Abstract
As considerable variation in the antimicrobial susceptibility of Haemophilus influenzae has been reported, the effects of various test media on the susceptibility of H. influenzae were studied. MICs were determined by three laboratories for 21 antimicrobial agents against a panel of 100 selected isolates. Testing was performed using a reference NCCLS frozen broth microdilution method with Haemophilus test medium (HTM) broth and dried commercial MIC trays rehydrated with the following media: in-house and commercially prepared HTM broth, Mueller-Hinton broth with 2% lysed horse blood and NAD, IsoSensitest broth with 2% lysed horse blood and NAD, and IsoSensitest broth-based HTM. Overall, all results were very reproducible, with the MIC at which 50% of the isolates tested are inhibited (MIC50), MIC90, and geometric mean MIC being within one doubling dilution by all six methods and at all three testing centers for 15 of the 21 agents tested. Interlaboratory differences were more marked than intralaboratory differences or differences among media. Cefprozil, cefaclor, and trimethoprim-sulfamethoxazole results differed the most, while results for ampicillin, amoxicillin-clavulanic acid, cefdinir, cefixime, ceftriaxone, and clarithromycin were the most reproducible. However, these variations in results caused considerable differences in susceptibility rates for agents for which NCCLS susceptible breakpoints were close to the geometric mean MIC, particularly for cefaclor and cefprozil. This was much less of a problem when pharmacokinetic-pharmacodynamic breakpoints were used. Reproducible susceptibility results were obtained for a wide range of agents against H. influenzae in three laboratories using a variety of media that support the growth of this fastidious species.
Assessment of the susceptibility of Haemophilus influenzae to various antimicrobial agents is relatively easy for agents for which defined resistance mechanisms are known and which result in high-level resistance, such as the activities of ampicillin and amoxicillin against β-lactamase-producing strains compared to those against β-lactamase-negative strains (1, 7, 10, 20). When bimodal MIC populations are found, susceptible and resistant strains can be readily differentiated, particularly if the MICs of susceptible strains are below clinically achievable levels of the agents. However, this is not the case for many agents for which unimodal MIC distributions are found and strains against which the MICs are higher are rare or absent (11, 12, 13, 22). This circumstance is further complicated by the fact that MIC ranges may include values that are close to clinically achievable levels and/or include MIC breakpoint concentrations (1, 4, 15). Examples of such agents include amoxicillin-clavulanate, cefuroxime, azithromycin, erythromycin, and clarithromycin. For the two β-lactam examples, the situation is further complicated by the fact that strains for which the MICs are at the high end of the distribution may have non-β-lactamase-mediated resistance due to altered penicillin-binding proteins or may be spheroplast-producing strains (2, 3, 11, 12, 14, 19, 22, 23, 24).
Considerable variation in the resistance rates of various agents against H. influenzae has been reported (8, 9, 12). For example, the resistance rates for amoxicillin-clavulanate varied from 0% in a large international study of 2,718 strains (9) to 4.5% in a U.S. study of 1,539 strains (8). Similarly, the resistance rates for cefuroxime varied from 3.2 to 6.4%. Although these resistance rates are low and differences between studies are not very large, the susceptible breakpoint for these agents (≤4 μg/ml) is close to the MICs of these agents at which 90% of the isolates tested are inhibited (MIC90s) (1 to 8 μg/ml), and normal population distributions could account for some MICs being in the resistant range (12).
This study examined the effects of various media on the susceptibility of this species to a wide range of antimicrobial agents, using media that would be acceptable in a large number of countries. The results are compared with those of the reference NCCLS method using Haemophilus test medium (HTM) in frozen microdilution trays (16, 17).
MATERIALS AND METHODS
Isolates.
The isolates tested were 100 untypeable H. influenzae isolates from stock cultures which have been used for the validation of MIC plates from several surveillance studies. Of the 100 isolates, 43 were β-lactamase positive and 4 were β-lactamase negative and ampicillin resistant.
Antimicrobial agents.
A total of 21 antimicrobial agents were tested, consisting of 9 β-lactam agents (ampicillin, amoxicillin, amoxicillin-clavulanic acid, ceftriaxone, cefuroxime, cefaclor, cefixime, cefprozil, and cefdinir), 3 macrolide agents (azithromycin, clarithromycin, and erythromycin), 6 fluoroquinolone agents (ciprofloxacin, gemifloxacin, grepafloxacin, levofloxacin, ofloxacin and trovafloxacin), chloramphenicol, doxycycline, and trimethoprim-sulfamethoxazole.
MIC methods.
All strains were tested with frozen trays utilizing the NCCLS broth microdilution method (15) with in-house-prepared HTM (frozen MH HTM [see below]) and with dried commercial MIC trays (Trek Diagnostics, Westlake, Ohio) rehydrated with five different media. Three different testing sites participated in the study. Frozen MH HTM was tested at Case Western Reserve University, Cleveland, Ohio, while the dried commercial trays were tested at all three sites. For the dried commercial trays, all of the sites used the same batch of in-house HTM (MH HTM), which was prepared by the investigators at Case Western Reserve University using Mueller-Hinton broth base (Difco), 0.5% yeast extract (Difco), 15 μg of NAD per ml, and 15 μg of hematin (Sigma) per ml. Case Western Reserve University (site 1) and M. S. Hershey Medical Center, Hershey, Pa. (site 2) also tested the strains with Mueller-Hinton broth (Trek Diagnostics) supplemented with 2% lysed horse blood (Cleveland Scientific, Bath, Ohio) and 15 μg of NAD/ml (MH LHB NAD). In addition, site 1 tested the strains with a commercial formulation of HTM broth (PML, Tualatin, Oreg.) (PML HTM). GR Micro, London, United Kingdom (site 3) also used IsoSensitest Broth (Oxoid Ltd., Basingstoke, United Kingdom) supplemented with 2% lysed horse blood (TCS Microbiology, Botolph Claydon, United Kingdom) and 15 μg of NAD (Sigma-Aldrich UK Ltd., Poole, United Kingdom)/ml (IST LHB NAD) and IsoSensitest broth-based HTM (IsoSensitest broth supplemented with 15 μg of NAD per ml, 0.5% yeast extract [Oxoid Ltd.], and 15 μg of hematin [Oxoid Ltd.] per ml) (IST HTM).
Inoculum checks were performed on all strains, and quality control strains (H. influenzae ATCC 49247 and 49766 and Escherichia coli ATCC 35218) were included in each testing run. The results were accepted if inocula were in the range of 3 × 105 to 7 × 105 CFU/ml and the MICs for the quality control strains were within published limits (17).
Data analysis.
Geometric mean MICs, MIC50s, MIC90s, and standard deviations for each method, based on the doubling-dilution (i.e., log2) values, were determined for antimicrobial agents with unimodal distributions and on-scale endpoints for >90% of the strains. Ampicillin, amoxicillin, and trimethoprim-sulfamethoxazole means were not calculated because of bimodal distributions. Results for all methods were compared to the results for frozen MH HTM, and doubling-dilution differences were calculated. Susceptibility rates were calculated based on both NCCLS (17) and pharmacokinetic-pharmacodynamic (PK-PD) breakpoints (4, 5, 18, 21). The PK-PD breakpoints were based on standard dosing regimens and criteria appropriate to each agent. For β-lactams, erythromycin, and clarithromycin, these breakpoints were based on drug concentrations in serum present for 40 to 50% of the dosing interval, while for azithromycin, fluoroquinolones, and doxycycline, they were based on 24-h area under the concentration-time curve/MIC ratios exceeding 25 (5, 18). For trimethoprim-sulfamethoxazole, the NCCLS breakpoint was used, as the PK-PD breakpoint was not available and the NCCLS breakpoint had been validated in bacteriologic outcome otitis media studies (6).
