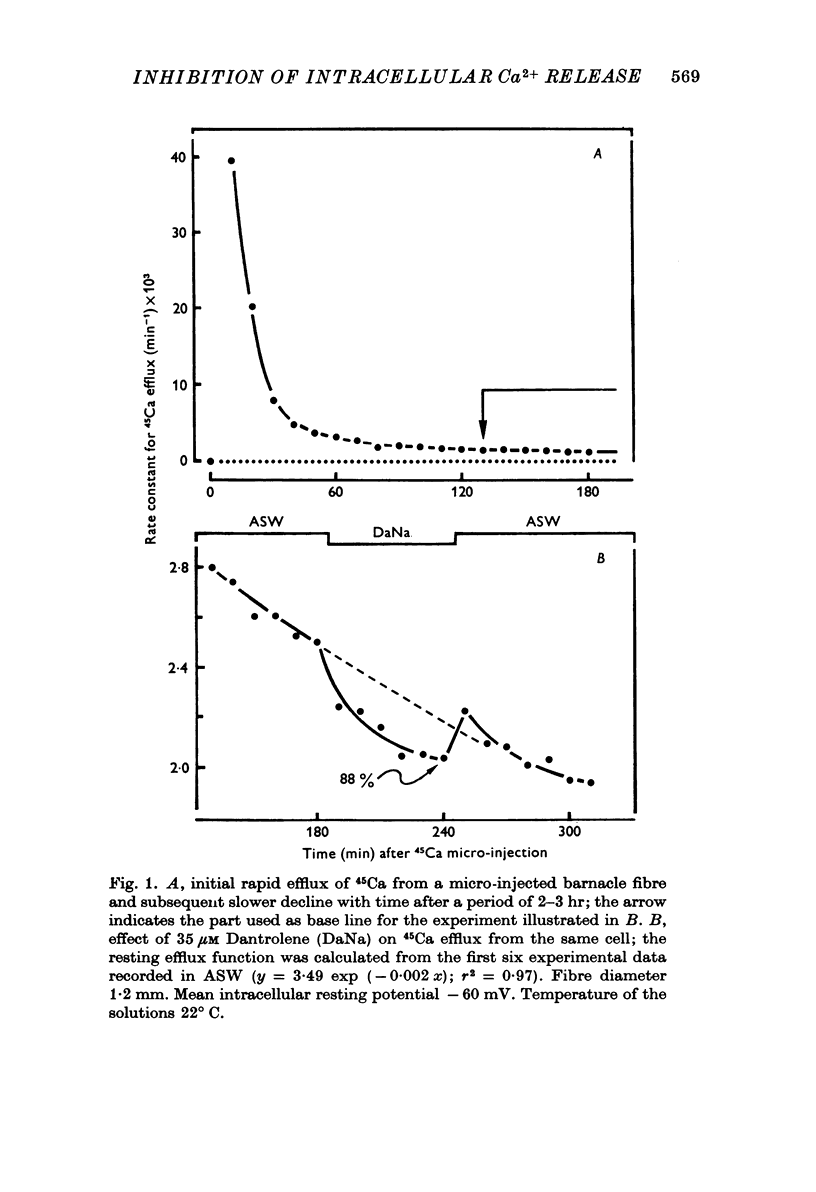
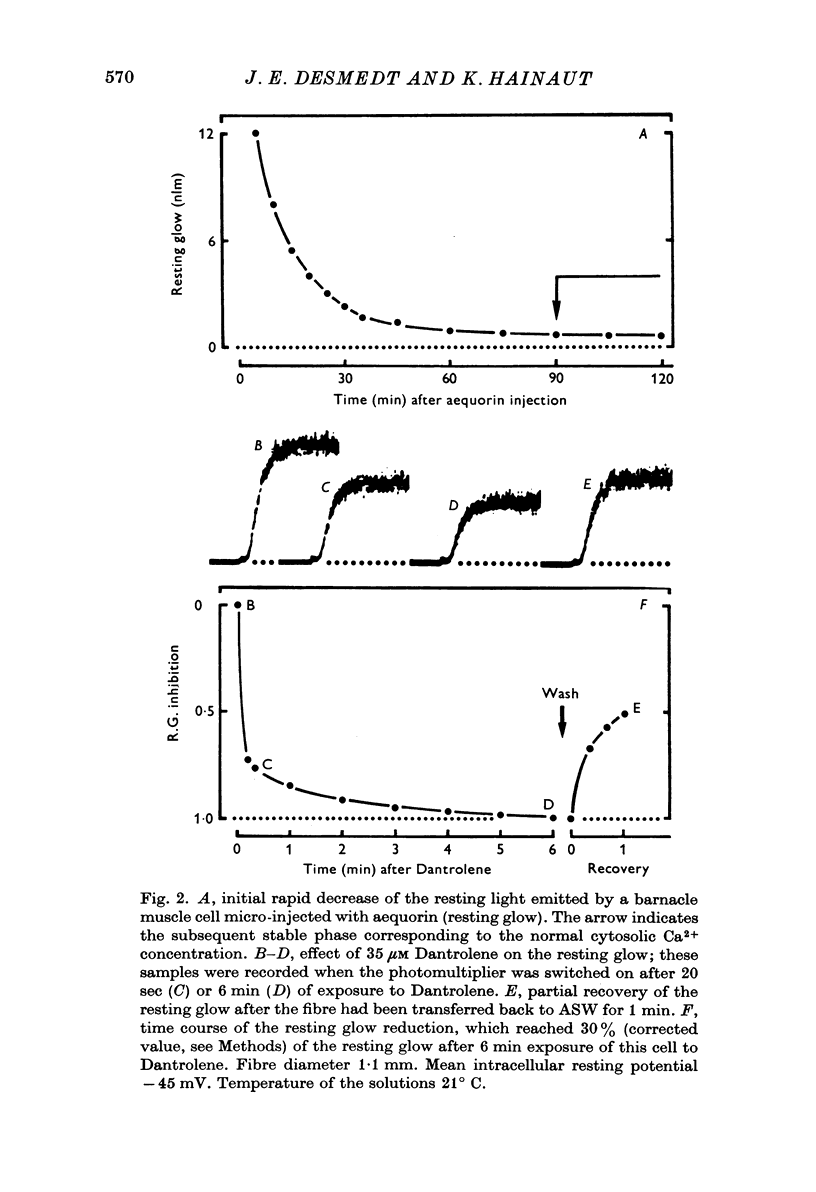
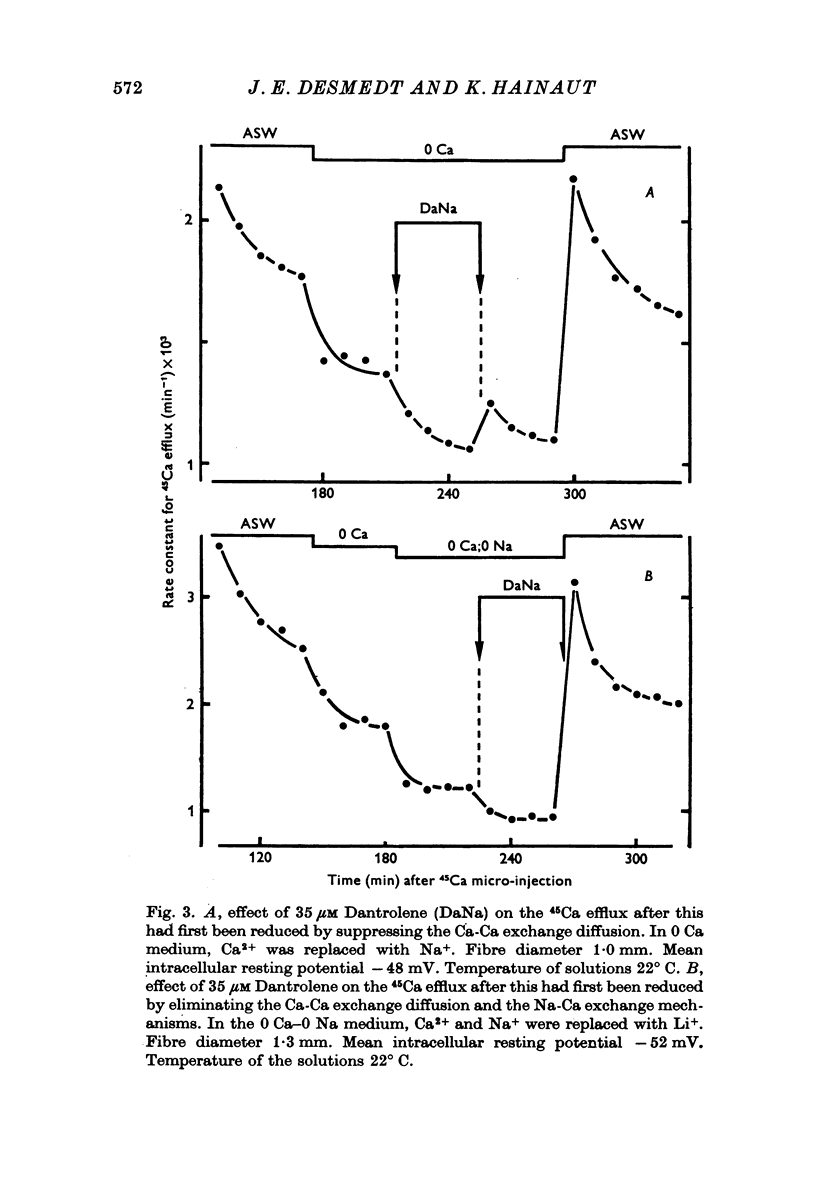
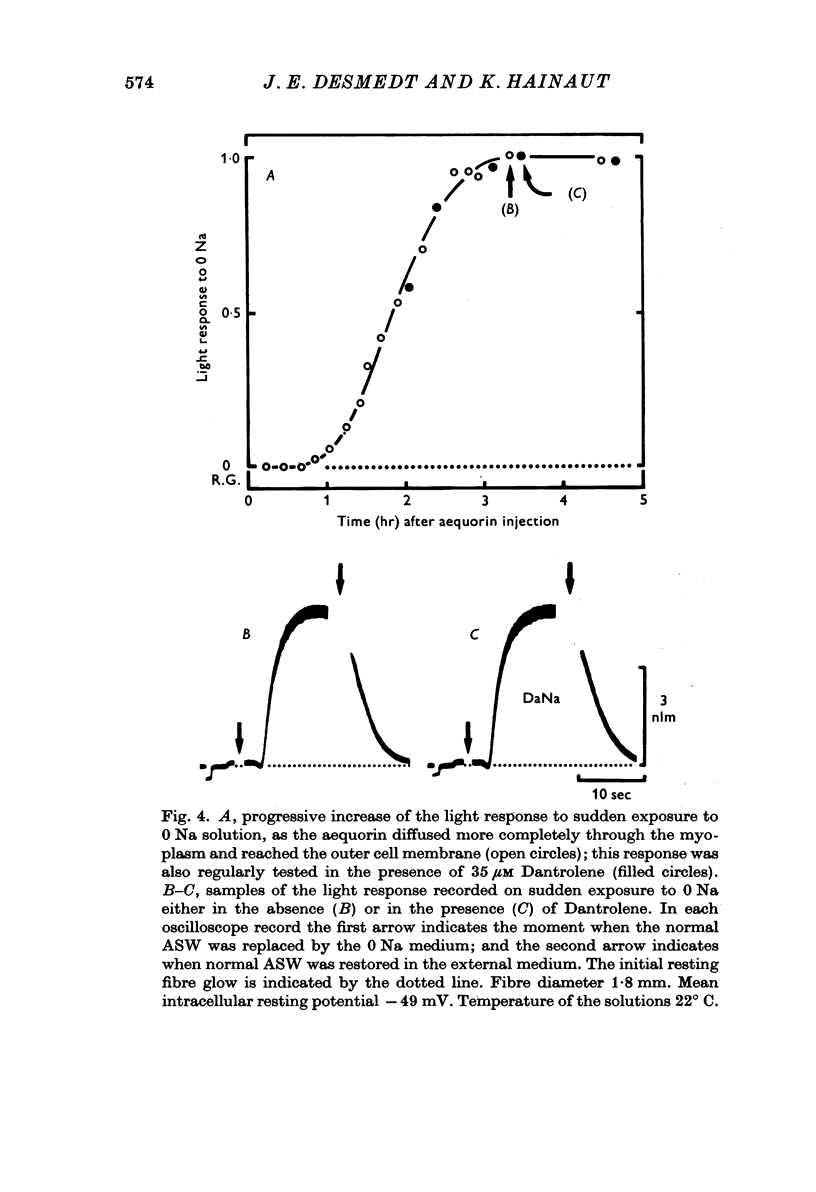
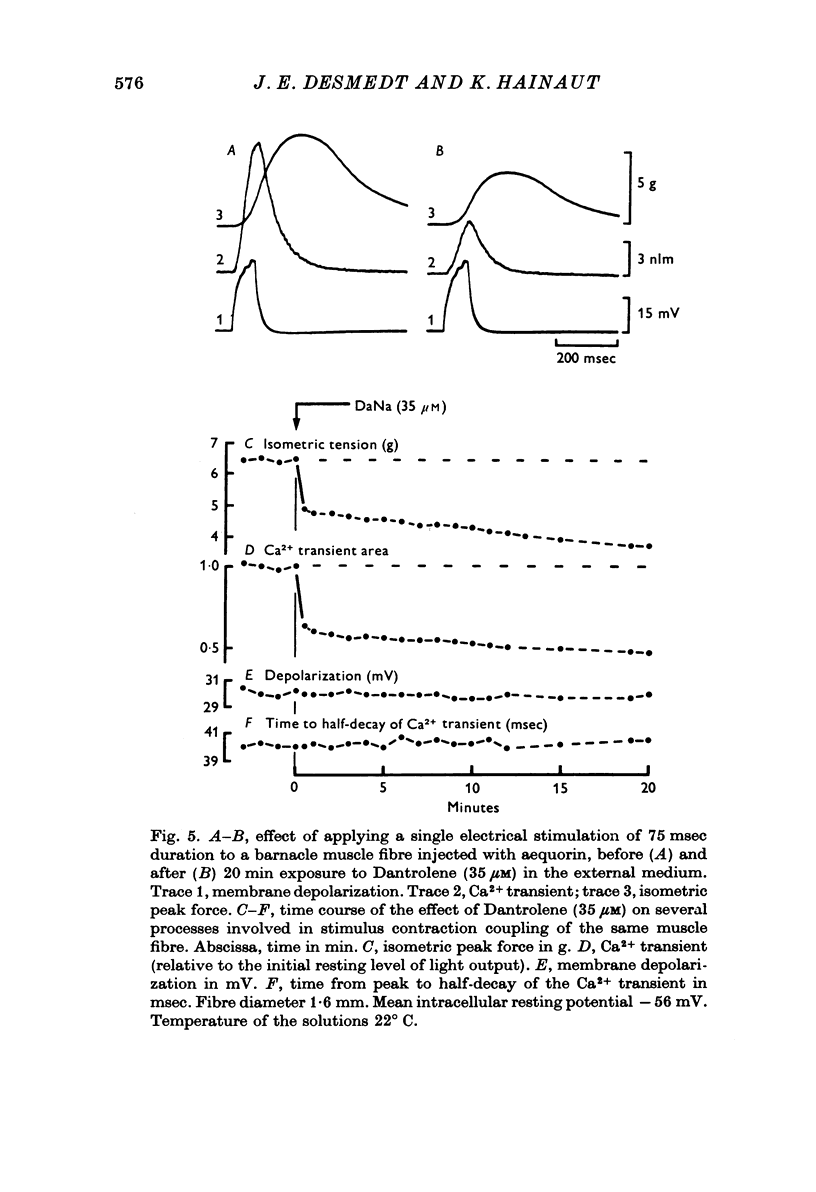
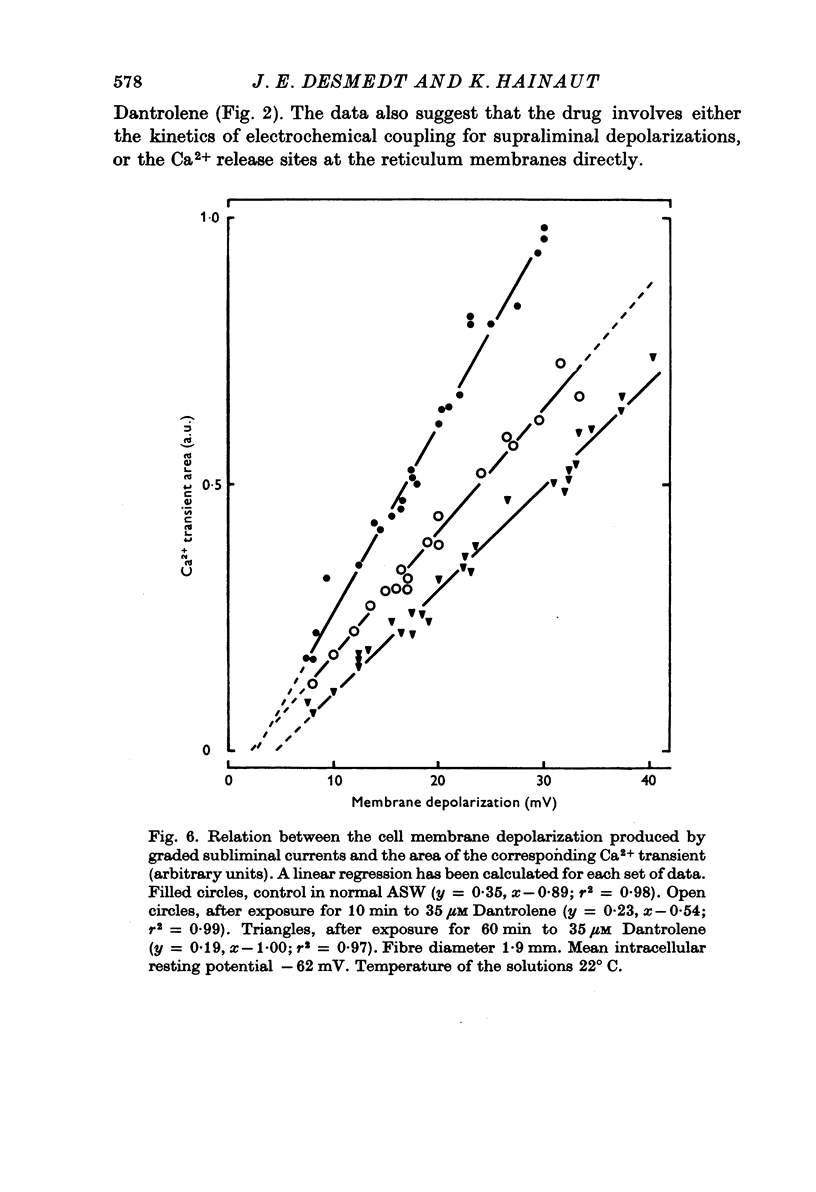
1. Ca movements in resting and in activated single giant muscle fibres of the barnacle were analysed before and after exposure to Dantrolene Na, a synthetic hydantoin derivative. 2. In fibres micro-injected with the photoprotein aequorin, the resting rate of light emission (resting glow) reversibly decreased upon exposure to Dantrolene. Similar results were obtained if the fibre had first been equilibrated in a O Ca-1 mM-EGTA medium. 3. The influx of 45Ca into resting muscle fibres was not modified by 35 micronM Dantrolene which also failed to significantly reduce the influx of 45Ca into muscle fibres which had been depolarized by exposure to external solutions in which K+ had been increased to 60 or 200 mM. 4. In fibres micro-injected with 45Ca, the calcium efflux was reversibly decreased by Dantrolene. This effect was still observed in O Ca medium and in O Ca-ONa medium. A possible effect of Dantrolene on the Na-Ca exchange process