Abstract
Human immunodeficiency virus (HIV)-positive women may represent one of the fastest-growing populations at risk for acquiring cervical cancer and thus require frequent screening. The purpose of the present studies was to validate a PCR-based urine assay by comparing detection and genotyping of human papillomavirus (HPV) DNA in urine samples and matching cervical swab specimens of HIV-positive women. Despite a difference in amplifiability, the prevalence of any HPV genotype (58% for the cervical swab specimens and 48% for the urine specimens) was not significantly different in this population. The levels of concordance were 70, 71, and 78% for detection of any HPV type, any high-risk HPV type, or any low-risk HPV type in the two specimen types, respectively. While instances of discordant detection were greater for the cervical swab specimens than for the urine specimens, this was not statistically significant. The distributions of HPV genotypes were similar in the cervix and the urine for the majority of types examined. Importantly, detection of HPV DNA in urine was associated with an abnormal Papanicolaou smear to the same extent that detection of HPV DNA in a cervical swab specimen was. These data provide preliminary support for the proposal to use urine testing as a primary or secondary screening tool for cervical cancer in HIV-positive women or as an epidemiological tool. Additional studies with larger sample sizes must be conducted in order to further verify these findings.
Human papillomavirus (HPV) is the cause of the most common sexually transmitted diseases (STDs) of viral etiology worldwide. Approximately 30 to 50% of the general population is positive for HPV DNA (13, 24). At least 30 known HPV types infect the anogenital region (6; Los Alamos National Laboratory HPV Sequence Database [http://hpv-web.lanl.gov/], 2001). These types are classified as being of low, intermediate, or high risk on the basis of their in vitro abilities to cause cellular transformation and their clinical association with cervical cancer. High-risk HPV types, like HPV types 16, 18, 31, and 45, are more closely associated with anogenital malignancies and have been implicated in the etiology of most, if not all, cervical cancers. Cervical cancer is the second most common cancer of women worldwide (25). Importantly, infection with HPV, even high-risk types, is asymptomatic in most people and usually does not lead to cancer. However, more than 35 HPV types have been found to be associated with at least 90% of cases of cervical intraepithelial neoplasia, which is a precursor lesion to cervical cancer (16, 21).
Although the medical standard for the diagnosis of HPV disease is the Papanicolaou (Pap) smear, screening by this type of method has inherent problems. Only 15 to 50% of patients with HPV infections are accurately identified by Pap smears (22, 26). Additionally, the efficacy of screening by use of the Pap smear relies on repeated (yearly) visits. Approximately 10% of women in the United States have never had a Pap smear, and about 30% of women do not have them on a regular basis (1, 2, 3, 29). Furthermore, Pap smear screening requires a pelvic examination, which is invasive and uncomfortable for the patient as well as time-consuming for the health care provider (27). Perhaps the use of urine sampling for routine detection of HPV could provide a preliminary screen for cervical cancer and thus circumvent the need for an annual Pap smear for women who are negative for HPV DNA. Alternatively, detection of HPV DNA in urine could possibly function as a secondary screening technique for cervical cancer in that it could be used to triage women with atypical squamous cells of undetermined significance (ASCUS).
Methods for the detection of DNA in patient urine have recently been used to diagnose other common STDs that affect the cervix, such as Chlamydia trachomatis and Neisseria gonorrhoeae infections (8). The success of these screening programs provides a good preliminary endorsement of attempts to monitor cervical pathology by detecting HPV DNA in urine specimens. One advantage to the detection of these organisms and the detection of HPV from urine specimens may be that a single specimen could perhaps be used to detect all three of these infectious agents simultaneously. Several investigators have attempted to use urine sampling for HPV DNA detection (10, 14, 30, 32, 35); however, no study to date has addressed the ability to detect HPV DNA in the urine of human immunodeficiency virus (HIV)-positive women.
HIV-positive women demonstrate increased rates of new HPV infections, persistence of high-risk HPV infections, multiple infections, dysplasia, and cervical cancer (4, 9, 18, 23, 31). Furthermore, once cervical cancer develops in these women, the disease tends to be more aggressive and less responsive to treatment (19, 28). In consideration of these factors, the importance of cervical cancer screening in this population is evident. The current strategy for baseline screening of HIV-positive women with normal cervical cytology is to repeat Pap smear testing every 6 months (17). The detection of HPV DNA in the urine of HIV-positive women could identify women at risk for HPV disease in a routine manner that is less invasive to the patient.
The purpose of the studies described here was to validate the urine-based HPV DNA detection method that was developed in our laboratory by comparing the results obtained by that method to those obtained by detection of HPV DNA from matching cervical swab specimens for a population of HIV-positive women for whom the ability to access cervical pathology due to HPV infection by urine testing has not been described previously.
MATERIALS AND METHODS
Patient population.