The data were also analyzed statistically using several approaches, with the frozen MH HTM as the reference method where applicable. First, the data were examined with generalized estimating equations (25) (GEE) (SAS, Cary, N.C.), a method of multiple regression analysis of the entire data set, which was used to assess overall differences between test sites and methods. GEE methodology was chosen because it could handle the lack of independence among the observations caused by the same isolates being analyzed multiple times. In effect, each isolate created a family of related observations. Since there will be more variability in MICs across isolates than across methods for a particular isolate, it is important to use analysis methods that recognize the relationship of the observations. The GEE methodology can be used to simultaneously examine the effects of method and sites, obtaining an estimated regression coefficient for each individual effect variable. Since all the effects were categorical variables, each effect would be measured as a difference from a reference method and site.
Second, sequential paired t tests were performed to compare data sets, using the frozen MH HTM as the reference method. This analysis was repeated using the site 1 MH HTM as the standard. Since the MH HTM was prepared at site 1 and distributed to each of the other testing sites, it provided a standard for measuring both methodological and within-site differences. Consequently, paired-t-test analysis was also used to examine within-laboratory differences using each site's MH HTM results as the reference method. To account for potential type 1 error resulting from the multiple t tests employed, a Bonferroni correction was used, requiring a critical P value of <0.0003 for statistical significance.
Third, the data were examined for overall mean population differences using the t distribution, since the test sample population MICs were normally distributed with similar variances. While this method is not appropriate for testing dependent data, it was thought to provide some useful information, since population mean data are used in microbial surveillance studies and repeated testing of the same sample would be expected to regress to the mean. The laboratory methods examined in this study are those that would likely be used in large-scale surveillance. The critical value used for this testing was that corresponding to a P value of <0.0003.
The robustness of some of the statistical differences found was tested by adjusting the data by ±0.25-, 0.5-, 0.75-, and 1-doubling-dilution differences. Since a one-doubling-dilution difference is considered an acceptable level of sample reproducibility in MIC testing, this analysis was performed to determine whether adjusting the data incrementally within the one-dilution range would effect statistical significance (9).
The percentages of MICs within one and two dilutions of the frozen reference values were calculated. The differences between sites and methods were examined by the binomial test for proportions for both one- and two-dilution differences. The differences in agreement with the frozen reference values were examined directly for each site-method combination, as well as for methods within each site, with the site-specific MH HTM difference from frozen reference values used as the baseline for these comparisons.
RESULTS
The geometric mean MICs were within one doubling dilution by all six methods and at all three testing centers for all antimicrobial agents except azithromycin, cefaclor, cefprozil, gemifloxacin, grepafloxacin, and levofloxacin (Table 1). At least 90% of MICs by all six methods and at all three testing centers were within two doubling dilutions compared with the frozen reference except for amoxicillin with IST HTM at site 3; cefuroxime and ampicillin with IST LHB NAD at site 3; cefaclor and cefprozil with in-house HTM at site 2, MH LHB NAD at site 2, IST HTM at site 3, and IST LHB NAD at site 3; gemifloxacin with MH LHB NAD at sites 1 and 2; and trimethoprim-sulfamethoxazole with in-house HTM at site 3, PML HTM at site 1, MH LHB NAD at site 2, and IST LHB NAD at site 3 (Table 2).
TABLE 1.
Geometric mean MICs and standard deviations (doubling dilutions) for each method for agents with unimodal populations and >90% of endpoints on scale
| Antimicrobial agent | MIC (μg/ml)a
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frozen MH HTM
|
In-house MH HTM
|
PML MH HTM
|
MH LHB NAD
|
IST LHB NAD
|
IST HTM
|
|||||||||||||
| Site 1
|
Site 1
|
Site 2
|
Site 3
|
Site 1
|
Site 1
|
Site 2
|
Site 3
|
Site 3
|
||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Amox-clav acidb | 1.18 | 1.52 | 1.05 | 1.44 | 0.697 | 1.299 | 0.829 | 1.118 | 1.007 | 1.31 | 1.73 | 1.3 | 0.908 | 1.12 | 0.907 | 1.21 | 1 | 1.2 |
| Azithromycin | 1.602 | 0.84 | 0.938 | 0.872 | 0.525 | 0.728 | 0.674 | 0.769 | 0.683 | 0.821 | 0.859 | 0.98 | 0.693 | 0.693 | 0.717 | 0.745 | 0.747 | 0.713 |
| Cefaclor | 10.63 | 1.71 | 10.48 | 1.421 | 3.58 | 1.383 | 6.233 | 1.679 | 9.126 | 1.331 | 13.36 | 1.488 | 5.426 | 1.635 | 6.105 | 1.413 | 5.579 | 1.367 |
| Cefdinir | NDc | NAa | 0.536 | 1.176 | 0.291 | 1.06 | 0.48 | 0.736 | 0.514 | 1.118 | 0.595 | 1.234 | 0.409 | 1.104 | 0.566 | 0.672 | 0.525 | 0.537 |
| Cefixime | 0.042 | 1.002 | 0.051 | 1.008 | 0.0298 | 0.956 | 0.037 | 0.933 | 0.055 | 0.982 | 0.058 | 1.014 | 0.035 | 0.961 | 0.034 | 0.956 | 0.033 | 0.918 |