at the outer membrane was excluded by showing that when the direction of the Ca2+ movement was inverted in aequorin-loaded fibres by the sudden removal of Na+ from the external medium, a marked increase in the resting glow was recorded which was not affected by exposure to Dantrolene. 5. It is argued that the reduction of Ca2+ efflux by Dantrolene does not result from any direct inhibitory effect on the metabolically driven Ca pump at the outer membrane, but that it is rather related to the reduction of the concentration of myoplasmic Ca2+ which is indeed demonstrated by the reduced resting glow. This in turn is thought to result from a shift in the balance between Ca2+ movements into and out of the intracellular storage sites, and namely the sarcoplasmic reticulum (SR). 6. The Ca2+ transient in aequorin-loaded fibres and the force of the isometric contraction elicited by imposed membrane depolarizations were markedly reduced by Dantrolene. The electrochemical threshold for eliciting intracellular Ca2+ release was not significantly modified. The linear relation between membrane depolarization and Ca2+ transient became less steep. The process of sequestration of myoplasmic Ca2+ back into SR was not significantly affected by Dantrolene which appeared to inhibit rather selectively the Ca2+ release from SR into the cytosol.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C. An estimate of calcium concentration changes during the contraction of single muscle fibres. J Physiol. 1970 Sep;210(2):133P–134P. [PubMed] [Google Scholar]
- Ashley C. C., Caldwell P. C., Lowe A. G. The efflux of calcium from single crab and barnacle muscle fibres. J Physiol. 1972 Jun;223(3):735–755. doi: 10.1113/jphysiol.1972.sp009872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley C. C., Ellory J. C., Hainaut K. Calcium movements in single crustacean muscle fibres. J Physiol. 1974 Oct;242(1):255–272. doi: 10.1113/jphysiol.1974.sp010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley C. C., Ridgway E. B. On the relationships between membrane potential, calcium transient and tension in single barnacle muscle fibres. J Physiol. 1970 Jul;209(1):105–130. doi: 10.1113/jphysiol.1970.sp009158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Horowicz P. Fluorescence intensity changes associated with contractile activation in frog muscle stained with Nile Blue A. J Physiol. 