All patients included in the study were enrolled in the HIV Outpatient Clinic at the Medical Center of Louisiana in New Orleans, La. The study included 101 patients with urine samples and matching cervical swab samples. At the time of their scheduled visit, which included a routine Pap smear, a cervical swab specimen and a non-clean-catch urine specimen were collected. Although the specimen collection sequence was not strictly enforced, the urine was usually collected prior to the pelvic examination. All study participants signed informed consents, and the study was approved by the Louisiana State University Health Sciences Center Institutional Review Board in compliance with federally regulated guidelines for research involving human subjects. Both cervical swab and urine specimens from all 101 patients were tested for the presence of HPV DNA regardless of Pap smear status. Demographic data were collected by questionnaire and from the Adult Spectrum of Disease database.
Cervical swab extractions.
One cervical swab specimen was collected from each patient by use of a Dacron swab; the swab was then placed in 1 ml of Digene transport medium and stored at 4°C until use. Sample extraction was based on the protocol by Ting and Manos (33). The samples were digested at 37°C in 50 mM proteinase K digestion solution (Gibco BRL, Gaithersburg, Md.) for 1 to 2 h. The DNA was then precipitated with a solution of 3 M ammonium acetate in 100% ethanol at twice the volume of the original sample. The DNA pellets were resuspended in 50 μl of TE buffer (10 mM Tris-HCl [pH 7.4], 1 mM EDTA [pH 8.0]) and stored at −20°C until use.
Extraction of DNA from urine.
All urine specimens collected were stored at −20°C for a minimum of 24 h. Two milliliters of each urine sample was then concentrated in an Amicon 100 protein concentration filter (Millipore Corporation, Bedford, Mass.) by centrifugation at 2,087 × g for 30 to 45 min at room temperature, according to the instructions of the manufacturer. The resulting filtrate was then resuspended in 200 μl of 1× phosphate-buffered saline (0.2 M phosphate, 1.5 M sodium chloride [pH 7.4]), and the DNA was extracted with a Qiagen DNA Mini kit (Qiagen Inc. Valencia, Calif.). These DNA extractions were performed according to the manufacturer's Blood and Body Fluid Spin Protocol and were stored at −20°C until use.
PCR.
Urine and matching cervical swab specimens were subjected to 40 cycles of PCR for amplification of the L1 open reading frame of HPV by using the PGMY09-PGMY11 consensus primer system labeled with biotin (final concentration, 1 μM) (11). The biotin-labeled GH20-PC04 (β-globin) primer system (final concentration, 0.025 μM) was also included in the reaction mixture. MgCl2 was used at a concentration of 4 mM, along with 1× PCR buffer II, 1.5 U of AmpliTaq Gold DNA polymerase (Perkin Elmer), and 1 μM deoxynucleoside triphosphates (Perkin-Elmer).
The DNA types of the HPV isolates from cervical swab specimens and the matching urine samples were determined by using the reverse line hybridization system by the protocol developed by Gravitt et al. (12). All reagents were provided by Roche Molecular Systems, Alameda, Calif. Briefly, probes for the L1 regions of the 27 HPV genital types were conjugated to a long strip of nylon membrane with bovine serum albumin. Biotinylated PCR products were denatured by adding EDTA (1.6%), sodium hydroxide (0.13 N), and thymol blue dye for 1 h at room temperature. Next, the samples were hybridized to the probes on the strips by using hybridization buffer containing 4× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.4]) and 0.1% sodium dodecyl sulfate for 30 min at 53°C. The samples were washed once with 1× SSPE-0.1% sodium dodecyl sulfate at 53°C and were then incubated for 0.5 h at room temperature with streptavidin-horseradish peroxidase conjugate solution. The strips were then washed twice at room temperature for 10 min. The pH of the samples was then lowered by the brief addition of sodium citrate buffer (3 ml; 0.1 M). Lastly, a developing solution (2 parts of part A [a citrate solution containing 0.01% H2O2] to 1 part of part B [0.1% 3,3′,5,5′-tetramethylbenzidine]) was added, and the strips were developed for 5 to 10 min. Finally, sodium citrate buffer (3 ml; 0.1 M) was added to stop the reaction.
Data analysis.
Statistical significance was determined with the SPSS program (version 9; SPSS Inc., Chicago, Ill.) for the McNemar test, the Fisher exact test, likelihood, and odds ratios. The Epi6 program (Centers for Disease Control and Prevention, Atlanta, Ga., and World Health Organization, Geneva, Switzerland) was used to determine P values generated from proportion tables.