| Cefprozil | 7.835 | 1.65 | 7.568 | 1.489 | 2.809 | 1.36 | 3.63 | 1.676 | 6.148 | 1.398 | 9.514 | 1.54 | 4.691 | 1.728 | 3.074 | 1.562 | 3.458 | 1.444 |
| Ceftriaxone | NA | NA | 0.008 | 1.157 | 0.0058 | 0.945 | 0.0069 | 1.083 | 0.009 | 1.125 | 0.008 | 1.093 | 0.006 | 0.971 | 0.007 | 1.07 | 0.006 | 0.986 |
| Cefuroxime | 1.257 | 1.69 | 1.778 | 1.326 | 0.901 | 1.14 | 0.92 | 1.085 | 1.591 | 1.146 | 1.815 | 1.414 | 1.197 | 1.268 | 0.865 | 1.149 | 0.871 | 0.985 |
| Chloramphenicol | 0.547 | 1.38 | 0.768 | 1.213 | 0.4175 | 1.203 | 0.624 | 1.246 | 0.642 | 1.25 | 0.807 | 1.237 | 0.483 | 1.258 | 0.62 | 1.2 | 0.629 | 0.12 |
| Ciprofloxacin | 0.017 | 0.36 | 0.016 | 0.243 | 0.0158 | 0.2 | 0.016 | 0.243 | 0.016 | 0.293 | 0.016 | 0.243 | 0.016 | 0.2 | 0.017 | 0.356 | 0.019 | 0.468 |
| Clarithromycin | 8.112 | 0.899 | 6.869 | 0.894 | 5.278 | 0.791 | 7.21 | 0.796 | 5.897 | 0.833 | 6.021 | 0.911 | 5.696 | 0.81 | 7.111 | 0.805 | 7.013 | 0.8 |
| Doxycycline | 0.574 | 0.71 | 0.570 | 0.788 | 0.4234 | 0.878 | 0.582 | 0.786 | 0.574 | 0.779 | 0.547 | 0.825 | 0.384 | 0.874 | 0.758 | 0.829 | 1.094 | 0.787 |
| Erythromycin | 8.168 | 0.81 | 5.242 | 0.777 | 3.387 | 0.74 | 4.891 | 0.756 | 4.438 | 0.783 | 5.278 | 0.865 | 4.141 | 0.757 | 5.169 | 0.8 | 4.857 | 0.726 |
| Gemifloxacin | 0.009 | 0.45 | 0.008 | 0.243 | 0.008 | 0.243 | 0.008 | 0.243 | 0.008 | 0.14 | 0.008 | 0.1 | 0.008 | 0.1 | 0.008 | 0.261 | 0.008 | 0.367 |
| Grepafloxacin | 0.017 | 0.53 | 0.009 | 0.465 | 0.008 | 0.293 | 0.009 | 0.505 | 0.009 | 0.428 | 0.008 | 0.293 | 0.008 | 0.243 | 0.01 | 0.563 | 0.013 | 0.661 |
| Levofloxacin | 0.028 | 0.44 | 0.017 | 0.321 | 0.016 | 0.223 | 0.016 | 0.293 | 0.017 | 0.308 | 0.016 | 0.293 | 0.016 | 0.2 | 0.019 | 0.482 | 0.022 | 0.559 |
| Ofloxacin | 0.054 | 0.54 | 0.035 | 0.435 | 0.033 | 0.308 | 0.034 | 0.393 | 0.036 | 0.465 | 0.035 | 0.435 | 0.033 | 0.293 | 0.044 | 0.577 | 0.049 | 0.555 |
| Trovafloxacin | 0.018 | 0.63 | 0.0098 | 0.566 | 0.009 | 0.52 | 0.009 | 0.552 | 0.009 | 0.539 | 0.009 | 0.5 | 0.009 | 0.42 | 0.01 | 0.603 | 0.013 | 0.704 |
For an agent with a geometric mean MIC of 4 μg/ml and an SD of one doubling dilution, the MIC range ±1 SD is 2 to 8 μg/ml and the MIC range ±2 SD is 1 to 16 μg/ml; if the SD is 0.5 doubling dilution, the MIC range ±1 SD is 2.8 to 5.7 μg/ml and the MIC range ±2 SD is 2 to 8 μg/ml.
Amox-clav acid, amoxicillin-clavulanic acid, shown as amoxicillin component.
ND, not determined.
NA, not applicable due to >10% off-scale endpoints.
TABLE 2.
GEE results; estimated regression coefficients and statistical values of significance for comparison to standard
| Antimicrobial agent | Estimated regression coefficients and significancea
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site-related differencesb
|
Method-related differencesc
|
|||||||||||
| Site 2
|
Site 3
|
PML MH HTM
|
MH LHB NAD
|
IST LHB NAD
|
IST HTM
|
|||||||
| Estimate | P value | Estimate | P value | Estimate | P value | Estimate | P value | Estimate | P value | Estimate | P value | |
| Amox-clav acidd | −0.4600 | <0.0001 | −0.2850 | 0.0934 | 0.4550 | <0.0001 | 0.2600 | <0.0001 | 0.1319 | 0.0046 | 0.2733 | <0.0001 |
| Azithromycin | −0.5550 | <0.0001 | −0.3175 | 0.0067 | 0.2575 | 0.0004 | 0.1550 | <0.0001 | 0.0900 | 0.1346 | 0.1500 | 0.0188 |
| Cefaclor | −1.4250 | <0.0001 | −0.6875 | 0.0005 | 1.2875 | <0.0001 | 0.4750 | <0.0001 | −0.0300 | 0.6856 | −0.1600 | 0.0690 |
| Cefixime | −0.7786 | <0.0001 | −0.4607 | 0.0078 | 0.8893 | <0.0001 | 0.2214 | <0.0001 | −0.1000 | 0.1556 | −0.1571 | 0.0287 |
| Cefprozil | −1.2250 | <0.0001 | −0.9575 | <0.0001 | 1.0275 | <0.0001 | 0.5350 | <0.0001 | −0.2400 | 0.0020 | −0.0700 | 0.3047 |
| Ceftriaxone | −0.5000 | <0.0001 | −0.2700 | 0.0901 | 0.5300 | <0.0001 | 0.0100 | 0.7455 | −0.0200 | 0.7387 | −0.1100 | 0.0134 |
| Cefuroxime | −0.7900 | <0.0001 | −0.8550 | <0.0001 | 0.7520 | <0.0001 | 0.2200 | 0.0001 | −0.0900 | 0.0673 | −0.0800 | 0.1122 |
| Chloramphenicol | −0.8100 | <0.0001 | −0.2650 | 0.0974 | 0.5850 | <0.0001 | 0.1400 | 0.0001 | −0.0100 | 0.8083 | 0.0100 | 0.7629 |
| Ciprofloxacin | −0.0200 | 0.0979 | 0.0000 | 1.0000 | 0.0500 | 0.0310 | 0.0000 | 1.0000 | 0.0800 | 0.0089 | 0.2300 | <0.0001 |
| Clarithromycin | −0.2300 | <0.0001 | 0.1450 | 0.2044 | 0.0850 | 0.0821 | −0.0400 | 0.2734 | −0.0200 | 0.6947 | 0.0400 | 0.4917 |
| Doxycycline | −0.4700 | <0.0001 | 0.0100 | 0.9278 | 0.4600 | <0.0001 | −0.1000 | 0.0003 | 0.3800 | <0.0001 | 0.9100 | <0.0001 |
| Erythromycin | −0.4900 | <0.0001 | −0.0300 | 0.7874 | 0.3200 | <0.0001 | 0.1500 | <0.0001 | 0.0800 | 0.1660 | −0.0100 | 0.8694 |
| Gemifloxacin | 0.0000 | 1.0000 | 0.0000 | 1.0000 | −0.0200 | 0.1531 | −0.0300 | 0.0786 | 0.0100 | 0.3149 | 0.0400 | 0.0979 |
| Grepafloxacin | −0.0750 | 0.0002 | 0.0925 | 0.1551 | 0.0775 | 0.0049 | −0.0750 | 0.0002 | 0.0500 | 0.2218 | 0.4800 | <0.0001 |
| Levofloxacin | −0.0550 | 0.0056 | −0.0175 | 0.6902 | 0.0475 | 0.0352 | −0.0150 | 0.1758 | 0.2300 | <0.0001 | 0.4500 | <0.0001 |
| Ofloxacin | −0.0750 | 0.0010 | −0.0225 | 0.7053 | 0.1125 | 0.0004 | −0.0050 | 0.7053 | 0.3500 | <0.0001 | 0.5300 | <0.0001 |
| Trovafloxacin | −0.0450 | 0.0786 | −0.0275 | 0.7171 | −0.0125 | 0.6951 | −0.1150 | <0.0001 | 0.1200 | 0.0199 | 0.4200 | <0.0001 |
Boldface, significant at >0.25-dilution difference. Regression coefficients are shown as doubling dilution differences above (positive values) or below (negative values) standard, and statistical values of significance (P values are compared to standard).
