Abstract
Toxin A variant strains (toxin A-negative, toxin B-positive strains) of Clostridium difficile have been reported to be responsible for diarrhea or pseudomembranous colitis in humans. These strains lack parts of the repeating sequences of the toxin A gene (tcdA) and are toxin A negative by commercial enzyme immunoassays (EIA). Here, we report the prevalence of the toxin A variant strains in 334 patients with C. difficile-associated diarrhea in France. The repeating segment of the tcdA gene (1,200 bp) was amplified by PCR using the primers NK9 and NK11 (H. Kato et al., J. Clin. Microbiol. 36:2178-2182, 1998). In the case of amplified fragments of unexpected size, the entire tcdA gene was studied by PCRs A1, A2, and A3 (Rupnik et al., J. Clin. Microbiol. 36:2240-2247, 1998), and strains were characterized by serotyping, pulsed-field gel electrophoresis and PCR ribotyping. By PCR with primers NK9 and NK11, C. difficile variant strains were detected in 2.7% of patients. Several variant types were found. A deletion of approximately 1,700 bp was observed in six strains from five patients. These strains belonged to serotype F and were characterized by the same pulsotype and the same PCR ribotype. They were toxin A negative by EIA and exhibited an atypical cytopathic effect on MRC-5 cells. Two other tcdA variant types that exhibited a positive result for toxin A by EIA were identified: one from serotype H with a longer amplified fragment (insertion of 200 bp) and one with a deletion of 600 bp. Diagnosis of C. difficile-associated diseases would have been missed in five patients (1.5%) by laboratories that screen the stools only for the presence of toxin A. This result underlines the need for testing stool by the cytotoxicity assay in patients with a high suspicion of C. difficile-associated diarrhea but a negative immunoassay for toxin A.
Clostridium difficile is responsible for 10 to 25% of antibiotic-associated diarrhea (AAD) and for virtually all cases of pseudomembranous colitis (PMC) (4, 24). C. difficile is the most common cause of nosocomial diarrhea. Toxigenic strains usually produce two toxins, A (enterotoxin) and B (cytotoxin), which are involved in the pathogenicity of this organism (25, 35). These toxins are encoded by two separate genes, tcdA and tcdB, which with three additional genes (tcdC, tcdD, and tcdE) form a locus of pathogenicity of 19.6 kb (8, 11).
Recently, toxin A variant strains have been reported which fail to produce detectable toxin A by enzyme immunoassay (EIA) yet produce toxin B (1, 7, 9, 19, 20, 22, 23, 32). Two types of toxin A-negative strains are well characterized: strain 1470 (reference strain of serogroup F from the classification of Delmée et al. [14]) and strain 8864, described by Borriello et al. and Lyerly et al. (7, 23). Both are truncated in the repetitive 3′-end domain, with 8864 having a 6.0-kb deletion (33) and strains from serotype F having a 1.7-kb deletion (29). This domain encodes the carboxy repetitive oligopeptide region that contains both the binding site to cell receptor (21) and the epitope recognized by the monoclonal antibody PGC-4 used in most of the EIAs (17). The clinical significance of these toxin A-negative strains still remains unclear. In the hamster model of C. difficile disease, strain 8864 was pathogenic (7) but strains from serotype F (including strain 1470) were reported to be nonpathogenic and have been mainly isolated from asymptomatic children (12). However, several authors have recently reported cases of PMC or diarrhea associated with toxin A-negative strains (1, 20, 22, 32). Prevalence of these variants seems to vary from one country to another. A recent Japanese study reported a prevalence of toxin-A negative strains of 12% in asymptomatic adults (20), whereas these strains accounted for only 3% of the total number of hospital strains sent for typing to the Anaerobe Reference Unit in Cardiff, Wales (9). Beside toxin A-negative strains, other tcdA variant types have been described by Rupnik et al. (29, 30). These variant types are characterized by mutations or deletions in the tcdA gene and have been assigned to 10 different toxinotypes (I to X). All these toxinotypes except toxinotype VIII (including strains from serotype F) and toxinotype X (including strain 8864) react in a toxin A-specific EIA.
The aims of this retrospective study were to determine the prevalence in France of toxin A variant strains that exhibit genetic deletion in the 3′ repeating sequences of tcdA gene in patients with C. difficile-associated diarrhea and to determine whether toxin A of these variant strains can be detected by EIA.