RESULTS
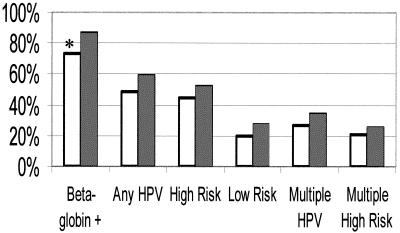
The study population consisted of 101 HIV-positive women recruited from the HIV Outpatient Clinic in New Orleans, La., who donated urine specimens and matching cervical swab specimens. The demographics of patients from the HIV Outpatient Clinic as well as the women in the urine study are presented in Table 1. There were no obvious differences in the characteristics of the urine study population compared to those of the HIV Outpatient Clinic population except that the urine study contained a significantly higher percentage of women in the 30- to 39-year-old age group (P = 0.05) (Table 1). The ability to amplify the samples was determined by visualization of the β-globin housekeeping gene by the reverse line hybridization assay. β-globin DNA was amplifiable from 87% of the cervical swab specimens and 73% of the urine specimens (P = 0.01). Thus, there was a significant difference in the ability to amplify DNA from urine specimens compared to the ability to amplify DNA from cervical swab specimens. However, the detection of any HPV type, any high-risk HPV type, and any low-risk HPV type between the two types of samples was not significantly different for this population (P = 0.12, 0.26, and 0.18, respectively) (Fig. 1). Interestingly, detection of HPV between the two sample types was better when one considered only urine samples (n = 74) from which β-globin DNA could be amplified or only cervical swab specimens (n = 88) from which β-globin DNA could be amplified. In this analysis, 48 of 74 (65%) urine samples and 59 of 88 (67%) cervical swab specimens contained any HPV type (P = 0.77); 45 of 74 (61%) urine samples from which β-globin DNA could be amplified contained any high-risk HPV DNA type, while 52 of 88 (59%) cervical swab specimens from which β-globin DNA could be amplified contained high-risk HPV DNA types (P = 0.82). Twenty of 74 (27%) urine samples from which β-globin DNA could be amplified were positive for low-risk HPV DNA types, whereas 28 of 88 (32%) cervical swab specimens were positive for low-risk HPV DNA types (P = 0.50) (data not shown). Lastly, Fig. 1 demonstrates that 27 of 101 (27%) urine specimens and 35 of 101 (35%) cervical swab specimens were infected with more than one HPV type and 21 of 101 (21%) urine specimens and 26 of 101 (26%) cervical swab specimens were infected with more than one of any of the high-risk HPV DNA types. Of the samples infected with multiple HPV DNA types, the average number of low-risk and high-risk HPV types detected in each sample were 3.4 (range, 2 to 4) and 3.2 (range, 2 to 6), respectively, for the urine specimens and 4.1 (range, 2 to 4) and 3.1 (range, 2 to 8), respectively, for the cervical swab specimens.
TABLE 1.
Demographic and clinical characteristics of current HIV Outpatient Clinic female population and urine study participants
| Characteristica | No. (%) of patients
|
|
|---|---|---|
| HIV Outpatient Clinic (n = 780) | Urine study (n = 101) | |
| Race | ||
| White | 87 (11.2)a | 14 (13.9) |
| African American | 676 (86.7) | 72 (71.3) |
| Hispanic | 16 (2.1) | 4 (4.0) |
| Asian/Pacific Islander | 1 (0.1) | 0 (0.0) |
| Missing | 0 (0) | 11 (10.9) |
| Age (yr) | ||
| <20 | 30 (3.8) | 0 (0.0) |
| 20-29 | 242 (31.0) | 22 (21.8) |
| 30-39 | 299 (38.3) | 49 (48.5)b |
| 40+ | 209 (26.8) | 27 (26.7) |
| Missing | 0 (0) | 3 (3) |
| CD4 count (no. of cells/mm3) | ||
| <200 | 147 (18.8) | 20 (19.8) |
| 200-500 | 315 (40.4) | 41 (40.6) |
| >500 | 247 (31.7) | 32 (31.7) |
| Missing | 71 (9.1) | 8 (7.9) |
| HIV load (no. of RNA copies/ml) | ||
| ≤400 | 200 (27.4) | 32 (31.7) |
| >400 | 531 (72.6) | 67 (66.3) |
| Missing | 49 (6.3) | 2 (2.0) |
| Pap smear | ||
| WNL | NAc | 53 (52.5) |
| ASCUS | NA | 17 (16.8) |
| LGSIL | NA | 22 (21.7) |
| HGSIL | NA | 5 (5.0) |
| Missing | NA | 4 (4.0) |
Demographic data are missing where indicated.
P = 0.05.
NA, data not available.
FIG. 1.
Prevalence of any HPV type, any high-risk HPV type, any low-risk HPV DNA type, or the presence of a multiple infection in urine or cervix for entire study population of 101 HIV-positive women. The amplifiability of HPV DNA from urine and cervical swab specimens is also compared in the first column. White bars, urine samples; thatched bars, cervical swab samples. Significant differences are denoted by an asterisk (P < 0.05).
The reverse line hybridization assay detects 27 different types of HPV (18 high-risk types and 9 low-risk types) (Table 2). None of the HPV DNA detected in this study was nontypeable (agarose gel electrophoresis positive, reverse line hybridization assay negative). When the overall distribution of HPV types detected in the urine specimens was compared with the overall distribution of HPV types detected in the cervical swab specimens, virtually no differences were observed for 21 of the 27 types represented. For types 53, MM4, MM7, 54, and 55, discordance was detected more frequently for the cervical swab specimens (incidence in cervical swab specimens versus incidence in urine specimens, >2.0). For type MM9, discordance was detected more frequently for the urine specimens (incidence in urine specimens versus incidence in cervical swab specimens, >2.0) (Table 2). However, these comparisons were not statistically significantly different (P ≥ 0.11 for all comparisons). It is possible that the relative insensitivity of the assay with urine for the detection of cervical HPV types 53, MM4, MM7, 54, and 55 may become a potential limitation of the assay.
TABLE 2.