Compared to site 1.
Compared to MH HTM.
Amox-clav acid, amoxicillin-clavulanic acid.
An analysis of the differences by antimicrobial agent demonstrates that the cefprozil, cefaclor, and trimethoprim-sulfamethoxazole results differed the most. The cefaclor and cefprozil results for sites 2 and 3 were one to two dilutions lower than those for site 1. The trimethoprim-sulfamethoxazole results were not reproducible within each laboratory at MICs of <0.5 μg/ml. The results for erythromycin and azithromycin for all media were one to two dilutions lower than those for frozen MH HTM; this was seen more frequently with erythromycin than with azithromycin. Clarithromycin agreement with the frozen reference method was very good for all media and only showed very slight differences from the frozen reference method (MICs in dried trays were lower than those in frozen MH HTM trays). The fluoroquinolone results (with the exception of ciprofloxacin and ofloxacin) for all media in the dried trays were lower (one to two dilutions) than those in the frozen MH HTM trays. The majority of fluoroquinolone results were at very low MICs of <0.03 μg/ml, which may explain the variation in results. The gemifloxacin and grepafloxacin MH LHB NAD results at sites 1 and 2 were lower by one dilution than those of HTM- and IsoSensitest-based media. The results for ampicillin, amoxicillin-clavulanic acid, cefdinir, cefixime, ceftriaxone, ciprofloxacin, clarithromycin, and ofloxacin were the most reproducible.
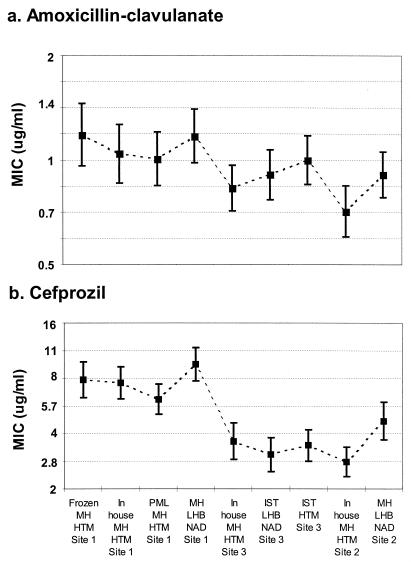
Less variation in mean MICs was found for all methods for amoxicillin-clavulanic acid (0.7 to 1.2 μg/ml), which was one of the most reproducible agents tested, than for cefprozil (2.7 to 9.5 μg/ml), which was one of the least reproducible agents tested. Less variation in ranges of mean ± 2 standard error values (approximate 95% confidence limits) was also noted for amoxicillin-clavulanic acid (approximately 1.5 doubling dilutions) than for cefprozil (approximately 2.5 doubling dilutions). Figure 1 provides a graphical example of these better and worse degrees of agreement, with confidence intervals included. Despite the differences in ranges of variation, the patterns of variability are strikingly similar. In addition, it is possible to see more clearly the similarities within sites and the differences among them.
FIG. 1.
Geometric mean MICs and upper and lower limits of 95% confidence limits (bars) for amoxicillin-clavulanic acid and cefprozil. Much less variation in mean MICs was found for amoxicillin-clavulanic acid ranges (0.7 to 1.4 μg/ml) for all methods and sites than for cefprozil (2.0 to 9.5 μg/ml). However, similar variations in the ranges of 95% confidence limits were noted for amoxicillin-clavulanate and cefprozil.
In addition to the differences with gemifloxacin and grepafloxacin in MH LHB NAD, the only other discernible medium differences were with amoxicillin and doxycycline for IsoSensitest-based medium results. Amoxicillin IST HTM varied most compared with other media (+6 to −4 dilution difference from the frozen reference). The amoxicillin IST HTM results were lower by one to two dilutions at MICs above 8 μg/ml. Doxycycline IST HTM results were one to two dilutions higher than other medium results, and the doxycycline IST LHB NAD results were also slightly higher by approximately one dilution.
Overall, the results for different media were comparable within each testing laboratory. The results for site 2 and site 3 were slightly lower overall than those for site 1.
GEE methodology examined the effects of testing sites and methods compared to the baseline of site 1 MH HTM, providing an estimated regression coefficient for each individual effect variable. This coefficient, stated as the number of doubling dilutions from the baseline, describes the degree of shift from the baseline caused by the specified variable. These coefficients and the statistical significances of the differences are summarized in Table 2. Site 2 and PML HTM were both significant contributors to differences seen with cefaclor and cefprozil, with shifts in MICs of greater than one doubling dilution for both drugs. The site 2 coefficient was greater than one dilution lower than the frozen standard for both drugs, while the PML HTM coefficient shifted the MICs greater than one dilution higher than the standard for both drugs. The quinolones (except for gemifloxacin and ciprofloxacin) and macrolides (except for clarithromycin) were also shifted nearly one dilution lower with the MH HTM than with the frozen medium. Statistically significant (P < 0.05) differences of ±0.25 dilutions are indicated in the table.
Table 3 summarizes the paired t test findings from the comparison within sites, using the MH HTM values as a baseline. Within site 1, the PML HTM medium was highly statistically different for azithromycin and also significantly different for cefprozil, chloramphenicol, clarithromycin, erythromycin, and gemifloxacin. The MH LHB medium was highly different for gemifloxacin and grepafloxacin and significantly different for cefaclor, clarithromycin, and trovafloxacin. Within site 2, MH LHB NAD was in good statistical agreement with MH HTM only for levofloxacin. Agreement for ceftriaxone and clarithromycin were borderline not significant, and that for ofloxacin and doxycycline was close to the level of statistical significance with the Bonferroni correction. All other agents were significantly or highly significantly different between the two methods used at site 2. Site 3 IST HTM was highly significantly different from MH HTM for the quinolones, doxycycline, and ciprofloxacin and significantly different for amoxicillin-clavulanic acid. All other agents tested with IST HTM at site 3 were not significantly different from the baseline. The site 3 IST LHB showed significance for only doxycycline, ofloxacin, levofloxacin, and cefdinir compared to MH HTM.
TABLE 3.