(This work has previously been presented in part [F. Barbut, V. Lalande, B. Burghoffer, N. Hidri, N. Vu Thien, E. Grimprel, and J.-C. Petit, poster P1477 from the 11th European Congress of Clinical Microbiology and Infectious Diseases, Istanbul, Turkey, 1 to 4 April 2001].)
MATERIALS AND METHODS
Strains and patients.
Three hundred sixty-four C. difficile strains were studied: 321 were isolated from 291 adults (136 in 1998 and 155 in 1999) presenting with AAD or antibiotic-associated colitis, and 43 were isolated from 43 children with diarrhea. Strains from adults were recovered from patients hospitalized in 25 different care facilities in Paris, France, or its surroundings. Strains from children came from the same hospital in Paris. All these strains were positive by the cytotoxicity assay.
In all experiments, C. difficile 8864 (Swedish Type Culture Collection no. 20309), ATCC 43596 (serogroup C), and ATCC 43598 (serotype F) served as reference strains.
Strains were isolated from stools on a selective medium (TCCA [brain heart infusion agar supplemented with 5% defibrinated horse blood, 0.1% sodium taurocholate, cefoxitin at 10 μg ml−1, and cycloserine at 250 μg ml−1]) that was incubated in an anaerobic atmosphere for 48 h. The colonies were identified by their enzymatic profile on a Rapid 32A gallery (bioMérieux, Marcy l'Etoile, France). PCR with primers PG48 and PG49 derived from a C. difficile 16S rRNA gene was used to confirm identification, as previously described (18).
Toxin assays.
C. difficile strains were cultured anaerobically in brain heart infusion broth for 5 to 7 days. Toxin B was detected by the cytotoxicity assay using human embryonic fibroblast (MRC-5) cell culture as previously described (2). The specificity of the cytopathic effect of toxin B was confirmed by its seroneutralization by an anti-Clostridium sordellii serum (prepared by M. R. Popoff at the Unité des Toxines, Institut Pasteur, Paris, France).
Detection of toxin A was performed using the Triage C. difficile panel (Biosite Diagnostic, San Diego, Calif.) (3). This test is a rapid EIA using a membrane onto which anti-toxin A antibodies are fixed. Briefly, broths were centrifuged for 5 min at 1,500 × g, and the supernatant was tested as a stool specimen according to the manufacturer's recommendations. A blue band appearing at the level of the toxin A zone was considered to be a positive result.
PCR assays.
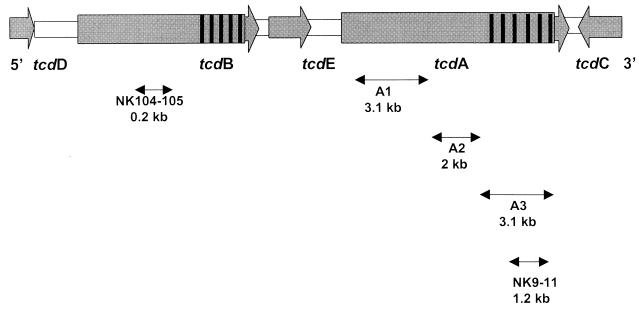
Primer sequences used to amplify regions of the toxin genes are shown in Table 1, and their locations on the pathogenicity locus are represented in Fig. 1.
TABLE 1.
Primers used for PCRs in this study
| Primer | Sequence | Fragment length (kb)a |
|---|---|---|
| NK9 | 5′-CCACCAGCTGCAGCCATA-3′ | 1.2 |
| NK11 | 5′-TGATGCTAATAATGAATCTAAAATGGTAAC-3′ | |
| NK104 | 5′-GTGTAGCAATGAAAGTCCAAGTTTACGC-3′ | 0.2 |
| NK105 | 5′-CACTTAGCTCTTTGATTGCTGCACCT-3′ | |
| A1C | 5′-GGAGGTTTTTATGCTTTAATATCTAAAGA-3′ | 3.1 |
| A2N | 5′-CCCTCTGTTATTGTAGGTAGTACATTTA-3′ | |
| A2C | 5′-TAAATGTACTACCTACAATAACAGAGGG-3′ | 2 |
| A3N | 5′-CTTGTATATAAATCAGGTGCTATCAATA-3′ | |
| A3C | 5′-TATTGATAGCACCTGATTTATATACAAG-3′ | 3.1 |
| A4N | 5′-TTATCAAACATATATTTTAGCCATATATC-3′ | |
| 16S | 5′-GTGCGGCTGGATCACCTCCT-3′ | |
| 23S | 5′-CCCTGCACCCTTAATAACTTGACC-3′ |
For the toxin gene of C. difficile strain VPI 10463.