1975 Apr;246(3):709–735. doi: 10.1113/jphysiol.1975.sp010912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P. The interrelationship between sodium and calcium fluxes across cell membranes. Rev Physiol Biochem Pharmacol. 1974;70:33–82. doi: 10.1007/BFb0034293. [DOI] [PubMed] [Google Scholar]
- Brocklehurst L. Letter: Dantrolene sodium and "skinned" muscle fibres. Nature. 1975 Mar 27;254(5498):364–364. doi: 10.1038/254364a0. [DOI] [PubMed] [Google Scholar]
- Desmedt J. E., Hainaut K. The effect of A23187 ionophore on calcium movements and contraction processes in single barnacle muscle fibres. J Physiol. 1976 May;257(1):87–107. doi: 10.1113/jphysiol.1976.sp011357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Ebashi S. Excitation-contraction coupling. Annu Rev Physiol. 1976;38:293–313. doi: 10.1146/annurev.ph.38.030176.001453. [DOI] [PubMed] [Google Scholar]
- Ellis K. O., Bryant S. H. Excitation-contraction uncoupling in skeletal muscle by dantrolene sodium. Naunyn Schmiedebergs Arch Pharmacol. 1972;274(1):107–109. doi: 10.1007/BF00501011. [DOI] [PubMed] [Google Scholar]