Paired t test P value results within sites, with results of MH HTM for each site as standard
| Antimicrobial agent | Paired t test P value results within sites compared to MH HTM for each siteb
|
||||
|---|---|---|---|---|---|
| Site 1
|
Site 2 MH LHB NAD | Site 3
|
|||
| PML MH HTM | MH LHB NAD | IST LHB NAD | IST HTM | ||
| Amox-clav Acida | 0.2410 | 0.0130 | <0.0001 | 0.0060 | <0.0001 |
| Azithromycin | <<0.0001 | 0.0860 | <<0.0001 | 0.1610 | 0.0200 |
| Cefaclor | 0.0059 | 0.0003 | <<0.0001 | 0.7910 | 0.0960 |
| Cefdinir | 0.3060 | 0.0540 | <<0.0001 | <0.0001 | 0.0150 |
| Cefixime | 0.3200 | 0.0020 | <0.0001 | 0.0390 | 0.0110 |
| Cefprozil | <0.0001 | 0.0010 | <<0.0001 | 0.0009 | 0.1980 |
| Ceftriaxone | 0.8199 | 0.5660 | 0.0960 | 0.7410 | 0.0160 |
| Cefuroxime | 0.0200 | 0.7270 | <<0.0001 | 0.0700 | 0.1170 |
| Chloramphenicol | <0.0001 | 0.1630 | <0.0001 | 0.8100 | 0.7650 |
| Ciprofloxacin | 0.0109 | 0.0005 | <0.0001 | 0.0004 | <<0.0001 |
| Clarithromycin | <0.0001 | <0.0001 | 0.0336 | 0.6970 | 0.4950 |
| Doxycycline | 1.0000 | 0.0896 | 0.0005 | <<0.0001 | <<0.0001 |
| Erythromycin | <0.0001 | 0.8290 | <0.0001 | 0.1710 | 0.8700 |
| Gemifloxacin | <0.0001 | <<0.0001 | <<0.0001 | 0.0118 | <<0.0001 |
| Grepafloxacin | 0.8199 | <<0.0001 | <<0.0001 | 0.1310 | <<0.0001 |
| Levofloxacin | 0.3680 | 0.0190 | 0.4410 | <0.0001 | <<0.0001 |
| Ofloxacin | 0.0517 | 0.7410 | 0.0019 | <<0.0001 | <<0.0001 |
| Trovafloxacin | 0.5930 | <0.0001 | <0.0001 | 0.0007 | <<0.0001 |
Amox-clav acid, amoxicillin-clavulanic acid.
Critical values of significance were P < 0.0003, which was based on P < 0.05 with Bonferroni correction for multiple tests, and are shown in boldface. Additionally, P < 10−9 values were regarded as highly significant (shown as <<0.0001 values).
Tables 4 and 5 summarize the statistical findings for the between-site comparisons of like media. Site 2 MICs for the MH HTM medium used at all three sites were significantly different from those seen at site 1, except for the quinolones. Site 3 showed some differences from site 1 for cefaclor, cefprozil, and cefuroxime. Compared to each other, sites 2 and 3 differed for cefaclor, cefdinir, clarithromycin, doxycycline, and erythromycin. When LHB media were compared, site 2 again showed greater differences from site 1 than did site 3. Compared to each other, sites 2 and 3 also showed several significant differences.
TABLE 4.
Paired t test P value results of MH HTM results between sites
| Antimicrobial agenta | Paired t test P value results of MH HTM results between sitesb
|
||
|---|---|---|---|
| Site 1 vs. site 2 | Site 1 vs. site 3 | Site 2 vs. site 3 | |
| Amox-clav acida | <<0.0001 | 0.0551 | 0.1703 |
| Azithromycin | <<0.0001 | 0.0005 | 0.0010 |
| Cefaclor | <<0.0001 | 0.0003 | 0.0002 |
| Cefdinir | <<0.0001 | 0.2551 | <0.0001 |
| Cefixime | <<0.0001 | 0.0007 | 0.0249 |
| Cefprozil | <<0.0001 | <0.0001 | 0.0753 |
| Ceftriaxone | <<0.0001 | 0.0814 | 0.0837 |
| Cefuroxime | <<0.0001 | <0.0001 | 0.8497 |
| Chloramphenicol | <<0.0001 | 0.0729 | 0.0004 |
| Ciprofloxacin | 0.1583 | 1.0000 | 0.5298 |
| Clarithromycin | <<0.0001 | 0.5465 | <0.0001 |
| Doxycycline | <<0.0001 | 0.7883 | 0.0002 |
| Erythromycin | <<0.0001 | 0.3757 | <0.0001 |
| Gemifloxacin | 1.0000 | 1.0000 | 1.0000 |
| Grepafloxacin | 0.0004 | 0.3096 | 0.0011 |
| Levofloxacin | 0.0136 | 0.6570 | 0.2873 |
| Ofloxacin | 0.0075 | 0.7407 | 0.3384 |
| Trovafloxacin | 0.0705 | 0.6019 | 0.6982 |
Amox-clav acid, amoxicillin-clavulanic acid.
Critical values of significance were P < 0.0003, which was based on P < 0.05 with Bonferroni correction for multiple tests, and are shown in boldface. Additionally, P < 10−9 values were regarded as highly significant (shown as <<0.0001 values).
TABLE 5.
Paired t test P value results for LHB-supplemented media
| Antimicrobial agent | Paired t test P value results for LHB-supplemented mediab
|
||
|---|---|---|---|
| Site 1 vs site 2 | Site 1 vs site 3 | Site 2 vs site 3 | |
| Amox-clav acida | <0.0001 | 0.0332 | 0.9460 |
| Azithromycin | 0.0008 | 0.0367 | 0.6595 |
| Cefaclor | <<0.0001 | <0.0001 | 0.3890 |
| Cefdinir | <<0.0001 | 0.6150 | 0.0003 |
| Cefixime | <<0.0001 | <0.0001 | 0.7870 |
| Cefprozil | <<0.0001 | <<0.0001 | 0.0041 |
| Ceftriaxone | <<0.0001 | 0.0825 | 0.2351 |
| Cefuroxime | <<0.0001 | <0.0001 | 0.0078 |
| Chloramphenicol | <<0.0001 | 0.0186 | 0.0301 |
| Ciprofloxacin | 0.1583 | 0.0590 | 0.0177 |
| Clarithromycin | 0.1583 | 0.0495 | 0.0059 |
| Doxycycline | <<0.0001 | 0.0001 | <<0.0001 |
| Erythromycin | <0.0001 | 0.7962 | 0.0039 |
| Gemifloxacin | 1.0000 | 0.1583 | 0.1583 |
| Grepafloxacin | 0.0832 | 0.0003 | <0.0001 |
| Levofloxacin | 0.0246 | 0.0001 | <0.0001 |
| Ofloxacin | 0.0042 | <0.0001 | <0.0001 |
| Trovafloxacin | 0.4823 | 0.0070 | 0.0016 |
Amox-clav acid, amoxicillin-clavulanic acid.
MN LHB NAD in sites 1 and 2; IST LHB NAD in site 3. Critical values of significance were P < 0.0003, which was based on P < 0.05 with Bonferroni correction for multiple tests, and are shown in boldface. Additionally, P < 10−9 values were regarded as highly significant (shown as <<0.0001 values).
When tested as a population rather than as discretely paired data, the groups showed greater statistical agreement (data not shown). Compared to the frozen HTM, the quinolones did not show the statistically significant differences that were seen when the data were paired, though the macrolides, cefaclor, and cefprozil remained statistically different. The comparison done within sites showed the best agreement at site 3, where only cefprozil was statistically different using the IST LHB NAD medium and cefaclor and doxycycline were significantly different using the IST HTM medium. At site 1 the PML HTM and MH LHB NAD were significantly different from the baseline MH HTM for cefaclor, cefprozil, and clarithromycin, and only the PML HTM was significantly different for erythromycin. At site 2, MH LHB NAD was significantly different from MH HTM for cefaclor, cefprozil, clarithromycin, and erythromycin.