FIG. 1.
Representation of the pathogenicity locus of C. difficile and the location of primers used for PCRs A1, A2, and A3 and PCRs with primers NK9-NK11 and NK104-NK105 (hatched areas represent repetitive domains of both toxin genes).
Strains were screened for variations in the 1.2-kb fragment spanning the 3′ repetitive domain of tcdA gene using PCR assay with NK9 and NK11 primers (20). DNA was extracted from three large C. difficile colonies by use of a Chelex resin-based commercial kit (InstaGene Matrix; Bio-Rad, Ivry, France) as recommended by the manufacturer. The PCR mix (total reaction volume, 100 μl) consisted of 1.5 mM MgCl2, 50 mM KCl, 10 mM Tris-HCl (pH 8.8), 200 μM deoxynucleoside triphosphate, 65 ng of each primer, 2.5 U of Taq polymerase (Pharmacia Biotech, Orsay, France), and 10 μl of DNA extract. PCR amplification was performed with 40 cycles, consisting of denaturation at 95°C for 15 s, annealing at 62°C for 120 s, and extension at 72°C for 40 s. Amplified products were fractionated by electrophoresis through 1.5% standard agarose (Eurobio, Les Ulis, France).
In the case of the amplified fragment of unexpected size obtained by PCR with primers NK9-NK11, the entire tcdA gene was amplified using three different overlapping PCRs (A1, A2, and A3) as described by Rupnik et al. (29, 30). PCRs A1, A2, and A3 were performed in a total volume of 50 μl containing 300 ng of DNA, 15 pmol of each paired primer, a 200 μM concentration of each deoxynucleoside triphosphate, and 2.5 U of Taq polymerase. Two-step PCR programs were as follows: initial denaturation at 93°C for 3 min and annealing and extension for 8 min at 56°C (fragments A1 and A2) or at 47°C (fragment A3), followed by a denaturation at 93°C for 4 s. Amplified fragments were visualized on 1.5% agarose.
The 3′ nonrepetitive portion of the toxin B gene was detected by PCR using primers NK104 and NK105 (270-bp fragment) (20). The amplification reactions were performed in a 100-μl volume containing 10 mM Tris-HCl (pH 8.8), 50 mM KCl, 1.5 mM MgCl2, a 200 μM concentration of each deoxynucleoside triphosphate, 50 pmol of each primer, 2.5 U of Taq polymerase, and 10 μl of DNA extract. The amplifications were performed with 1 cycle of 5 min at 94°C for denaturation followed by 40 cycles (20 s at 94°C, 60 s at 62°C, and 40 s at 74°C) and a final extension of 5 min at 74°C. The amplification products were visualized on 1.5% gel agarose and analyzed with a UV table after ethidium bromide staining.
Serotyping of C. difficile strains.
Serotyping was performed by enzyme-linked immunosorbent assay according to the method described by Delmée et al. (13) using the antisera A1, A5, A8, A9, A10, C, D, F, G, H, and K.
Genotyping of C. difficile strains.
C. difficile strains were analyzed by PCR ribotyping and pulsed-field gel electrophoresis (PFGE) as previously described by Bidet et al. (5).
The primer sequences for PCR ribotyping were 5′-GTG CGG CTG GAT CAC CTC CT-3′ (16S primer) and 5′-CCC TGC ACC CTT AAT AAC TTG ACC-3′ (23S primer) (Table 1). Amplification reactions were performed in a 100-μl volume containing 10 mM Tris-HCl (pH 8.8), 50 mM KCl, 1.5 mM MgCl2, a 200 μM concentration of each deoxynucleoside triphosphate, 50 pmol of each primer, 2.5 U of Taq polymerase, and 10 μl of DNA extract. Amplifications were carried out in the thermal cycler for 1 cycle of 6 min at 94°C for denaturation, followed by 35 cycles (1 min at 94°C, 1 min at 57°C, and 1 min at 72°C) and a final extension of 7 min at 72°C. Amplification products were fractionated by electrophoresis through 3% Resophor agarose (Eurobio) and analyzed on UV table after ethidium bromide staining. Comparison of PCR ribotyping patterns was performed visually. Strains with patterns differing at least by one band were assigned to different types.