- Ellis K. O., Carpenter J. F. Studies on the mechanism of action of dantrolene sodium. A skeletal muscle relaxant. Naunyn Schmiedebergs Arch Pharmacol. 1972;275(1):83–94. doi: 10.1007/BF00505069. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. The electrical properties of crustacean muscle fibres. J Physiol. 1953 Apr 28;120(1-2):171–204. doi: 10.1113/jphysiol.1953.sp004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C. Membrane particles and transmission at the triad. Fed Proc. 1975 Apr;34(5):1382–1389. [PubMed] [Google Scholar]
- HAGIWARA S., NAKA K. I. THE INITIATION OF SPIKE POTENTIAL IN BARNACLE MUSCLE FIBERS UNDER LOW INTRACELLULAR CA++. J Gen Physiol. 1964 Sep;48:141–162. doi: 10.1085/jgp.48.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYLE G., SMYTH T., Jr NEUROMUSCULAR PHYSIOLOGY OF GIANT MUSCLE FIBERS OF A BARNACLE, BALANUS NUBILUS DARWIN. Comp Biochem Physiol. 1963 Dec;10:291–314. doi: 10.1016/0010-406x(63)90229-9. [DOI] [PubMed] [Google Scholar]
- Hainaut K., Desmedt J. E. Calcium ionophore A23187 potentiates twitch and intracellular calcium release in single muscle fibres. Nature. 1974 Nov 29;252(5482):407–408. doi: 10.1038/252407a0. [DOI] [PubMed] [Google Scholar]