The MIC adjustments done on azithromycin, erythromycin, grepafloxacin, levofloxacin, ofloxacin, and trovafloxacin to test for the robustness of the statistical differences required no more than a one-doubling-dilution adjustment to change from a very high degree of statistical significance to none (data not shown). The MIC distributions of these drugs also show a left shift in the normally distributed curve of approximately one doubling dilution.
Table 6 summarizes the percentage of MICs found to be within one and two dilutions of the frozen standard. Many statistically significant differences were found when the site-method combinations were compared to the MH HTM site 1 baseline. When comparisons were made within each laboratory, using the site-specific MH HTM difference from the frozen standard as a baseline, fewer significant differences were seen. At site 1, only 4 of the 19 antimicrobial agents tested with PML HTM broth—azithromycin, erythromycin, gemifloxacin, and trovafloxicin—were statistically different from the baseline at one dilution difference of agreement. At agreement to two dilutions difference, only amoxicillin and azithromycin were significantly different from the baseline for PML HTM. At site 2, there were a larger number of drugs that did not agree to within one or two dilutions difference for the two methods tested.
TABLE 6.
Percentages of MICs within one or two dilutions from reference method based on test site and medium
| Antimicrobial agent | % MICs within 1 dilution/2 dilutions from reference method
|
|||||||
|---|---|---|---|---|---|---|---|---|
| In-house MH HTM
|
PML MH HTM (Site 1) | MH LHB NAD
|
IST LHB NAD (Site 3) | IST HTM (Site 3) | ||||
| Site 1 | Site 2 | Site 3 | Site 1 | Site 2 | ||||
| Amox-clav acida | 97/100 | 82/98 | 82/93 | 98/100 | 91/100 | 89/99 | 81/92 | 81/94 |
| Amoxicillin | 95/100 | 74/91 | 80/90 | 97/98 | 86/100 | 86/97 | 80/90 | 72/86 |
| Ampicillin | 100/100 | 83/100 | 79/100 | 100/100 | 100/100 | 93/100 | 84/89 | 87/95 |
| Azithromycin | 91/100 | 40/92 | 69/95 | 71/97 | 97/98 | 70/94 | 68/93 | 73/95 |
| Cefaclor | 91/98 | 49/84 | 67/97 | 86/97 | 80/95 | 71/87 | 67/86 | 67/85 |
| Cefprozil | 97/99 | 47/81 | 90/98 | 90/98 | 81/96 | 67/89 | 43/73 | 52/78 |
| Ceftriaxone | 99/100 | 96/100 | 100/100 | 100/100 | 100/100 | 99/100 | 90/100 | 91/100 |
| Cefuroxime | 81/97 | 86/97 | 72/90 | 72/90 | 87/99 | 91/99 | 70/89 | 72/90 |
| Chloramphenicol | 87/100 | 90/100 | 95/100 | 95/100 | 82/100 | 92/100 | 93/96 | 94/96 |
| Ciprofloxacin | 99/100 | 100/100 | 99/100 | 99/100 | 99/100 | 96/99 | 99/100 | 100/100 |
| Clarithromycin | 99/100 | 96/100 | 99/100 | 99/100 | 98/100 | 96/100 | 94/98 | 93/98 |
| Doxycycline | 99/100 | 99/100 | 97/100 | 97/100 | 97/99 | 96/100 | 85/96 | 67/96 |
| Erythromycin | 98/100 | 67/99 | 88/100 | 88/100 | 95/99 | 87/99 | 88/96 | 83/96 |
| Gemifloxacin | 64/97 | 41/98 | 41/92 | 41/92 | 12/80 | 5/67 | 75/97 | 87/98 |
| Grepafloxacin | 79/99 | 54/99 | 81/100 | 81/100 | 41/98 | 23/98 | 95/100 | 99/100 |
| Levofloxacin | 99/100 | 78/100 | 99/100 | 99/100 | 98/100 | 74/100 | 95/100 | 98/99 |
| Ofloxacin | 100/100 | 100/100 | 100/100 | 100/100 | 99/100 | 98/100 | 99/100 | 99/100 |
| Trim./Sulfa.b | 71/92 | 89/96 | 52/83 | 52/83 | 74/92 | 54/84 | 65/78 | 69/91 |
| Trovafloxacin | 71/94 | 66/91 | 85/99 | 68/94 | 61/91 | 52/90 | 90/100 | 96/100 |
Amox-clav acid, amoxicillin-clavulanic acid.
Trim./Sulfa., trimethoprim-sulfamethoxazole.
DISCUSSION
The results of this study indicate that MICs of a wide variety of antimicrobial agents for H. influenzae can be reproducibly determined using several media. Most variations in MICs were due to site-to-site differences and not due to differences in media. The site-to-site differences, however, did not significantly impact the susceptibility rates for the majority of the antimicrobial agents tested, as many of the differences occurred at drug concentrations below susceptibility breakpoints (Tables 7 and 8). However, considerable variation in the percentages of isolates susceptible to cefprozil and cefaclor occurred, as the geometric mean MICs were close to NCCLS susceptible breakpoints. Less variation occurred when PK-PD breakpoints were used.
TABLE 7.