Distribution of high- and low-risk HPV types in urine and cervix of HIV-positive women infected with single and multiple HPV types
| HPV type | No. of patients positive for HPV in:
|
||
|---|---|---|---|
| Both samples | Cervical swab specimens only | Urine specimens only | |
| 16 | 4 | 6 | 5 |
| 18 | 3 | 5 | 4 |
| 26 | 0 | 2 | 0 |
| 31 | 1 | 0 | 0 |
| 33 | 4 | 0 | 1 |
| 35 | 6 | 3 | 1 |
| 39 | 0 | 2 | 0 |
| 45 | 4 | 2 | 2 |
| 51 | 4 | 3 | 3 |
| 52 | 5 | 1 | 1 |
| 55 | 2 | 4 | 0 |
| 56 | 1 | 2 | 0 |
| 58 | 4 | 4 | 4 |
| 59 | 5 | 2 | 4 |
| 68 | 3 | 2 | 1 |
| MM4 | 1 | 4 | 0 |
| MM7 | 6 | 4 | 1 |
| MM9 | 3 | 1 | 4 |
| 6 | 3 | 2 | 4 |
| 11 | 3 | 0 | 0 |
| 40 | 2 | 1 | 0 |
| 42 | 2 | 0 | 1 |
| 53 | 4 | 7 | 1 |
| 54 | 8 | 6 | 2 |
| 57 | 0 | 0 | 0 |
| 66 | 4 | 3 | 1 |
| MM8 | 2 | 4 | 3 |
Concordance is a measure of whether both samples had any HPV type, any high-risk HPV type, or any low-risk HPV type or whether no HPV was present in the paired samples. The rates of concordance were 70% for any HPV type, 71% for any high-risk HPV type, and 78% for any low-risk HPV type. The rates at which HPV occurred more frequently in the cervix than in the urine are as follows for any HPV type, any high-risk HPV type, or any low-risk HPV type: 21 versus 9% (P = 0.04), 19 versus 11% (P = 0.20), and 15 versus 7% (P = 0.13), respectively (data not shown). Although the test with urine specimens detected fewer instances of discordance for any HPV type, it was not statistically significantly different from the instances of discordant results for cervical swab specimens for the detection of any high- or low-risk HPV type in this analysis.
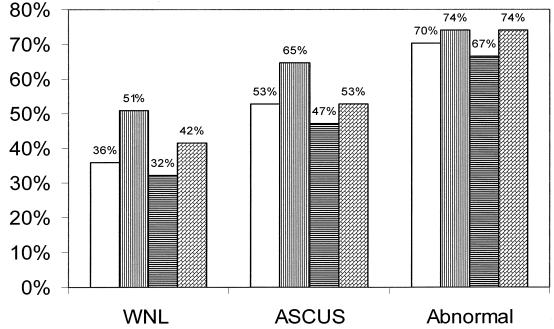
Table 3 illustrates the risk of detection of HPV DNA in urine and the cervix in association with an abnormal Pap smear. Of the 97 patients in the study for whom Pap smear data were available, 53 had Pap smears within normal limits (WNL), 17 had Pap smear results indicating ASCUS, and 27 patients had Pap smears graded low-grade squamous intraepithelial lesion (LGSIL) or high-grade squamous intraepithelial lesion (HGSIL) (Table 1). There were no significant differences between urine and the cervix in terms of the percentage of any HPV type or a high-risk HPV type of the different cytologic grades detected (Fig. 2). Figure 2 also illustrates that for women with normal Pap smears, the rate of detection of any HPV type was 15% higher in the cervix than in urine.
TABLE 3.
Association of Pap smear status with detection of HPV in urine and cervixa
| Parameter | Sample | Adjusted odds ratio (95% CI)
|
||
|---|---|---|---|---|
| WNL (n = 53) | LGSIL or HGSIL (n = 27) | ASCUS, LGSIL, or HGSIL (n = 44) | ||
| Any HPV type | Urine | 1.0 | 3.30b (1.16-9.40) | 2.89b (1.18-7.07) |
| Cervix | 1.0 | 3.44b (1.09-10.82) | 2.98b (1.16-7.66) | |
| High-risk HPV type | Urine | 1.0 | 3.31b (1.17-9.36) | 2.79b (1.14-6.84) |
| Cervix | 1.0 | 5.30b (1.68-16.73) | 3.48b (1.39-8.69) | |
| Multiple HPV types | Urine | 1.0 | 5.73b (1.50-21.81) | 3.89b (1.38-10.98) |
| Cervix | 1.0 | 5.46b (1.85-16.10) | 3.43b (1.36-8.61) | |
| Multiple high-risk HPV types | Urine | 1.0 | 5.73b (1.50-21.81) | 5.82b (1.70-19.85) |
| Cervix | 1.0 | 4.60b (1.54-13.75) | 2.87b (1.08-7.66) | |
Adjusted for age, race, CD4+ T-cell count, and HIV load. CI, confidence interval.
Statistical significance.
FIG. 2.