- Hainaut K., Desmedt J. E. Effect of dantrolene sodium on calcium movements in single muscle fibres. Nature. 1974 Dec 20;252(5485):728–730. doi: 10.1038/252728a0. [DOI] [PubMed] [Google Scholar]
- Hoyle G., McNeill P. A., Selverston A. I. Ultrastructure of barnacle giant muscle fibers. J Cell Biol. 1973 Jan;56(1):74–91. doi: 10.1083/jcb.56.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Biancri C. P. Site of action of dantrolene in frog sartorius muscle. J Pharmacol Exp Ther. 1974 Apr;189(1):202–212. [PubMed] [Google Scholar]
- Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970 Oct 23;170(3956):404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- Russell J. M., Blaustein M. P. Calcium efflux from barnacle muscle fibers. Dependence on external cations. J Gen Physiol. 1974 Feb;63(2):144–167. doi: 10.1085/jgp.63.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMOMURA O., JOHNSON F. H., SAIGA Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol. 1962 Jun;59:223–239. doi: 10.1002/jcp.1030590302. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Weber A., Murray J. M. Molecular control mechanisms in muscle contraction. Physiol Rev. 1973 Jul;53(3):612–673. doi: 10.1152/physrev.1973.53.3.612. [DOI] [PubMed] [Google Scholar]