MIC50 and MIC90 for each method
| Antimicrobial agent | MIC50/MIC90 (μg/ml)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Frozen MH HTM (Site 1) | In-house MH HTM
|
PML MH HTM (Site 1) | MH LHB NAD
|
IST LHB NAD (Site 3) | IST HTM (Site 3) | ||||
| Site 1 | Site 2 | Site 3 | Site 1 | Site 2 | |||||
| Amox-clav acida | 1/8 | 1/4 | 0.5/2 | 1/2 | 1/4 | 1/4 | 1/2 | 1/4 | 1/4 |
| Amoxicillin | 8/>16 | 4/>16 | 2/>16 | 2/>16 | 4/>16 | 4/>16 | 2/>16 | 2/>16 | 4/16 |
| Ampicillin | 2/>16 | 2/>16 | 1/>16 | 1/>16 | 2/>16 | 2/>16 | 1/>16 | 1/>16 | 1/>16 |
| Azithromycin | 2/4 | 1/2 | 0.5/1 | 0.5/1 | 1/1 | 1/2 | 0.5/2 | 1/1 | 1/1 |
| Cefaclor | 16/64 | 8/32 | 4/8 | 4/32 | 8/32 | 16/64 | 4/32 | 8/16 | 4/16 |
| Cefdinir | N/A | 0.5/2 | 0.25/1 | 0.5/1 | 0.5/1 | 0.5/2 | 0.25/1 | 0.5/1 | 0.5/1 |
| Cefixime | 0.03/0.12 | 0.06/0.12 | 0.03/0.06 | 0.03/0.06 | 0.06/0.12 | 0.06/0.12 | 0.03/0.06 | 0.03/0.06 | 0.03/0.06 |
| Cefprozil | 8/32 | 8/32 | 2/8 | 4/16 | 4/32 | 8/32 | 4/32 | 4/32 | 4/16 |
| Ceftriaxone | 0.008/0.03 | 0.008/0.03 | 0.004/0.015 | 0.004/0.015 | 0.008/0.015 | 0.008/0.015 | ≤0.004/0.015 | ≤0.004/0.03 | ≤0.004/0.015 |
| Cefuroxime | 1/8 | 2/4 | 1/2 | 1/2 | 1/4 | 1/8 | 1/4 | 1/2 | 1/2 |
| Chloramphenicol | 0.5/1 | 0.5/1 | 0.25/0.5 | 0.5/1 | 0.5/1 | 0.5/1 | 0.5/0.5 | 0.5/1 | 0.5/1 |
| Ciprofloxacin | 0.015/0.03 | 0.015/0.015 | 0.008/0.015 | 0.015/0.015 | 0.015/0.015 | 0.008/0.015 | 0.008/0.015 | 0.015/0.03 | 0.015/0.03 |
| Clarithromycin | 8/16 | 8/16 | 4/8 | 8/16 | 8/8 | 8/16 | 8/16 | 8/8 | 8/8 |
| Doxycycline | 0.5/1 | 0.5/1 | 0.5/1 | 0.5/2 | 0.5/1 | 0.5/1 | 0.25/1 | 0.5/2 | 1/4 |
| Erythromycin | 8/16 | 4/8 | 4/4 | 4/8 | 4/8 | 4/8 | 4/8 | 4/8 | 4/8 |
| Gemifloxacin | 0.008/0.015 | 0.004/0.008 | 0.004/0.004 | 0.004/0.008 | 0.004/0.008 | 0.002/0.004 | 0.002/0.004 | 0.004/0.008 | 0.008/0.008 |
| Grepafloxacin | 0.015/0.015 | 0.008/0.015 | 0.008/0.008 | 0.008/0.015 | 0.008/0.015 | 0.004/0.008 | 0.004/0.008 | 0.008/0.015 | 0.015/0.03 |
| Levofloxacin | 0.03/0.03 | 0.015/0.015 | 0.015/0.015 | 0.015/0.015 | 0.015/0.015 | 0.015/0.015 | 0.015/0.015 | 0.015/0.03 | 0.015/0.03 |
| Ofloxacin | 0.06/0.06 | 0.03/0.06 | 0.03/0.03 | 0.03/0.06 | 0.03/0.06 | 0.03/0.06 | 0.03/0.03 | 0.03/0.06 | 0.06/0.06 |
| Trim./Sulfa.b | 0.12/>4 | 0.12/>4 | 0.06/>4 | 0.12/>4 | 0.25/>4 | 0.03/>4 | 0.03/4 | 0.03/4 | 0.12/4 |
| Trovafloxacin | 0.015/0.03 | 0.008/0.015 | 0.008/0.015 | 0.008/0.015 | 0.008/0.015 | 0.008/0.015 | 0.008/0.015 | 0.008/0.015 | 0.015/0.015 |
Amox-clav acid, amoxicillin-clavulanic acid.
Trim./Sulfa., trimethoprim-sulfamethoxazole.
TABLE 8.
Ranges and mean percentages of susceptible isolates for all methods based on NCCLS and PK-PD breakpoints
| Antimicrobial agent | NCCLS
|
PK-PD
|
||||
|---|---|---|---|---|---|---|
| Susceptible breakpoint (μg/ml) | % Susceptible range | % Susceptible mean | Susceptible breakpoint (μg/ml) | % Susceptible range | % Susceptible mean | |
| Amoxicillin-clavulanic acida | 4 | 89.0-99.0 | 96.9 | 2 | 75.0-96.0 | 85.7 |
| Amoxicillin | NAb | NA | NA | 2 | 41-57 | 49.3 |
| Ampicillin | 1 | 44.0-57.0 | 50.3 | NA | NA | NA |
| Azithromycin | 4 | All at 100 | 100 | 0.12 | 0.0-6.0 | 2.2 |
| Cefaclor | 8 | 47.0-90.0 | 67.7 | 0.5 | 0.0-3.0 | 0.78 |
| Cefdinir | 1 | 79.0-100.0 | 93.5 | 0.5 | 61.0-87.9 | 74.2 |
| Cefixime | 1 | All at 100 | 100 | 1 | All at 100 | 100 |
| Cefprozil | 8 | 52.0-91.0 | 73.2 | 1 | 1.0-27.0 | 13.2 |
| Ceftriaxone | 2 | All at 100 | 100 | NA | NA | NA |
| Cefuroxime | 4 | 83.0-99.0 | 93.8 | 1 | 47.0-75.8 | 62.7 |
| Chloramphenicol | 2 | 92.9-93.0 | 93.0 | NA | NA | NA |
| Ciprofloxacin | 1 | All at 100 | 100 | 1 | All at 100 | 100 |
| Clarithromycin | 8 | 77.0-96.0 | 90.3 | 0.25 | All at 0 | 0 |
| Doxycycline | NA | NA | NA | 0.25 | 78.0-89.0 | 80.9 |
| Erythromycin | NA | NA | NA | 0.25 | All at 0 | 0 |
| Gemifloxacin | NA | NA | NA | 1 | All at 100 | 100 |
| Grepafloxacin | 0.5 | All at 100 | 100 | 1 | All at 100 | 100 |
| Levofloxacin | 2 | All at 100 | 100 | 1 | All at 100 | 100 |
| Ofloxacin | 2 | All at 100 | 100 | 1 | All at 100 | 100 |
| Trim./Sulfa.c | 0.5 | 71.0-82.0 | 76.0 | NA | NA | NA |
| Trovafloxacin | 1 | All at 100 | 100 | 1 | All at 100 | 100 |
Breakpoints shown as amoxicillin component.
NA, not applicable.
Trim./Sulfa., trimethoprim-sulfamethoxazole.
There were few significant differences as a result of the compositions of media. The results obtained using Mueller-Hinton broth with 2% LHB and 15 μg of NAD/ml and IST with 2% LHB and 15 μg of NAD/ml were equivalent to the results obtained using HTM for determination of the MICs of agents evaluated against H. influenzae. However, HTM is more difficult to manufacture and has a short shelf life at 4°C (4 to 6 weeks) before the hematin component degrades, whereas LHB-containing media can be kept for at least 6 months at 4°C; both media are stable at −20°C (10-12). Additional advantages of LHB-based media are that endpoints are easier to read and that the same medium can be used to test streptococci.
A necessary consideration when examining data with a permissible range of reproducibility difference statistically is at what point a conventionally significant result is scientifically meaningful. MIC tests are generally regarded as being in agreement when they are reproducible within one doubling dilution, while for statistical tests, such a difference is generally considered highly significant; there is consequently an inherent tension between what is statistically significant and what is scientifically significant. For the purposes of this study, a shift of 0.25-doubling-dilution difference was regarded as representing a scientifically noteworthy methodological or site bias.
There appears to be a greater degree of difference between testing sites than among methods within one site. Sites 1 and 3, for the most part, had good reproducibility across methods, while site 2 showed more variability. This is especially apparent when looking at the GEE results, where site 2 is more frequently a contributor of significant difference than the other two sites. Site 2 shows a consistent bias in the negative direction, suggesting that the MICs for all drugs tested at that site would be consistently lower than those found at the other sites. Examination of method-specific differences shows only PML HTM to be a significant contributor of difference. In contrast to the site 2 bias, that seen with this medium is in the positive direction. MICs with PML HTM broth would be expected to be higher than those found with the other methods.