Detection of any HPV DNA type or any high-risk HPV DNA type in urine and cervix of women with normal Pap smears (WNL; n = 53), ASCUS (n = 17), or abnormal Pap smears (n = 27). White bars, any HPV DNA type in urine; bars with vertical stripes, any HPV DNA type in the cervix; bars with horizontal stripes, any high-risk HPV DNA type in urine; bars with angled thatching, any high-risk HPV DNA type in the cervix. The percentage of urine or cervical swab samples positive for HPV DNA is indicated above each bar for each Pap smear classification.
The ages, races, CD4 T-cell counts, and HIV loads of the patients included in the urine study were examined and were found by multivariate analysis not to be independent risk factors for having either an abnormal Pap smear or an abnormal Pap smear including ASCUS. When these factors were adjusted for, the detection of any HPV type, any high-risk HPV type, multiple HPV types, or multiple high-risk HPV types was significantly associated with having an abnormal Pap smear or an abnormal Pap smear including ASCUS for both urine and cervical swab specimens (Table 3). Incidentally, neither HPV DNA nor β-globin DNA could be amplified from 27 urine specimens and 13 cervical swab specimens. The Pap smear results for these patients were as follows: for urine specimens, 3 of 27 (11%) patients did not have Pap smear data available, 16 of 27 (59%) patients had WNL, 4 of 27 (15%) patients had ASCUS, and 4 of 27 (15%) patients had LGSIL; for the cervical swab specimens, 6 of 13 (46%) patients had WNL, 3 of 13 (23%) patients had ASCUS, 3 of 13 (23%) patients had LGSIL, and 1 of 13 (7%) patients had HGSIL. There was no further investigation as to why neither HPV DNA nor β-globin DNA could be amplified from these samples by the PCR assay in this study.
DISCUSSION
The validity of a PCR utilizing urine for the detection of HPV DNA was examined in these studies. The β-globin housekeeping gene was amplified from 87% of the cervical swab specimens but only 73% of the urine specimens. This demonstrates that despite our best efforts, the rate of amplifiability of HPV DNA from a cervical swab specimen is significantly higher than that from a urine specimen. This could simply be due to the fact that urine samples from healthy women contain relatively few cells [Microscopic examination of sediment, p. 78, H. M. Free (ed.), Modern urine chemistry, Bayer Diagnostics Division, Bayer Corporation, Tarrytown, N.Y., 1996] or that urine contains inhibitors of the PCR (7, 15, 34). The rates of detection of any HPV DNA type, any high-risk HPV type, and any low-risk HPV type were not statistically different between the urine specimens and the cervical swab specimens for this population. This may imply that a patient whose cervix is infected with HPV is more likely to shed HPV-infected cervical cells that can then be detected in the urine of that patient. This possibility is not particularly surprising when one considers other virus infection systems, like the cytomegalovirus infection system, in which virus is often shed in the urine (36). Conversely, the fact that significant differences in rates of HPV DNA detection between the urine and the cervix were not detected in the present cohort may simply be due to a lack of power due to the size of the population studied. Future studies with increased numbers of paired samples are needed in order to strengthen these observations.
The overall prevalence of detection of any HPV DNA was 48% for the urine specimens and 58% for the cervical swab specimens. These rates are higher than those found in other recent studies. One study conducted by Strauss et al. (32) with 144 women attending a genitourinary clinic demonstrated HPV DNA in 40% of cervical swab samples and 15% of urine specimens by first-round PCR amplification. Another study conducted with 489 patients who were referred to a colposcopy clinic detected HPV DNA in 49% of the cervical swab specimens and 38% of the urine specimens (10). The differences in prevalence between these studies and the present study may simply be due to the different populations that were evaluated. Because of the sexually acquired nature of their disease, HIV-positive women probably experience increased levels of exposure to HPV as well as other STDs. This would result in a higher overall prevalence of HPV infection in this population.
The prevalence rates found in the present study also differ from those found in a recent study conducted by Sellors et al. (30) with a population of women who were attending a colposcopy clinic. The overall prevalence of HPV of any type in the study by Sellors et al. (30) was 35% in urine specimens and 63% in physician-obtained cervical swab specimens. The discrepancy between the two studies can again be explained in part by the different populations examined. The study of women attending a colposcopy clinic contained only women who had an abnormal Pap smear (ASCUS, LGSIL, HGSIL, or adenocarcinoma). Conversely, 52.5% of the HIV-positive population evaluated in the present study had Pap smear gradings within normal limits (Table 1), which would lead to overall lower prevalence rates in cervical swab samples. Additionally, the higher prevalence seen in the urine specimens in the urine study with HIV-positive women may simply reflect differences in specimen handling and extraction techniques between the two studies.
Lastly, the prevalence of HPV DNA in the present study was lower than that found in another recent study conducted by Jacobson et al. (14). They detected HPV DNA at relatively high rates in urine (75%) and cervical swab (90%) specimens from an inner-city adolescent population. Interestingly, although no patients under 20 years of age were included in the present study with HIV-infected subjects, the rate of detection of any HPV type in urine was highest (79%) for women ages 20 to 30 years (data not shown).