For PFGE analysis, strains were cultured in prereduced Schaedler broths for 17 h at 37°C and pelleted by centrifugation at 5,000 × g. Bacterial cells were embedded in agarose plugs and lysed for 18 h at 37°C with lysozyme-lysostaphin and overnight at 50°C with proteinase K using a GenePath Group 1 kit (Bio-Rad) according to the manufacturer's recommendations. Plugs were digested in a volume of 300 μl by 25 U of SmaI endonuclease overnight at 25°C. Electrophoresis was performed in a contour-clamped homogeneous electric field device (CHEF DRII; Bio-Rad) using the gel kit (Bio-Rad) with program no. 14 (50 to 500 V, 19 h 7 min). Gels were then stained with ethidium bromide and analyzed on a UV table. PFGE patterns were interpreted according to the criteria proposed by Tenover et al. (36)
RESULTS
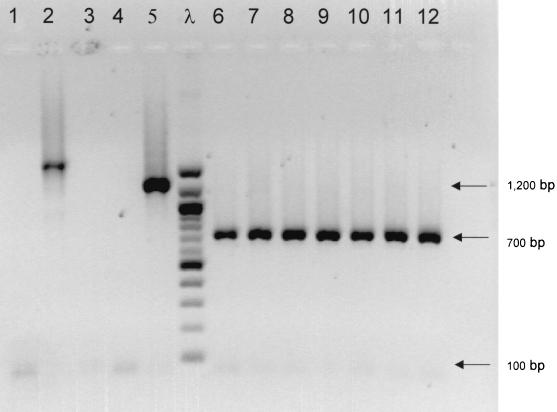
We used the PCR primer set NK9-NK11 as a screening method to detect tcdA variant strains of C. difficile. Among the 364 strains studied (isolated from 291 adults and 43 children), 9 (2.5%) strains isolated from seven patients (2.1%) exhibited an amplified fragment different from the expected 1.2 kb obtained with toxin A-positive, toxin B-positive strain ATCC 43596 (Table 2; Fig. 2). These nine strains tested positive by the cytotoxicity assay, and the nonrepetitive domain of the tcdB gene was detected in all the strains by PCR (NK104-NK105). The entire tcdA genes of these strains were studied using PCR A1, A2, A3.
TABLE 2.
Analysis of tcdA gene from C. difficile variant strains
| Straina | Age (yr) | Clinical data | Amplicon sizeb by:
|
Triage toxin A | CPEc | Sero- type | PCR ribotype | Pulso- type | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PCR A1 | PCR A2 | PCR A3 | PCR with primers NK9-NK11 | ||||||||
| Serotype C (ATCC 43596) | Reference | 3.1 | 2 | 3.1 | 1.2 | + | Typical | C | 3 | II | |
| Serotype F (ATCC 43598) | Reference | 3.1 | 2 | 1.3 | 700 bp | − | Atypical | F | 1 | I | |
| 8864 | Reference | No amplicon | No amplicon | No amplicon | No amplicon | − | Typical | NTd | 6 | III | |
| 60 | Child | AAD | 3.1 | 2 | 1.3 | 700 bp | − | Atypical | F | 1 | I |
| 98-4318 | 94 | AAD | 3.1 | 2 | 1.3 | 700 bp | − | Atypical | F | 1 | I |
| 98-7426 | 94 | AAD | 3.1 | 2 | 1.3 | 700 bp | − | Atypical | F | 1 | I |
| 98-16948 | 60 | PMC | 3.1 | 2 | 1.3 | 700 bp | − | Atypical | F | 1 | I |
| 98-15632 | 87 | AAD | 3.1 | 2 | 1.3 | 700 bp | − | Atypical | F | 1 | I |
| 98-11522 | 64 | PMC | 3.1 | 2 | 2.5 | No amplicon | + | Typical | NT | 2 | IV |
| 98-15845 | 64 | PMC | 3.1 | 2 | 2.5 | No amplicon | + | Typical | NT | 2 | IV |
| 98-15323 | 66 | AAD | 3.1 | 2 | 3.3 | 1.4 | + | Typical | H | 4 | V |
| 99-3050 | 56 | AAD | 3.1 | 2 | 1.3 | 700 bp | − | Atypical | F | 1 | I |
Strains 98-4318 and 98-7426 were isolated from the same patient; strains 98-11522 and 98-15845 were also isolated from the same patient.