Examination of the MICs of many of the antimicrobial agents tested by paired t test remained consistent across both methods and testing sites. The quinolones, while very statistically different in the comparison between MH HTM and the frozen standard, did not differ much in the between-site analysis, the GEE analysis, or when tested as population means. Because these drugs are active at very low concentrations, a difference of one dilution is much less important than for drugs with activity at higher concentrations and therefore closer to or crossing breakpoints.
The data adjustment to test robustness and the resulting loss of statistical significance reinforced the data summarized in Table 6, showing that the groups, while statistically different from each other, largely agree within the acceptable range of differences for antimicrobial MIC testing of 95% within one doubling dilution (25). The results of these tests, as well as the examination of differences in group means, suggest that there is a systematic shift of less than one dilution difference in several of the drugs. While this is within the acceptable range for reproducibility, it could be problematic if the shift occurs at or near a breakpoint.
Acknowledgments
This work was supported by a grant from SmithKline Beecham Pharmaceuticals.
REFERENCES
- 1.Barry, A. L., M. A. Pfaller, P. C. Fuchs, and R. R. Packer. 1994. In vitro activities of 12 orally administered antimicrobial agents against four species of bacterial respiratory pathogens from U.S. medical centers in 1992 and 1993. Antimicrob. Agents Chemother. 38:2419-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bottone, E. J., Z. Brandman, and S. S. Schneierson. 1976. Spheroplasts of Haemophilus influenzae induced by cell wall-active antibiotics and their effect upon interpretation of susceptibility tests. Antimicrob. Agents Chemother. 9:327-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bulger, R. R., and J. A. Washington. 1980. Effect of inoculum size and β-lactamase production on in vitro activity of new cephalosporins against Haemophilus species. Antimicrob. Agents Chemother. 17:393-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craig, W. A. 1998. Pharmacokinetic/pharmacodyamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. [DOI] [PubMed] [Google Scholar]
- 5.Craig, W. A., and D. Andes. 1996. Pharmacokinetics and pharmacodynamics of antibiotics in otitis media. Pediatr. Infect. Dis. J. 15:255-259. [DOI] [PubMed] [Google Scholar]
- 6.Dagan, R., O. Abramson, E. Leibovitz, D. Greenberg, R. Lang, S. Goshen, P. Yagupsky, A. Leiberman, and D. M. Fliss. 1996. Impaired bacteriologic response to oral cephalosporins in acute otitis media caused by pneumococci with intermediate resistance to penicillin. Pediatr. Infect. Dis J. 15:980-985. [DOI] [PubMed] [Google Scholar]
- 7.Doern, G. V., J. H. Jorgensen, C. Thornsberry, D. A. Preston, T. Tubert, J. S. Redding, and L. A. Maher. 1988. National collaborative study of the prevalence of antimicrobial resistance among clinical isolates of Haemophilus influenzae. Antimicrob. Agents Chemother. 32:180-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doern, G. V., A. B. Brueggemann, G. Pierce, H. P. Holley, and A. Rauch. 1997. Antibiotic resistance among clinical isolates of Haemophilus influenzae in the United States in 1994 and 1995 and detection of β-lactamase-positive strains resistant to amoxicillin-clavulanate: results of a national multicenter surveillance study. Antimicrob. Agents Chemother. 41:292-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doern, G. V., and the Alexander project Collaborative Group. 1996. Antimicrobial resistance among lower respiratory tract isolates of Haemophilus influenzae: results of a 1992-93 Western Europe and USA collaborative surveillance study. J. Antimicrob. Chemother. 38A:59-69. [DOI] [PubMed]
- 9a.Food and Drug Administration. 2000. Guidance on review criteria for assessment of antimicrobial susceptibility devices. Food and Drug Administration, Washington, D.C.
- 10.Geiger, O., J. E. Mortensen, R. B. Clark, and A. Evangelista. 1996. Comparison of five different susceptibility test methods for detecting antimicrobial agent resistance among Haemophilus influenzae isolates. Diagn. Microbiol. Infect. Dis. 24:145-153. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs, M. R., and S. Bajaksouzian. 1997. Evaluation of Haemophilus influenzae isolates with elevated MICs to amoxicillin/clavulanic acids. Diagn. Microbiol. Infect. Dis. 28:105-112. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs, M. R. 1999. Assessing the quality of the Alexander Project. J. Chemother. 11:26-34. [DOI] [PubMed] [Google Scholar]
- 13.Jones, R. N., M. R. Jacobs, J. A. Washington, and M. A. Pfaller. 1997. A 1994 to 1995 survey of Haemophilus influenzae susceptibility to ten orally administered agents: a 187 clinical laboratory center sample in the United States. Diagn. Microbiol. Infect. Dis. 27:75-84. [DOI] [PubMed] [Google Scholar]
- 14.Jorgensen, J. H. 1992. Update on mechanisms and prevalence of antimicrobial resistance in Haemophilus influenzae. Clin. Infect. Dis. 14:1119-1123. [DOI] [PubMed] [Google Scholar]
- 15.Jorgensen, J. H., G. V. Doern, L. A. Maher, A. W. Howell, and J. S. Redding. 1990. Antimicrobial resistance among respiratory isolates of Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae in the United States. Antimicrob. Agents Chemother. 34:2075-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved Standard M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 17.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial susceptibility testing. 10th information supplement M100-S10. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.Nightingale, C. H. 1995. Pharmacokinetics and pharmacodynamics of newer macrolides. Pediatr. Infect. Dis. J. 16:438-443. [DOI] [PubMed] [Google Scholar]
- 19.Roberts, D. E., A. Ingold, S. V. Want, and J. R. May. 1974. Osmotically stable L-forms of Haemophilus influenzae and their significance in testing sensitivity to penicillins. J. Clin. Pathol. 27:560-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rolinson, G. N. 1994. A review of the microbiology of amoxicillin/clavulanic acid over the 15 year period 1978-1993. J. Chemother. 6:283-318. [DOI] [PubMed] [Google Scholar]
- 21.Sinus and Allergy Health Partnership. 2000. Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Otolaryngol. Head Neck Surg. 123:S1-S32. [PubMed]
- 22.Sykes, R. B., A. Griffiths, and D. M. Ryan. 1977. Comparative activity of ampicillin and cefuroxime against three types of Haemophilus influenzae. Antimicrob. Agents Chemother. 11:599-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Syriopoulou, V. P., D. W. Scheifele, C. M. Sack, and A. L. Smith. 1979. Effect of inoculum size on the susceptibility of Haemophilus influenzae b to β-lactam antibiotics. Antimicrob. Agents Chemother. 16:510-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu, P. K. W., and J. A. Washington. 1981. Bactericidal activity of cefoperazone with CP-45,899 against large inocula of β-lactamase-producing Haemophilus influenzae. Antimicrob. Agents Chemother. 20:63-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeger, S. L., and K. Y. Liang. 1986. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42:121-130. [PubMed] [Google Scholar]