Examination of the samples for the 27 different HPV types that are detected by the reverse line hybridization assay revealed the presence of 26 of these types in the patients in the urine study. The diversity of HPV types represented in this study is most likely a reflection of the high-risk behaviors of these women. When the overall distribution of HPV types that were detected in the urine samples was compared with the overall distribution of HPV types that were detected in the cervical swab samples (Table 2), potential preferences for the detection of types 53, MM4, MM7, 54, and 55 were seen for the cervical swab specimens and a potential preference for the detection of type MM9 was seen for the urine samples. These differences did not reach statistical significance due to the small sample sizes. HPV type MM9 is considered to be of intermediate to high risk as a cause of cervical cancer (20). Type MM9 may represent a viral type that is tropic for the vagina or that primarily infects the urethra. Types 53 and 54 are considered low risk; and types 55, MM4, and MM7 are thought to be of intermediate or high risk as causes of cervical cancer. Perhaps infections caused by these viral types are more likely to be localized only to the cervix or have very low copy numbers so that the virus is not shed in the urine. This lack of sensitivity for these types could prove to be a limitation of urine testing, which could potentially be compensated for by taking multiple urine specimens from the same patient (5). Future studies are needed not only to increase the sample size but also to compare the site(s) of anogenital HPV infection in women whose urine is positive for HPV DNA. Finally, HPV type 16 was commonly found in both the cervix and urine, as seen previously (14). However, the other most common types found in the present study (types 35 and MM7 in the cervix and type 59 in urine) were either not tested for or found less often in the inner-city adolescent population. This most likely reflects the differences in the populations tested between the two studies.
When the rates of concordance of the results between the samples was compared, the results of the test with urine were not statistically different from those of tests with cervical swabs for the detection of any high-risk or low-risk HPV types for specimen pairs with discordant results. However, when the detection of any HPV type is considered, HPV DNA of any type was detected significantly more frequently in the cervical swab specimens. This is most likely due to the increased number of comparisons for this group and indicates that the assay with urine specimens may be a slightly less sensitive method for detecting HPV DNA than the assay with cervical swab specimens. This finding may also be a reflection of the natural history of HPV infection, which may be detected more readily in urine as a function of the higher viral loads and increased shedding of infected cells. Determination of the local HPV load would help prove this theory. The inability to detect low-risk HPV types in urine is not believed to be a serious limitation of this assay.
Table 3 examined the risk associated with having an abnormal Pap smear when HPV DNA was detected in urine or cervical swab samples. For both sample types, there were significant associations between having an abnormal Pap smear or an abnormal Pap smear result including ASCUS and detection of any HPV type, any high-risk HPV type, multiple HPV types, or multiple high-risk HPV types. Importantly, for these categories the percentage of samples in which HPV DNA was detected did not differ significantly between the urine specimens and the cervical swab specimens (Fig. 2 and data not shown). These data suggest that testing of urine may be comparable to testing of a cervical swab specimen for HPV DNA detection and that, like the cervical swab specimen, the rate of HPV DNA detection increases with the severity of the abnormal Pap smear or the presence of HPV disease. These data corroborate the findings of Vossler et al. (35), who demonstrated HPV DNA in urine specimens from 13 of 15 women who had evidence of condylomata, dysplasia, or invasive carcinoma but in only 3 of 6 women who had normal Pap smears. A potential limitation of this analysis is that our study lacks biopsy data to verify the findings of an abnormal Pap smear. Such data could be important for determination of the true positives in the group with abnormal Pap smears. Unfortunately, an ASCUS or even a low-grade abnormal Pap smear is not always cause for an immediate biopsy in this HIV-positive population, and women with normal smears are not normally subject to a biopsy procedure. Thus, with so few biopsy data available for this study, Pap smear status was used to stratify the population.
Interestingly, in women with normal Pap smears the rate of detection of any HPV type was approximately 15% greater in the cervix than in urine (Fig. 2). In the present study, this finding was not significant. However, it be will interesting to monitor this observation in future studies in order to determine if screening by use of a cervical swab specimen compared to screening by use of a urine sample would produce a higher percentage of false-positive results.
In summary, we have developed a urine-based assay for detection of the DNA of HPV, which is responsible for nearly all cases of cervical cancer and which is of particular concern in the HIV-positive population. The assay with urine appears to be as adept as the assay with a cervical swab specimen for the detection of any HPV type or any high-risk HPV type. Given that instances of discordant results for HPV DNA detection between the urine specimens and the cervical swab specimens may potentially be rectified by testing multiple urine specimens from the same patient, the urine assay merits further study to assess whether it may be an appropriate cervical cancer screening technique for HIV-positive women. Primary screening of HIV-positive women could involve the identification of patients infected with high-risk HPV types by urine assay and then monitoring of these patients more closely by use of Pap smears (every 6 months). In addition, this assay could potentially be used as a secondary screen for those HIV-positive women with abnormal Pap smears to monitor the course of their HPV infections. Additional studies need to be performed in order to solidify the role of urine screening in the HIV-positive population. Future studies also need to be undertaken to ascertain the role of testing of urine for HPV DNA in women not infected with HIV.
Acknowledgments
This research was supported by the Doris Duke Charitable Foundation, NIH grant CA86378-01, and the Stanley Scott Cancer Center.