In kilobases unless otherwise noted.
CPE, cytopathic effect on MRC-5 cells.
NT, nontypeable.
FIG. 2.
PCR amplification of C. difficile variant strains using tcdA primers NK9-NK11. Lanes: 1, strain 98-11522; 2, strain 98-15523; 3, strain 98-15845; 4, negative control; 5, ATCC 43596 (serotype C); 6, ATCC 43598 (serotype F); 7, strain 98-15632; 8, strain 98-16948; 9, strain 98-3050; 10, strain 98-4318; 11, strain 98-7426; 12, strain 60; λ, 100-bp ladder (Biolabs, Saint Quentin en Yvelines, France).
A deletion of 1.7 kb located in the repeating domain of the tcdA gene was observed by PCR A3 in six strains (strains 60, 98-24318, 98-7426, 98-16948, 98-15632, and 99-3050) isolated from five patients. Among these five patients, one presented with an endoscopically proven PMC. All these strains belonged to serotype F and were characterized by the same pulsotype and PCR ribotype. All these strains were negative for toxin A by EIA. They exhibited an atypical cytopathic effect on MRC-5 cells compared to regular strains, with clusters of cells and no spindle formation.
A deletion of 600 bp was observed by PCR A3 in the repeating domain of the tcdA gene in two nontypeable strains (strains 98-11522 and 98-15845). These strains were isolated from the same patient who presented with two episodes of pseudomembranous colitis over a period of 2 months. Despite this deletion, toxin A could be detected by EIA, but the positive signal (blue band) was weak even after repeating the test.
An insertion of approximately 200 bp in the repetitive domain of the tcdA gene was observed in one strain (strain 98-15323) belonging to serotype H (3.3 kb by PCR A3). This strain was positive for toxin A by EIA.
These two tcdA variant types exhibited different banding patterns in PFGE and PCR ribotyping that were different from the patterns of serotype F strains.
DISCUSSION
Using PCR with primers NK9-NK11 derived from the repeating sequences of the toxin A gene, we successfully discriminated nine tcdA variant strains. These strains were isolated in 2.7% of adults with C. difficile-associated diarrhea and in 2.3% of children with diarrhea. Among these nine variant strains, toxin A was not detected by EIA except for three strains (two with the 600-bp deletion and one with the 200-bp insertion). Diagnosis of C. difficile diarrhea would have been missed in five patients (1.5%) by laboratories that screen the stools only for the presence of toxin A. This prevalence is lower than that of 3% reported by Brazier et al. in Wales (9), using an immunoenzymatic test and stool cytotoxicity assay as screening methods. The prevalence of toxin A-negative, toxin B-positive strains in Europe seems to be much lower than that found in Japan (6.7 to 12% in children and 12.5% in asymptomatic adults) but higher than the prevalence of 0.2% found during a multicenter study in the United States (20, 26). This discrepancy may be explained by differences in the studied populations.
Most of the toxin A-negative variant strains (six of nine) belong to serogroup F and were characterized by a deletion of 1.7 kb by PCR A3. This large deleted region encodes the repeating units that contain the epitope for the monoclonal antibody used in commercial EIAs (16, 17). All these strains (including reference strain ATCC 43598 from serotype F) exhibited the same banding pattern by PCR ribotyping and PFGE. However, these strains were isolated from patients hospitalized in different care facilities and were epidemiologically unrelated. We can hypothesize that these strains are derived from a common ancestor that spread in Paris and the surroundings. In a previous study performed in Japan and Indonesia, Kato et al. (20) found only 19 different PFGE types among the 48 toxin A-negative, toxin B-positive strains, including 44 strains from serotype F and 4 strains from serotype X. More recently, Moncrief et al. showed that a number of serotype F strains around the world were identical by PCR analysis with a series of primers designed to analyze various regions of both toxin genes (28). All these results support the hypothesis of a low genetic diversity of serotype F strains and the possible international spread of this clone. Strains from serotype F reported in our study are very similar to those described by Kato et al. (20) and probably correspond to toxinotype VIII according to the toxinotyping scheme established by Rupnik et al. (29). As previously reported, these strains exhibit a very atypical effect on MRC-5 cells, distinctive from the one produced by regular strains (ATCC 43596), and characterized by a rounding of the cell with no spindle formation and a trend towards a discrete cluster of cells. This atypical effect has been shown to be comparable to the effect of the lethal toxin of Clostridium sordellii (10). It has been hypothesized that toxin B from serotype F strains could be a functional hybrid of toxin B of C. difficile and lethal toxin of C. sordellii. Although these strains were reported to be nonpathogenic in the rabbit ileal loop test and unable to alter tight junctions by using in vitro tissue culture testing, they have been recently incriminated in a nosocomial outbreak of C. difficile-associated diarrhea in Canada (1) and in two well-described cases of PMC (22, 32). The review of clinical data of serotype F cases reported in our study suggests that these strains were truly responsible for PMC in one patient and AAD in four patients. We can hypothesize that toxin B could act as the sole pathogenic factor as has already been postulated from studies performed with strain 8864 (23). Pathogenesis could be also explained by additional virulence factors such as the production of an actin-specific ADP ribosyltransferase (binary toxin) or hydrolytic enzymes (6, 34). In all patients, C. difficile was the only enteropathogen found in stool culture, and diarrhea of these patients resolved after antibiotic treatment with metronidazole or vancomycin. These findings emphasize the clinical potential of these strains and underline the need of using the cytotoxicity assay to detect them. EIAs that simultaneously detect toxins A and B could be considered as an alternative to the cytotoxicity assay for laboratories lacking cell culture facilities. However, even if the sensitivities of EIAs detecting both toxins A and B has been shown to be greater than the sensitivity of EIA detecting toxin A only, their sensitivities compared to that of the cytotoxicity assay range from 79 to 89% (2, 15, 27).
We also found two other variant types: one exhibited a deletion of 600 bp by PCR A3. This variant could be very close to toxinotype VII described by Rupnik et al. (29). The other tcdA variant exhibited an insertion of approximately 200 bp by PCR A3. Nevertheless, all these genetic modifications did not affect detection of toxin A by EIA. All these strains are currently under investigation to better characterize their genetic background. This is the second time, to our knowledge, that an insertion has been described in the C. difficile tcdA gene. The first case was reported by Rupnik et al. in 2001 with the description of a novel toxinotype (XIV) characterized by a large insertion at the beginning of the tcdA gene (31).
In conclusion, diagnosis of C. difficile-associated diseases would have been missed in five patients (1.5%) by laboratories that screen the stools only for the presence of toxin A. This result underlines the need for testing stool by the cytotoxicity assay in patients with a high suspicion of C. difficile-associated diarrhea but a negative immunoassay for toxin A.
Acknowledgments
This study was supported by grants from the Unité Propre de Recherche de l'Enseignement Supérieur (UPRES) EA 2392.
We are deeply indebted to F. Espinasse, P. Baune, and L. Raskine for reviewing clinical data of patients with C. difficile-associated diarrhea and to M. Rupnik for helpful discussion about toxinotypes.