We acknowledge Roche Molecular Systems for graciously donating the reagents used for amplification and detection of HPV.
REFERENCES
- 1.Ackermann, S. P., R. M. Brackbill, B. A. Bewerse, N. E. Cheal, and L. M. Sanderson. 1992. Cancer screening behaviors among US women: breast cancer, 1987-1989 and cervical cancer, 1988-1989. Morb. Mortal. Wkly. Rep. 41:17-25. [PubMed] [Google Scholar]
- 2.Anderson, L. M. 1995. Has the use of cervical, breast, and colorectal cancer screening increased in the United States? Am. J. Public Health 85:840-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anonymous. 1996. National Institutes of Health Consensus Development Conference statement on cervical cancer. Gynecol. Oncol. 66:351-361. [DOI] [PubMed] [Google Scholar]
- 4.Arany, I., and S. Tyring. 1998. Systemic immunosuppression by HIV infection influences HPV transcription and thus local immune responses in condyloma acuminatum. Int. J. STD AIDS 9:268-271. [DOI] [PubMed] [Google Scholar]
- 5.Burstein, G. R., G. Waterfield, A. Joffe, J. M. Zenilman, T. C. Quinn, and C. A. Gaydos. 1998. Screening for gonorrhea and chlamydia by DNA amplification in adolescents attending middle school health centers. Opportunity for early intervention. Sex. Transm. Dis. 25:395-402. [DOI] [PubMed] [Google Scholar]
- 6.Chan, S. Y., H. Delius, A. L. Halpern, and H. U. Bernard. 1995. Analysis of genomic sequences of 95 papillomavirus types: uniting typing, phylogeny, and taxonomy. J. Virol. 69:3074-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chernesky, M. A., D. Jang, J. Sellors, K. Luinstra, S. Chong, S. Castriciano, and J. B. Mahony. 1997. Urinary inhibitors of polymerase chain reaction and ligase chain reaction and testing of multiple specimens may contribute to lower assay sensitivities for diagnosing Chlamydia trachomatis infected women. Mol. Cell Probes 11:243-249. [DOI] [PubMed] [Google Scholar]
- 8.Crotchfelt, K. A., L. E. Welsh, D. DeBonville, M. Rosenstraus, and T. C. Quinn. 1997. Detection of Neisseria gonorrhoeae and Chlamydia trachomatis in genitourinary specimens from men and women by a coamplification PCR assay. J. Clin. Microbiol. 35:1536-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellerbrock, T., M. Chaisson, T. Bush, X.-W. Sun, D. Sawo, K. Brudney, et al. 2000. Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA 283:1031-1037. [DOI] [PubMed] [Google Scholar]
- 10.Forslund, O., B. G. Hansson, P. Rymark, and B. Bjerre. 1993. Human papillomavirus DNA in urine samples compared with that in simultaneously collected urethra and cervix samples. J. Clin. Microbiol. 8:1975-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gravitt, P. E., C. L. Peyton, T. Q. Alessi, C. M. Wheeler, F. Coutlee, A. Hildesheim, M. H. Schiffman, D. R. Scott, and R. J. Apple. 2000. Improved amplification of genital human papillomaviruses. J. Clin. Microbiol. 38:357-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gravitt, P. E., C. L. Peyton, R. J. Apple, and C. M. Wheeler. 1998. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. J. Clin. Microbiol. 36:3020-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrero, R. 1996. Epidemiology of cervical cancer. J. Natl. Cancer Inst. Monogr. 21:1-6. [PubMed] [Google Scholar]
- 14.Jacobson, D. L., S. D. Womack, L. Peralta, J. M. Zenilman, K. Feroli, J. Maehr, R. W. Daniel, and K. V. Shah. 2000. Concordance of human papillomavirus in the cervix and urine among inner city adolescents. Pediatr. Infect. Dis. J. 19:722-728. [DOI] [PubMed] [Google Scholar]
- 15.Khan, G., H. O. Kangro, P. J. Coates, and R. B. Heath. 1991. Inhibitory effects of urine on the polymerase chain reaction for cytomegalovirus DNA. J. Clin. Pathol. 44:360-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorincz, A. T., R. Reid, A. B. Jenson, M. D. Greenberg, W. D. Lancaster, and R. J. Kurman. 1992. Human papillomavirus infection of the cervix: relative risk associations of 15 common anogenital types. Obstet. Gynecol. 79:328-337. [DOI] [PubMed] [Google Scholar]
- 17.Maiman, M. 1998. Management of cervical neoplasia in human immunodeficiency virus-infected women. J. Natl. Cancer Inst. Monogr. 23:43-49. [DOI] [PubMed] [Google Scholar]
- 18.Maiman, M., R. G. Fruchter, M. Clark, C. D. Arrastia, R. Matthews, and E. J. Gates. 1997. Cervical cancer as an AIDS-defining illness. Obstet. Gynecol. 89:76-80. [DOI] [PubMed] [Google Scholar]