REFERENCES
- 1.Alfa, M. J., Kabani, A., D. Lyerly, S. Moncrief, L. M. Neville, A. Al-Barrak, G. K. H. Harding, B. Dyck, K. Olekson, and J. M. Embil. 2000. Characterization of a toxin A-negative, toxin B-positive strain of Clostridium difficile responsible for a nosocomial outbreak of Clostridium difficile-associated diarrhea. J. Clin. Microbiol. 38:2706-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbut, F., C. Kajzer, N. Planas, and J.-C. Petit. 1993. Comparison of three enzyme immunoassays, a cytotoxicity assay, and toxigenic culture for diagnosis of Clostridium difficile associated diarrhea. J. Clin. Microbiol. 31:963-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbut, F., V. Lalande, G. Daprey, P. Cohen, N. Marle, B. Burghoffer, and J. C. Petit. 2000. Usefulness of simultaneous detection of toxin A and glutamate dehydrogenase for the diagnosis of Clostridium difficile-associated diarrhea. Eur. J. Clin. Microbiol. Infect. Dis. 19:481-484. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett, J. G., N. Moon, T. W. Chang, N. Taylor, and A. B. Onderdonk. 1978. Role of Clostridium difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology 75:778-782. [PubMed] [Google Scholar]
- 5.Bidet, P., F. Barbut, V. Lalande, B. Burghoffer, and J. C. Petit. 1999. Development of a new PCR-ribotyping method for Clostridium difficile. FEMS Microbiol. Lett. 175:261-266. [DOI] [PubMed] [Google Scholar]
- 6.Borriello, S. P. 1998. Pathogenesis of Clostridium difficile infection. J. Antimicrob. Chemother. 41(Suppl. C):13-19. [DOI] [PubMed] [Google Scholar]
- 7.Borriello, S. P., B. W. Wren, S. Hyde, S. V. Seddon, P. Sibbons, M. M. Krishna, S. Tabaqchali, S. Manek, and A. B. Price. 1992. Molecular, immunological, and biological characterization of a toxin A-negative, toxin B-positive strain of Clostridium difficile. Infect. Immun. 60:4192-4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun, V., T. Hundsberger, P. Leukel, M. Sauerborn, and C. von Eichel-Streiber. 1996. Definition of the single integration site of the pathogenicity locus in Clostridium difficile. Gene 181:29-38. [DOI] [PubMed] [Google Scholar]
- 9.Brazier, J. S., S. L. Stubbs, and B. I. Duerden. 1999. Prevalence of toxin A negative/B positive Clostridium difficile strains. J. Hosp. Infect. 42:248-249. [PubMed] [Google Scholar]
- 10.Chaves-Olarte, E., P. Low, E. Freer, T. Norlin, M. Weidmann, C. von Eichel-Streiber, and M. Thelestam. 1999. A novel cytotoxin from Clostridium difficile serogroup F is a functional hybrid between two other large clostridial toxins. J. Biol. Chem. 274:11046-11052. [DOI] [PubMed] [Google Scholar]
- 11.Cohen, S. H., Y. J. Tang, and J. Silva. 2000. Analysis of the pathogenicity locus in Clostridium difficile strains. J. Infect. Dis. 181:959-963. [DOI] [PubMed] [Google Scholar]
- 12.Delmée, M., and V. Avésani. 1990. Virulence of ten serogroups of Clostridium difficile. J. Med. Microbiol. 33:85-90. [DOI] [PubMed] [Google Scholar]
- 13.Delmée, M., C. Depitre, G. Corthier, A. Ahoyo, and V. Avésani. 1993. Use of an enzyme-linked immunoassay for Clostridium difficile serogrouping. J. Clin. Microbiol. 31:2526-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delmée, M., M. Homel, and G. Wauters. 1985. Serogrouping of Clostridium difficile strains by slide agglutination. J. Clin. Microbiol. 21:232-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doern, G. V., R. T. Coughlin, and L. Wu. 1992. Laboratory diagnosis of Clostridium difficile-associated gastrointestinal disease: comparison of a monoclonal antibody enzyme immunoassay for toxins A and B with a monoclonal antibody enzyme immunoassay for toxin A only and two cytotoxicity assays. J. Clin. Microbiol. 30:2042-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dove, C. H., S. Z. Wang, S. B. Price, C. J. Phelps, and D. M. Lyerly. 1990. Molecular characterization of the Clostridium difficile toxin A gene. Infect. Immun. 58:480-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frey, S. M., and T. D. Wilkins. 1992. Localization of two epitopes recognized by monoclonal antibody PCG-4 on Clostridium difficile toxin A. Infect. Immun. 60:2488-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gumerlock, P. H., Y. J. Tang, F. J. Meyers, and J. Silva, Jr. 1991. Use of the polymerase chain reaction for the specific and direct detection of Clostridium difficile in human feces. Rev. Infect. Dis. 13:1053-1060. [DOI] [PubMed] [Google Scholar]
- 19.Kato, H., N. Kato, S. Katow, T. Maegawa, S. Nakamura, and D. M. Lyerly. 1999. Deletions in the repeating sequences of the toxin A gene of toxin A-negative, toxin B-positive Clostridium difficile strains. FEMS Microbiol. Lett. 175:197-203. [DOI] [PubMed] [Google Scholar]
- 20.Kato, H., N. Kato, K. Watanabe, N. Iwai, H. Nakamura, T. Yamamoto, K. Suzuki, S. M. Kim, Y. Chong, and E. B. Wasito. 1998. Identification of toxin A-negative, toxin B-positive Clostridium difficile by PCR. J. Clin. Microbiol. 36:2178-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krivan, H. C., G. F. Clark, D. F. Smith, and T. D. Wilkins. 1986. Cell surface binding site for Clostridium difficile enterotoxin: evidence for a glycoconjugate containing the sequence Galα1-3Galβ1-4GlcNAc. Infect. Immun. 53:573-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Limaye, A. P., D. K. Turgeon, B. T. Cookson, and T. R. Fritsche. 2000. Pseudomembranous colitis caused by a toxin A− B+ strain of Clostridium difficile. J. Clin. Microbiol. 38:1696-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyerly, D. M., L. A. Barroso, T. D. Wilkins, C. Depitre, and G. Corthier. 1992. Characterization of a toxin A-negative, toxin B-positive strain of Clostridium difficile. Infect. Immun. 60:4633-4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyerly, D. M., H. C. Krivan, and T. D. Wilkins. 1988. Clostridium difficile: its disease and toxins. Clin. Microbiol. Rev. 1:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyerly, D. M., D. E. Lockwood, S. H. Richardson, and T. D. Wilkins. 1982. Biological activities of toxins A and B of Clostridium difficile. Infect. Immun. 35:1147-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyerly, D. M., L. M. Neville, D. T. Evans, J. Fill, and S. Allen. 1998. Multicenter evaluation of the Clostridium difficile TOX A/B TEST. J. Clin. Microbiol. 36:184-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merz, C. S., C. Kramer, M. Forman, L. Gluck, K. Mills, K. Senft, I. Steiman, N. Wallace, and P. Charache. 1994. Comparison of four commercially available rapid enzyme immunoassays with cytotoxin assay for detection of Clostridium difficile toxin(s) from stool specimens. J. Clin. Microbiol. 32:1142-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moncrief, J. S., L. Zheng, L. M. Neville, and D. M. Lyerly. 2000. Genetic characterization of toxin-A-negative, toxin-B-positive Clostridium difficile isolates by PCR. J. Clin. Microbiol. 38:3072-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rupnik, M., V. Avésani, M. Janc, C. von Eichel-Streiber, and M. Delmée. 1998. A novel toxinotyping scheme and correlation of toxinotypes with serogroups of Clostridium difficile isolates. J. Clin. Microbiol. 36:2240-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rupnik, M., V. Braun, F. Soehn, M. Janc, M. Hofstetter, R. Laufenberg-Feldmann, and C. von Eichel-Streiber. 1997. Characterization of polymorphisms in the toxin A and B genes of Clostridium difficile. FEMS Microbiol. Lett. 148:197-202. [DOI] [PubMed] [Google Scholar]
- 31.Rupnik, M., J. S. Brazier, B. I. Duerden, M. Grabnar, and S. L. J. Stubbs. 2001. Comparison of toxinotyping and PCR ribotyping of Clostridium difficile strains and description of novel toxinotypes. Microbiology 147:439-447. [DOI] [PubMed] [Google Scholar]
- 32.Sambol, S. P., M. M. Merrigan, D. Lyerly, D. N. Gerding, and S. Johnson. 2000. Toxin A gene analysis of a variant strain of Clostridium difficile that causes human clinical disease. J. Clin. Microbiol. 68:5480-5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soehn, F., A. Wagenknecht-Wiesner, P. Leukel, M. Kohl, M. Weidmann, C. von Eichel-Steiber, and V. Braun. 1998. Genetic rearrangements in the pathogenicity locus of Clostridium difficile strain 8864: implications for transcription, expression and enzyme activity of toxins A and B. Mol. Gen. Genet. 258:222-232. [DOI] [PubMed] [Google Scholar]
- 34.Stubbs, S., M. Rupnik, M. Gibert, J. Brazier, B. Duerden, and M. Popoff. 2000. Production of actin-specific ADP-ribosyltransferase (binary toxin) by strains of Clostridium difficile. FEMS Microbiol. Lett. 186:307-312. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan, N. M., S. Pellett, and T. D. Wilkins. 1982. Purification and characterization of toxins A and B of Clostridium difficile. Infect. Immun. 35:1032-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]