- 19.Maiman, M., R. G. Fruchter, E. Serur, J. C. Remy, G. Feuer, and J. Boyce. 1990. Human immunodeficiency virus infection and cervical neoplasia. Gynecol. Oncol. 38:377-382. [DOI] [PubMed] [Google Scholar]
- 20.Manos, M. M., J. Waldman, T. Y. Zhang, C. E. Greer, G. Eichinger, M. H. Schiffman, and C. M. Wheeler. 1994. Epidemiology and partial nucleotide sequence of four novel human papillomaviruses. J. Infect. Dis. 170:1096-1099. [DOI] [PubMed] [Google Scholar]
- 21.Matsukura, T., and M. Sugase. 1995. Identification of genital human papillomaviruses in cervical biopsy specimens: segregation of specific virus types in specific clinicopathologic lesions. Int. J. Cancer 61:13-22. [DOI] [PubMed] [Google Scholar]
- 22.Meisels, A. 1983. The story of a cell. The George N. Papanicolaou Award lecture. Acta Cytol. 27:584-596. [PubMed] [Google Scholar]
- 23.Minkoff, H., J. Feldman, J. DeHovitz, S. Landesman, and R. D. Burk. 1998. A longitudinal study of human papillomavirus carriage in human immunodeficiency virus-infected and human immunodeficiency virus-uninfected women. Am. J. Obstet. Gynecol. 178:982-986. [DOI] [PubMed] [Google Scholar]
- 24.Munoz, N., and F. X. Bosch. 1989. Epidemiology of cervical cancer, p. 9-40. In N. Munoz, F. X. Bosch, and O. M. Jensen (ed.), Human papillomaviruses and cervical cancer. IARC Scientific Publications, Lyon, France. [PubMed]
- 25.Orth, G., and M. Favre. 1985. Human papillomaviruses. Biochemical and biologic properties. Clin. Dermatol. 3:27-42. [DOI] [PubMed] [Google Scholar]
- 26.Purola, E., and E. Savia. 1977. Cytology of gynecologic condyloma acuminatum. Acta Cytol. 21:26-31. [PubMed] [Google Scholar]
- 27.Reddy, D. M., and S. A. Wasserman. 1997. Patient anxiety during gynecologic examinations. Behavioral indicators. J. Reprod. Med. 42:631-636. [PubMed] [Google Scholar]
- 28.Rellihan, M. A., D. P. Dooley, T. W. Burke, M. E. Berkland, and R. N. Longfield. 1990. Rapidly progressing cervical cancer in a patient with human immunodeficiency virus infection. Gynecol. Oncol. 36:435-438. [DOI] [PubMed] [Google Scholar]
- 29.Segnan, N. 1997. Socioeconomic status and cancer screening. IARC Sci. Publ. 138:369-376. [PubMed] [Google Scholar]
- 30.Sellors, J. W., A. T. Lorincz, J. B. Mahony, I. Mielzynska, A. Lytwyn, P. Roth, M. Howard, S. Chong, D. Daya, W. Chapman, and M. Chernesky. 2000. Comparison of self-collected vaginal, vulvar and urine samples with physician-collected cervical samples for human papillomavirus testing to detect high-grade squamous intraepithelial lesions. Can. Med. Assoc. J. 163:513-518. [PMC free article] [PubMed] [Google Scholar]
- 31.Six, C., I. Heard, C. Bergeron, G. Orth, J., Poveda, P. Zagury, P. Cesbron, C. Crenn-Hebert, R. Pradinaud, M. Sobesky, C. Marty, M. Babut, J. Malkin, A. Odier, S. Fridmann, J. Aubert, J. Brunet, and I. de Vincenzi. 1998. Comparative prevalence, incidence and short-term prognosis of cervical squamous intraepithelial lesions amongst HIV-positive and HIV-negative women. AIDS 12:1047-1056. [PubMed] [Google Scholar]
- 32.Strauss, S., J. Z. Jordens, D. McBride, C. Sonnex, S. Edwards, U. Desselberger, P. Watt, and J. J. Gray. 1999. Detection and typing of human papillomavirus DNA in paired urine and cervical scrapes. Eur. J. Epidemiol. 6:537-543. [DOI] [PubMed] [Google Scholar]
- 33.Ting, Y., and M. M. Manos. 1990. Detection and typing of genital human papillomaviruses, p. 356-367. In M. Innis, D. Gelfand, J. Sninsky, and T. White (ed.), PCR protocols: a guide to methods and applications. Academic Press, Inc., San Diego, Calif.
- 34.Toye, B., W. Woods, M. Bobrowska, and K. Ramotar. 1998. Inhibition of PCR in genital and urine specimens submitted for Chlamydia trachomatis testing. J. Clin. Microbiol. 36:2356-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vossler, J. L., B. A. Forbes, and M. D. Adelson. 1995. Evaluation of the polymerase chain reaction for the detection of human papillomavirus from urine. J. Med Virol. 45:354-360. [DOI] [PubMed] [Google Scholar]
- 36.Yamaguchi, Y., T. Hironaka, M. Kajiwara, E. Tateno, H. Kita, and K. Hirai. 1992. Increased sensitivity for detection of human cytomegalovirus in urine by removal of inhibitors for the polymerase chain reaction. J. Virol. Methods 37:209-218. [DOI] [PubMed] [Google Scholar]